Abstract
Background
Gallbladder cancer (GBC) is an aggressive disease with dismal results of surgical treatment mainly because of advanced stage at presentation. The objective of this study was to investigate whether aggressive surgical treatment can be associated with reasonable survival for patients with GBC at acceptable morbidity and mortality.
Methods
A total of 113 patients with proven or presumptive diagnosis of GBC were recruited prospectively over a period of 2 years and evaluated for diagnosis and staging by appropriate investigations. Seven out of 113 patients were found to have benign pathology either intraoperatively or on histopathological examination hence excluded from follow-up and survival analysis. Out of 32 potentially resectable patients, only 21 patients could finally be resected with curative intent. Patients found unresectable/metastatic disease intraoperatively (n = 11) were treated with palliative chemotherapy if eligible for the same. Short-term morbidity, perioperative mortality, disease-free survival (DFS), and median overall survival (OS) of surgically resected patients were analyzed. Median OS of resected patients was compared with that of unresectable patients.
Results
Overall resectability rate in this study cohort was 19.8 % (21/106). Overall mortality was 4.7 % and morbidity was 42.8 %. Stage distribution of resected patients was as follows: stage II (3), stage IIIA (9), stage IIIB (8), and stage IVA (1). DFS at 12 and 18 months was found to be 82.5 and 73.3 %, respectively. Mean DFS was 19.9 months (SE 1.42, 95 % CI). Mean OS for resected patients was 21 months and that for unresectable patients was 11.3 months only. Both groups were compared using log rank (Mantel-cox) test and statistically significant difference in OS was observed (p value <0.0001).
Conclusion
Since curative resection is the only chance of cure, aggressive surgical approach adopted by us is justified with acceptable mortality and morbidity and encouraging overall survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer of the gallbladder is a potentially lethal disease, usually unresectable at presentation and has a dismal prognosis. The surgical treatment of gallbladder cancer has traditionally been viewed with nihilism due to poor survival results. However, complete surgical resections offers the only chance for cure but unfortunately, only 10 % of patients present with early-stage disease and are considered surgical candidates. Among those patients who do undergo “curative” resection, recurrence rates are high. Patients with unresectable or metastatic GBC have a poor prognosis. In 1978, a review of nearly 6000 cases revealed a 5 % 5-year survival rate with a median survival of between 5 and 8 months [1]. Recent publications during the 1990s have shown the same poor results, with 5 and 12 % 5-year survival rates reported from France and Australia [2, 3]. Factors contributing to these dismal outcomes include the anatomic proximity of the gallbladder to the porta hepatis and the aggressive biologic nature of this cancer. Furthermore, direct extension of the tumor into the liver, the structures of the hepatoduodenal ligament, as well as organs in close proximity such as the duodenum, the hepatic flexure of the colon, and the pancreas contribute to technical challenges in achieving a margin negative, potentially curative (R0) resection.
However, improvements in surgical and anesthetic techniques over the last 10 to 15 years have made it safer to perform extensive liver resections and multi-organ resection with decreased morbidity and low mortality, particularly in high-volume centers [4–6]. The aggressive surgical approach to gallbladder carcinoma practiced by Japanese centers has yielded encouraging results. Recent publications have shown improved survival with this strategy. The group from Memorial Sloan Kettering in New York has reported a median survival of 26 months and a 5-year survival of 38 % for radically resected patients. A similar result was obtained by a group from Canada that reported a 5-year survival of 35 % during the last 6 years. Some hepatobiliary specialty units have broadened an already aggressive approach that includes liver resection in combination with resection of the gallbladder, bile duct, and regional lymphatics by including pancreaticoduodenectomy. The addition of pancreaticoduodenectomy allows complete resection of the extrahepatic biliary tree and its regional lymph node drainage to obtain an R0 resection that would not otherwise be possible [7–19].
