Abstract
Background
Cancer stem cells proved to have a vital role in cell migration, invasion, metastasis, and treatment resistance of colorectal cancer (CRC) that subsequently lead to poor clinical outcomes. These stem cells may be a novel therapeutic target for the management of CRC progression. Signals of the Notch-1 pathway are responsible for acquisition of stem cell characters. ALDH1 and CD44 are usually detected in stem cells in colorectal cancer.
Aim
The aims of this work are to evaluate the immunohistochemical expression of cancer stem cell markers ALDH1, Notch1, and CD44 in colorectal cancer and investigate their correlation with clinicopathological characters and patient survival.
Methods
Paraffin-embedded specimens of 70 patients with primary colorectal carcinoma were analyzed for Notch 1, ALDH1, and CD44 expressions by immunohistochemistry.
Results
Notch1 was mainly located in the cytoplasm of CRC tissues, rarely expressed in adjacent normal tissues. A highly statistically significant relationship was found between grading, lymphovascular invasion, the degree of lymphocytic infiltration, peritumoral budding, lymph node ratio, lymph node metastasis, and Notch1 expression (p < 0.001). There was a highly statistically significant relationship found between AJCC stage and Notch1 expression (p < 0.001). CD44 was mainly located in the cell membrane of CRC tissues. A highly statistically significant relationship was found between grading (p = 0.006), lymphovascular invasion, the degree of lymphocytic infiltration, peritumoral budding, lymph node metastasis, lymph node ratio, and CD44 expression (p < 0.001). There was a highly statistically significant relationship found between AJCC stage and CD44 expression (p < 0.001). ALDH1 was detected in the cytoplasm of the CRC tissue. A highly statistically significant relationship was found between grading, lymphovascular invasion, the degree of lymphocytic infiltration, peritumoral budding, lymph node metastasis, lymph node ratio, and ALDH1 expression (p < 0.001). There was a highly statistically significant relationship found between AJCC stage and ALDH1 expression (p < 0.001). There is a highly statistically significant direct correlation between Notch1, CD44 expression, and ALDH1 expression (p < 0.001).
Conclusions
There is a substantial correlation between Notch 1, ALDH1, and CD44 as cancer stem cell markers and lymph node metastasis, advanced stage and tumor recurrence in colorectal carcinoma.
Conclusion
Expression of stem cell markers ALDH1, Notch1, and CD44 correlates with poor prognosis in a CRC and represents an independent prognostic factor. They are associated with a feature of epithelial-mesenchymal transition evidenced by their association with high tumor burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women worldwide [8]. In Egypt, CRC was identified in 11–15% of patients who underwent colonoscopy [11]. The cancer stem cells (CSCs) that represent tumor-initiating cells expected to survive after radiochemotherapy, with subsequent local recurrences and metastasis despite treatment [1]. Metastasis in CRC is the primary cause of poor prognosis [23]. That is why identification and targeting these cells are needed to improve survival. Signals through the Notch pathway play a vital role in the stem cell maintenance in achieving a balance between cell proliferation and apoptosis [19]. Notch usually triggered by binding of a ligand to adjacent cells. It is also vital in intestinal development and renewal of epithelium [33]. The ALDH1 which is a detoxifying enzyme is responsible for resistance to alkylating chemotherapeutic agents with guarding against oxidative damage. ALDH1 positivity usually associated with stem cell characters as self-renewal, tumorigenicity, and chemoradiotherapy resistance and correlate withs a poor prognosis of breast cancer [12]. ALDH1 also has a role in epithelial-mesenchymal transition (EMT associating with the capability of the tumor for invasion and metastasis [27]. CD44 is an adhesion glycoprotein responsible for controlling cell-matrix interactions. Mutation in it is closely related to the invasion and metastasis of tumor cells [31].
Patients and Methods
For this retrospective cohort study, 70 formalin-fixed, paraffin-embedded tissue blocks of primary colorectal carcinoma cases and concordant cancer adjacent normal mucosa were retrieved from the archives of the Pathology Department, Faculty of Medicine, Zagazig University, in the period from 2012 to 2014. Typing and grading of colorectal carcinomas were based on WHO 2010 published criteria [14]. Staging of colorectal carcinomas was defined according to TNM staging systems [7]. Tumor budding was assessed semiquantitatively on H&E-stained sections. The tumor border was scanned at the 10× power, and the area of densest budding was identified. In the center of this area, tumor buds (single cells or clusters of up to 5 cells) were counted at × 20 magnification [28]. Cases were then divided into two groups: counts of 0–9 tumor buds were classified as low-grade, while numbers of ≥ ten tumor buds were classified as high-grade budding [26]. Lymph node ratio (LNR) categorization was performed. A cut-off value of 0.25 was chosen to facilitate case assessment to subgroups because 0.25 represents the number of metastatic lymph nodes (Pn1 category) based on 12 lymph nodes harvested (3/12), which are recommended by the TNM staging system. Cases assigned to two groups based on LNR: LNR1, less than or equal to 0.25; LNR2, more significant than 0.25 [25]. Lymphocytic infiltration was assessed and divided into mild, moderate, and severe [21]. Lymphovascular invasion was evaluated whether present or absent [21]. We identified sex, age, tumor size, histological subtype, grade, stage, lymph node, and distant metastasis of the cases by a retrospective examination of the patient’s and the slide files of the Pathology Department. Most patients had follow-up records for 3 years at the Clinical Oncology Department, Faculty of Medicine, Zagazig University. Patients were followed up until death or their most recent medical examination. The follow-up was up to 2017. Pre-treatment staging included an examination under anesthesia, colonoscopy, cystoscopy, chest X-ray, abdominal, and pelvic computed tomography. Clinical, radiological, and pathological data were abstracted from the files of the corresponding departments. Clinical follow-up was done every 3 months to all cases and information concerning follow-up was abstracted from hospital records or patient contact. Patients with deficient data were omitted. None of the patients had received chemo- or radiotherapy preceding surgery.
Immunohistochemistry
Thick sections of 5 μm were cut from paraffin blocks, placed on positively charged slides and then deparaffinized in xylene and rehydrated in increasing grades of ethyl alcohol. Antigen retrieval was performed by boiling sections in citrate buffer (pH 6.0) for 20 min and then after washing with phosphate-buffered saline, the slides were incubated with monoclonal primary anti-CD44 antibodies (Novacastra; Newcastle, UK) at 1:100, primary goat polyclonal antibodies against Notch1 (1:100 dilution; sc-6014; Santa Cruz Biotechnology, Santa Cruz, Calif) overnight at 4C, a primary rabbit monoclonal anti-ALDH1 antibody (1:400; Earth Ox; cat no. HZ348711).
The sections were then washed in phosphate-buffered saline and incubated with polyperoxidase-anti-mouse/rabbit IgG (Zymed Laboratories, San Francisco, California, USA) for 20 min. 3, 30-diaminobenzidine was used as the chromogen. Finally, the sections were counterstained with hematoxylin. Normal tonsils were used as a positive control for CD44; Rat Brain Tissue used as the positive control for Notch1; rat liver used as the positive control for ALDH1. The negative controls were replacing the primary antibodies by the non-immune serum.
Evaluation of Immunohistochemical Expressions
All slides were evaluated by two pathologists separately. Positive cells were counted in 10 high-power fields.
Notch 1 Immunohistochemistry
Intensity of staining that divided into (0, 1, 2, 3) and extent of positive cells given a score 0 if 5% of cells showed positivity; 1 if 6–25%; 2 if 26–50%; 3 if 51–75%; 4 when < 75%. The net score by multiplying extent and intensity and graded as (0 score, absent), + (1–4 score, weak), ++ (5–8 score, moderate), and + + + (9–12 score, strong) [33].
ALDH1 Immunohistochemistry
ALDH1 expression was considered low if < 20% of tumor cells were positive, while cases with > 20% considered a high ALDH1 expression [2].
CD44 Immunohistochemistry
CD44 considered positive if more than 5% of the tumor with (membrane and/or cytoplasm immunoreactivity) [22].