Radical surgery is advocated for patients with T2 and T3 disease and includes cholecystectomy, lymphadenectomy, and en bloc hepatic resection, with or without bile duct resection. T4 tumors are those that invade the main portal vein or hepatic artery and those that invade two or more extrahepatic organs or structures. A significant subset of T4 tumors is those which involve multiple adjacent organs other than the liver, e.g., the biliary tree, stomach, duodenum, and colon. These patients need special consideration as they can now be safely offered multi-organ resection with improvements in modern surgical therapy, anesthetic management, and intensive care facilities. Provided the patient is fit and properly staged, en bloc resection should be indicated for this subset of patients. On the other hand, T4 tumors with invasion of major vessels to the liver are usually not amenable to surgical resection or carry substantial morbidity and mortality when approached surgically. Conventionally, involvement of N2 nodal stations (paracaval, superior mesenteric, and celiac groups) was considered a contraindication for surgery but few studies from Japan has shown survival benefit for these patients with surgical resection when compared with unresected patients [20]. However, surgical indications in such advanced disease should be determined on an individual basis, based on clinical status. One must consider postoperative hospital mortality when determining indications for surgery. The overall mortality rate after surgery for stage IV gallbladder carcinoma in most series is 10 to 14 %. Thus, we must balance long-term survival with the risk of hospital death. Clinical status and ability to tolerate extensive surgical procedures must be weighed against the advantages of a potentially curative procedure. Therefore, resection is not recommended for all patients with paraaortic lymph node metastasis, but instead, the decision should be individualized on a case-by-case basis.
The indications for excision of the bile duct can be either gross involvement or to facilitate lymph node dissection in the hepatoduodenal ligament. Though resection of the bile duct simplifies the skeletonization of the hepatoduodenal ligament, it also necessitates a bilioenteric anastomosis with its attendant problems of biliary leak and recurrent biliary infections. Removing the bile duct improves node clearance, and some surgeons prefer to do this routinely. A recent report examined the survival benefits associated with elective bile duct resection and noted no benefit to this approach [21].
Methods
Study Design
This was a single-center prospective non-randomized cohort study conducted at a tertiary care center of Northern India, the endemic zone of GBC. Study data was prospectively collected and informed consent was gathered in accordance with the Declaration of Helsinki and approved by the institute’s ethics committee.
Inclusion Criteria and Treatment
Gallbladder cancer patients presenting to our center were assessed clinically and radiologically for resectability, operability, and fitness for therapy. Imaging included ultrasonography and triple-phase CECT abdomen and supplemented with MRI/MRCP, FDG-PET scan, and upper GI endoscopy/ERCP in selected patients. Select patients with high index of suspicion for peritoneal disease underwent staging/diagnostic laparoscopy. Based upon evaluation, non-metastatic patients were divided into two groups:
Early GBC
Gallbladder Confined Disease, i.e., Stages I and II (AJCC 7th Edition)
This group also included patients with incidental GBC who had pathological T1/T2 disease. Early GBC patients (except T1a) were offered surgical treatment in the form of radical cholecystectomy which entailed en bloc resection of gallbladder with wedge resection of the liver bed and hepatoduodenal lymphadenectomy.
Locally Advanced GBC
Patients with T3/T4 lesion with or without lymphadenopathy and any lesion with significant regional lymphadenopathy (radiological size >10 mm) were considered locally advanced. Locally advanced GBC with definite invasion of major vessels at porta were deemed unresectable and merited consideration of palliative therapy if qualified for the same.
Remainders of locally advanced GBC patients were subjected to surgical exploration; resectability and extent of surgery was guided by intraoperative findings. Surgical procedures ranged from mere biopsy/FS of N2 nodes/peritoneal nodules to multi-organ resection including pancreaticoduodenectomy and radical lymphadenectomy of hepatoduodenal ligament.
Patients who underwent curative resection were assessed for adjuvant therapy. Adjuvant therapy was offered to those patients with good postoperative recovery. The nature of adjuvant therapy was decided purely on discretion of medical and radiation oncologist in willing patients. Patients who received curative treatment were followed up with 3-monthly clinical evaluations and 6-monthly CT scan for a period of maximum 2 years. Those who had unresectable or metastatic disease on surgical exploration were subjected to palliative chemotherapy if they qualify for the same.
Outcome
Clinically important short-term outcomes following surgery included mortality, morbidity, need for ICU care and positive-pressure ventilation, blood transfusion, and duration of hospital stay.
Disease-free survival and overall survival rates were used to define long-term outcomes for curative resection. Disease-free survival (DFS) is defined as the length of time of survival from gallbladder cancer diagnosis without clinical or radiological evidence of locoregional recurrence or distant metastases. Overall survival (OS) was defined as the time from GB cancer diagnosis to death from any cause. Stage distribution of resected patients was as follows: stage II (3), stage IIIA (9), stage IIIB (8), and stage IVA (1). Minimum follow-up of operated patients was 1 month and maximum follow up was 24 months. For survival analyses, surviving patients were censored on the date of data analysis.