Statistical Analysis
Continuous variables were expressed as the mean ± SD and median (range), and the categorical variables were expressed as a number (percentage). Continuous variables were checked for normality by using the Shapiro-Wilk test. Independent samples Student’s t test was used to compare two groups of normally distributed variables while Mann-Whitney U test was used for non- normally distributed variables. The Kruskal-Wallis H test was used to compare between more than two groups of non-normally distributed variables. Percent of categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test when was appropriate. The trend of change in the distribution of relative frequencies between ordinal data was compared using the chi-square test for trend. Disease-free survival (DFS) was calculated as the time from surgery to the date at which local recurrence or distant metastasis were detected or most recent follow-up in which local recurrence or distant metastasis was not detected (censored). Overall survival (OS) was calculated as the time from diagnosis to death or the most recent follow-up contact (censored). Stratification of OS and DFS was done according to all clinicopathological features and immunohistochemical markers. These time-to-death distributions were estimated using the method of Kaplan-Meier plot and compared using two-sided exact log-rank test. All tests were two-sided. P value < 0.05 was considered significant. All statistics were performed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA) and MedCalc windows (MedCalc Software bvba 13, Ostend, Belgium).
Results
Seventy cases were fitting into the selection criteria. Clinicopathologic characteristics and staging data of primary colorectal carcinoma cases are summarized in Table 1. The mean age was 54.14 ± 8.73 years (range 29–68), 27 males and 43 females were included in the study. The commonest site is in the Rectum representing 41 cases (58.6%), followed by Left colon representing 21 cases (30%), then Right colon representing 8 cases (11.4%). Most of the tumor 48/70 (68.6%) had a tumor size ≤ 5 mm, whereas 22/40 (31.4%) had a tumor size > 5 mm. Adenocarcinoma (NOS) is the commonest histologic type representing 56 cases (80%), followed by mucinous carcinoma representing 9 cases (12.9%) then signet-ring carcinoma representing 5 cases (7.1%). Histologic grading showed 9 cases (12.9%) with grade 1 (well differentiated), 45 cases (64.3%) with grade 2 (moderately differentiated), and 16 cases (22.9%) with grade 3 (poorly differentiated) adenocarcinoma. Evidence of lymphovascular invasion was present only in 49 cases (70%). As for neural invasion, it was present in 12 cases (17.1%). Most of the tumor 31/70 (44.3%) showed peritumoral moderate lymphocytic infiltration. High-grade peritumoral budding was present in 42 cases (60%), while 28 cases (40%) showed low-grade peritumoral budding. Most of the tumor 37/70 (52.9%) had a lymph node ratio > 0.25, whereas 33/70 (47.1%) had a lymph node ratio < 0.25. Evidence of lymph node metastasis was detected in 37 cases (52.9%). Eight cases (11.4%) were diagnosed at TNM stage I, 12 cases (17.1%) at stage TNM IIA, four cases (5.7%) at stage TNM IIB, six cases (8.6%) at stage IIC, 14 cases (20%) at stage TNM IIIA, five cases (7.1%) at stage TNM IIIB, 14 cases (20%) at stage TNM IIIC, and 7 cases (10%) at stage TNM IV.
Notch1 Expression
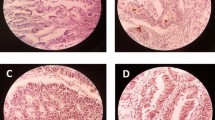
Analysis of Notch1 expression with clinicopathologic characteristics was presented in Table 2. Notch1 was mainly located in the cytoplasm of CRC tissues, rarely expressed in adjacent normal tissues. Negative Notch1 expression was detected in 15 out of 70 (21.4%) cases. Seventeen cases (24.3%) showed mild Notch1 expression (Fig. 1), 30 cases (42.9%) showed moderate Notch1 expression (Fig. 2), and eight cases (11.4%) showed marked Notch1 expression (Fig. 3). No statistically significant relationship was found between histological type and Notch1 expression (p = 0.144). A statistically significant relationship was found between the size and the Notch1 expression (p = 0.020). A highly statistically significant relationship was found between grading, lymphovascular invasion, the degree of lymphocytic infiltration, peritumoral budding, lymph node ratio, lymph node metastasis, and Notch1 expression (p < 0.001). The association between neural invasion and Notch1 expression was found to be highly statistically significant (p = 0.001). Notch1 showed high specific association with tumor staging; there was a highly statistically significant relationship was found between AJCC stage and Notch1 expression (p < 0.001).
CD44 Expression
CD44 was mainly located in the cell membrane of CRC tissues. Negative CD44 expression was detected in 25 out of 70 (35.7%) cases (Fig. 4), and 45 out of 70 (64.3%) cases showed positive CD44 expression (Figs. 5 and 6). No statistically significant relationship was found between the histological type (p = 0.660) and CD44 expression. There was a statistically significant difference between the size (p = 0.002), site (p = 0.038), perineural invasion (p = 0.045) and CD44 expression. A highly statistically significant relationship was found between grading (p = 0.006), lymphovascular invasion, the degree of lymphocytic infiltration, peritumoral budding, lymph node metastasis, lymph node ratio, and CD44 expression (p < 0.001). CD44 showed high specific association with tumor staging. There was a highly statistically significant relationship was found between AJCC stage and CD44 expression (p < 0.001) (Table 3).
ALDH1 Expression (Figs. 7 and 8)
ALDH1 was detected in the cytoplasm of the CRC tissue. No statistically significant relationship was found between the histological type (p = 0.163) and ALDH1 expression. There was a statistically significant difference between the size (p = 0.046), neural invasion (p = 0.02), and ALDH1 expression. The association between the site and ALDH1 expression was found to be highly statistically significant (p = 0.001). A highly statistically significant relationship was found between grading, lymphovascular invasion, the degree of lymphocytic infiltration, peritumoral budding, lymph node metastasis, lymph node ratio, and ALDH1 expression (p < 0.001). ALDH1 showed high specific association with tumor staging. There was a highly statistically significant relationship found between AJCC stage and ALDH1 expression (p < 0.001) (Table 3).
Correlation Between Notch1 Expression, CD44 Expression, and ALDH1 Expression
There is a highly statistically significant direct correlation between Notch1, CD44 expression, and ALDH1 expression (p < 0.001).
The relationship Between Notch1 Expression and Outcome in 70 Patients with CRC (Table 4)
Relapse was observed significantly in notch1 positive expressed patients than those had a negative expression (p < 0.001); the mean DFS in notch1 negative expressed patients was 33.73 months significantly better than in positive cases with mean DFS (26, 11.07, and 9 months for +, ++, and +++ notch1 expressed patients respectively. Overall survival was significantly better in patients had negative notch1 expression with a mean of 34.53 months than those expressed notch1 +, ++, and +++, (mean 30.12, 18.8, 11 months) respectively (p < 0.001) at 95%CI.
The relationship Between CD44 Expression, ALDH1 Expression, and Outcome in 70 Patients with CRC (Table 5)
The relapse was observed significantly in CD44 positive and highly expressed ALDH1 cases than CD44 negative and low expressed ALDH1 cases, p < 0.001. The mean DFS in CD44 - and low expressed ALDH1- were 32.08 and 31.32 months significantly better than CD44+ and highly expressed ALDH1+ (mean DFS were 12.71 and 11.66 months, respectively). The OS was considerably better in CD44 - and low expressed ALDH1- expressed patients (mean OS 34.32 and 33.57 months respectively in comparison to CD44 + and highly expressed ALDH1+ (mean OS 18.25 and 17.58 months respectively), p < 0.001 at 95%C I (Fig. 9).
Discussion
Cancer colon represents the third leading cause of cancer death worldwide [30]. Mortality in colorectal cancer in most of the cases is due to the distant spread. [6] concluded that stem cells could initiate tumor and reproduced the full morphological and phenotypic heterogeneity. These cells can escape the effect of chemo- and radiotherapy, that is why targeting these cells will improve the prognosis of CRC [10]. The role of cancer stem cells in the development of a tumor is proved, but the relation of between CSC marker expression with disease prognosis is still not clear [24].
Notch1 is one of stem cell markers. Its function is to control the balance between cell proliferation, differentiation, and apoptosis [29]. It was observed that its activation is observed in CRC associated with chemoresistance [34].
In contrast to Zheng et al [34] who found no difference in Notch 1 expression in the different colonic site, in this study, Notch1 was overexpressed in rectal cancer p < 0.001 than other colonic sites. This may be due to the difference in numbers of the studied cases in each colonic location in their study.
High Notch1 expression correlates with the large size of the primary tumor (p = 0.020) while Zheng et al [34] found no correlation between size and Notch1 expression.
In agreement with Zheng et al [34], no statistically significant relationship was found between histological type and Notch1 expression (p = 0.144) while Zhang et al [33] found that signet and mucinous subtypes have higher Notch1 expression.
Zhang et al [33] concluded that knockdown of Notch1 inhibited cell proliferation with cell cycle arrest in G0/G1 phase and retarded the development and growth of implanted while its induction promotes cell proliferation and inhibited spontaneous apoptosis and accelerated the development and growth of implanted colon cancers in vivo.
In line with the finding of Zhang et al and Zheng et al [33, 34] we found a direct correlation between Notch1 and lymph node metastasis expression and tumor grade (p < 0.001).
The association between neural and lymphovascular invasion and Notch1 expression was found to be highly statistically significant (p = 0.001). Highly statistically significant (p = 0.001) correlation was detected between Notch1 appearance and degree of lymphocytic infiltration and lymph node ratio.
Peritumoral budding in CRC reflects the ability of cells for invasion and usually associated with lymph node metastasis, lymphovascular invasion and distant metastasis with a high rate of local recurrence and poor outcome.
According to our result, a significant correlation was detected between Notch1 expression peritumoral budding. This finding explained by the observation of Fender et al [9] who concluded that introducing Notch-1 into the colon tumor cell line resulted in increased migration and growth by increased expression of the EMT/stemness associated proteins as Slug and Smad-3.
In concordance with Chu et al and Zhang et al [5, 34] there was a highly statistically significant relationship found between AJCC stage and Notch1 expression (p < 0.001). This is reasonable because of the association of the Notch signaling pathway and NF-κB facilitating its nuclear retention, and it was proved that Notch-1 control MMP-9 expression that promotes cancer cell invasion.
According to our finding, level of Notch1 expression correlates with poor overall survival. Chu et al [5] reported the same results.
According to Dalerba et al [6], CD44 could be one of the stem cell markers for colorectal cancer. In agreement with Zavrides et al [32], there was a statistically significant correlation between high CD44 expression and size (p = 0.038) and stage (p < 0.001) of colorectal cancer, in contrast, Hong et al [17], found no correlation between CD44 expression and stage and size of the tumor.
CD44 expression showed a statistically significant difference concerning its appearance in different colonic sites (p = 0.002), being expressed more in the rectum than colon; this was in agreement with the finding of Nour El Houda et al [24]. However, in contrast, Hong et al [17] found that CD44 expression rate was lower in the rectum than colonic tumors.
Nour El Houda et al [24], found that adenocarcinoma cases showed the highest rates of CD44 expression (68.9%) than mucoid and signet ring subtypes, but in this study, no variation detected (p = 0.660). This may be explained by a low number of cases of the unconventional type involved in this study.
In agreement with Huh et al and Nour El Houda et al [20, 24] CD44 expression correlated with lymph node metastasis and (p < 0.001).
A statistically significant relationship was found between perineural invasion (p = 0.045), lymphovascular invasion, the degree of lymphocytic infiltration, peritumoral budding and lymph node ratio and CD44 expression (p < 0.001). As far as we know, this work is the first that investigates this association.
In contrast to Hong et al. [17], we found that high CD44 expression correlates with the reduced OS. Huh, et al. [20] reported the same finding.
Aldehyde dehydrogenase 1 (ALDH1) is a member of a group of intracellular enzymes that are involved in differentiation, and drug resistance through the oxidation of cellular aldehydes [16].
The association between rectal ALDH1 expression rather than colonic in this work found to be of high significance (p = 0.001). On the contrary Gossens-Beumer et al [13], found that high ALDH1 expression was associated with colonic tumor rather than rectal tumor.
No statistically significant relationship was found between the histological type (p = 0.163) and ALDH1 expression, a finding also detected by Holah et al [16].
A relationship found between lymph node metastasis, and ALDH1 expression was highly significant (p < 0.001). This agreed with other studies [3, 4, 18] and also agreed with Hessman et al [15] who found that 52.9% of cases with nodal metastasis showed high expression of ALDH1. On the contrary, Zhou et al., [35], found that no association was identified between lymph node metastasis and ALDH1 expression.
There was a highly statistically significant relationship was found between AJCC stage and ALDH1 expression (p < 0.001).
ALDH1 showed high specific association with lymphovascular invasion (p < 0.001). Finding also detected by Holah et al [16] who found that ALDH1 had a significant association with vascular invasion in breast cancer.
ALDH1 induces epithelial-mesenchymal transition (EMT) in cancer cells that contribute to the invasive and metastatic tumor. This process is considered to promote cancer cell progression through the basement membrane and invasion into the surrounding microenvironment, such as the lymph and blood vascular systems [3, 4].
Statistically significant relationship between ALDH1 expression and degree of lymphocytic infiltration, peritumoral budding, lymph node metastasis, lymph node ratio invasion (p = 0.02). As far as we know, it is the first paper that examined these relations.
In line with Gossens- Beumer et al [13], ALDH1 expression correlated with poor survival. In our study, we found that ALDH1 expression associated with poor clinical outcome in the form of mean DFS was 11.66 months and mean 3 years overall survival was 17.5 months.
Conclusion
Expression of ALDH1, Notch1, and CD44 which is known as stem cell markers, correlate with poor prognosis in a CRC and represent an independent prognostic factor. They are associated with a feature of epithelial-mesenchymal transition evidenced by their association with high tumor burden.
References
Boman B, Wicha M. Cancer stem cells: a step toward the cure. J Clin Oncol. 2008;26(17):2795–9.
Chang B, Liu G, Xue F, Rosen D, Xiao L, Wang X, et al. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol. 2009;9(22):817–23.
Chen J, Xia Q, Jiang B, Chang W, Yuan W, Ma Z, et al. Prognostic value of cancer stem cell marker ALDH1 expression in colorectal cancer: a systematic review and meta-analysis. PLoS One. 2015a;10(12):e0145164.
Chen L, Jiang B, Wang Z, Liu M, Yang H, Xing J. Combined preoperative CEA and CD44v6 improves prognostic value in patients with stage I and stage II colorectal cancer. Clin Transl Oncol. 2015b;16(3):285–92.
Chu D, Zhang Z, Zhou Y, Wang W, Li Y, Zhang H, et al. Notch1 and Notch2 have opposite prognostic effects on patients. With colorectal cancer. Ann Oncol. 2011;22(11):2440–7.
Dalerba P, Dylla S, Park I, Liu R, Wang X, Cho R, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci. 2007;104(24):10158–63.
Edge S, Compton C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Fender A, Nutter J, Fitzgerald T, Bertrand F, Sigounas G. Notch1 promotes stemness and epithelial to mesenchymal transition in colorectal cancer. J Cell. 2015;116(11):2517–27.
Frank N, Schatton T, Frank M. The therapeutic promise of the cancer stem cell concept. J Clin Investig. 2010;120(1):41.
Gado A, Ebeid B, Abdelmohsen A, Axon A. Colorectal cancer in Egypt is commoner in young people: is this cause for alarm? Alex J Med. 2014;50(3):197–201.
Ginestier C, Hur M, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cancer Stem Cell. 2007;1(5):555–67.
Goossens-Beumer I, Zeestraten E, Benard A, Christen T, Reimers M, Keijzer R, et al. The clinical prognostic value of combined analysis of Aldh1, Survivin, and EpCAM expression in colorectal cancer. Br J Cancer. 2014;110(12):2935–44.
Hamilton S, Bosman F, Boffetta P, et al. Carcinoma of the colon and rectum. In: Bosman F, Carneiro F, Hruban R, Theise N, editors. WHO classification of tumors of the digestive system. 4th ed. Lyon: IARC; 2010. p. 134–46.
Hessman C, Bubbers E, Billingsley K, Herzig D, Wong M. Loss of expression of the cancer stem cell marker aldehyde dehydrogenase 1 correlates with advanced-stage colorectal cancer. Am J Surg. 2012;203(5):649–53.
Holah N, Aiad H, Asaad N, Elkhouly E, Lasheen A. Evaluation of the role of ALDH1 as cancer stem cell marker in colorectal carcinoma: an immunohistochemical study. J Clin Diagn Res. 2017;11(1):EC17.
Hong I, Hong S, Chang Y, Lee W, Lee B, Kang Y, et al. Expression of the cancer stem cell markers CD44 and CD133 in colorectal cancer: an immunohistochemical staining analysis. Ann Coloproctol. 2015;31(3):84–91.
Hou Y, Liu Y, Zhao X. Expression of aldehyde dehydrogenase 1 in colon cancer. Asian Pac J Trop Med. 2013;6(7):574–7.
Huang F, Zhu X, Hu X, Fang Z, Tang L, Lu X, et al. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med. 2013;31(2):484–92.
Huh J, Kim H, Kim Y, Lee J, Park Y, Cho S, et al. Expression of standard CD44 in human colorectal carcinoma: association with prognosis. Pathol Int. 2009;59(4):241–6.
Lee S, Choi S, Kim W, et al. Combined aberrant expression of E-cadherin and S100A4, but not beta-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn Pathol. 2013;8(1):99.
Liu D, Sun J, Zhu J, Zhou H, Zhang X, Zhang Y, et al. Expression and clinical significance of colorectal cancer stem cell marker EpCAMhigh/CD44+ in colorectal cancer. Oncol Lett. 2014;7:1544–8.
Mina L, Sledge G. Rethinking the metastatic cascade as a therapeutic target. Nat Rev Clin Oncol. 2011;8(6):325–32.
Nour El Hoda S, Sharaf W, Helmy D, Zaki M, Badawi M, Soliman A. Detection of cancer stem cells in colorectal cancer: Histopathological and Immunohistochemical Study. Open Access Maced J Med Sci. 2016;4(4):543.
Park I, Yu C, Lim S, et al. Ratio of metastatic lymph nodes is more important for rectal cancer patients treated with preoperative Chemoradiotherapy. World J Gastroenterol. 2015;21(11):3274–81.
Shinto E, Mochizuki H, Ueno H, Matsubara O, Jass JR. A novel classification of tumor budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology. 2005;47:25–31.
Ueda K, Ogasawara S, Akiba J, Nakayama M, Todoroki K, Ueda K, et al. Aldehyde dehydrogenase 1 identifies cells with cancer stem cell-like properties in a human renal cell carcinoma cell line. PloS one. 2013;8(10):e75463. https://doi.org/10.1371/journal.pone.0075463.
Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–94.
Van Dussen K, Carulli A, Keeley T, Patel S, Puthoff B, Magness S, et al. nNotch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139(3):488–97.
Vinson K, George D, Fender A, Bertrand F, Sigounas G. The notch pathway in colorectal cancer. Int J Cancer. 2016;138(8):1835–42.
Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell R, et al. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther. 2015;8:3783.
Zavrides H, Zizi-Sermpetzoglou A, Panousopoulos D, Athanasas G, Elemenoglou I, Peros G. Prognostic evaluation of CD44 expression in correlation with bcl-2 and p53 in colorectal cancer. Folia Histochem Cytobiol. 2005;43(1):31–6.
Zhang Y, Li B, Ji Z, Zheng P. Notch1 regulates the growth of human colon cancers. Cancer. 2010;116(22):5207–18.
Zheng C, Chen R, Xie J, Liu C, Jin Z, Jin C. Immunohistochemical expression of Notch1, Jagged1, NF-κB and MMP-9 in colorectal cancer patients and the relationship to clinicopathological parameters. Cancer Biomark. 2015;15(6):889–97.
Zhou F, Mu Y, Liang J, Liu Z, Chen H, Zhang J. Expression and prognostic value of tumor stem cell markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett. 2014;7:507–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohamed, S.Y., Kaf, R., Ahmed, M.M. et al. The Prognostic Value of Cancer Stem Cell Markers (Notch1, ALDH1, and CD44) in Primary Colorectal Carcinoma. J Gastrointest Canc 50, 824–837 (2019). https://doi.org/10.1007/s12029-018-0156-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-018-0156-6