Data Analysis
The descriptive statistics is presented using mean (with SD) and median (with range) for quantitative variables, and categorical variables are presented in frequencies along with respective percentages. The statistical comparisons for quantitative variables were done by using Student’s t test. For categorical variables, chi-square test or Fisher’s exact test was used according to the nature of data. OS was calculated from date of entry to date of death or censoring at the date last known for being alive for all the patients. For survival analysis, Kaplan-Meier survival curve was plotted to see the survival pattern in different subgroups and log-rank test was used for comparison of survival. Data were entered and coded in MS Excel (Version, 2007), and all statistical analyses were performed by using SPSS software (Version 22, SPSS Inc, Chicago, IL, USA). The p values less than 0.05 were considered statistically significant.
Results
A total of 113 patients with proven or presumptive diagnosis of carcinoma gallbladder were recruited and evaluated for diagnosis and staging by appropriate investigations. Seven out of 113 patients were found to have benign pathology either intraoperatively or on histopathological examination hence excluded from follow-up and survival analysis. Depending upon the abovementioned evaluation, 39 patients with proved or presumptive diagnosis of GBC were supposed to be resectable and underwent exploratory laparotomy. Intraoperatively, 11 patients were either unresectable or metastatic; 5 patients did not have any clinical evidence of malignancy. Hence, simple cholecystectomy was performed in four of them and partial cholecystectomy in one who had pyocele with dense inflammation around porta due to previous biliary stenting and repeated cholangitis. All surgical specimens were opened up in operation theater to rule out any sinister lesion.
The reasons for not offering curative intent surgery in 11 cases were metastatic disease in 7 patients and surgically unresectable disease in 4 patients. Major vascular invasion or frozen portal structures were the cause of unresectability. Four patients had peritoneal or omental nodules and three had liver nodules. In addition, three patients had paraaortic lymph nodes proved to be metastatic on frozen section examination.
A total of 23 patients were considered to have resectable GBC intraoperatively and underwent curative intent surgery, though final histopathology report showed two patients to have benign granulomatous disease, i.e., xanthogranulomatous cholecystitis and tubercular cholecystitis. Thus, only 21 patients of GBC underwent curative resection. Overall resectability rate at presentation was found to be 19.8 % (21/106). The extent of resection ranged from radical cholecystectomy (9 patients) to multi-organ resection (12 patients). Multi-organ resection was defined as resection of any adjacent structures beyond standard radical cholecystectomy, to get gross negative margin. Extra hepatic biliary radical excision was done in eight patients either because of direct invasion or to perform adequate lymphadenectomy. Colon (hepatic flexure and transverse colon) and distal stomach with proximal duodenum (D1) were excised in five and three patients, respectively (Fig. 1). The head of pancreas and D2 invasion necessitated to perform pancreaticoduodenectomy in two patients. One patient with extensive contiguous invasion of the liver underwent right liver lobectomy though histopathology report turned out to be xanthogranulomatous cholecystitis in this patient. The most common intraoperative finding of GBC was GB fossa mass followed by contiguous liver invasion and regional lymphadenopathy (Table 1).
Extent of Lymphadenectomy for GBC
For all patients who underwent curative resection for GBC, hepatoduodenal lymphadenectomy was performed which included removal of all fibro fatty tissue along hepatoduodenal ligament. One patient underwent Whipple’s procedure to get clearance of nodal metastasis at posterior superior pancreatic group. The median yield of lymphadenectomy was found to be 8 (Table 2).
A total of 9 patients out of 21 were found to have nodal metastasis in HPE report. Advanced T stage was found to have two times increased risk of nodal metastasis over early T stage (Table 3). The rate of LVE and PNI positivity was 24 and 38 %, respectively, in this cohort of patients.
The most common histology of gallbladder cancer in resected patients was adenocarcinoma; most were grade 2 (11/21). Mucinous adenocarcinoma and adenosquamous carcinoma was reported in one patient each.
Short-Term Outcomes of Surgery
Clinically important short-term outcomes following surgery included mortality, morbidity, need for ICU care and positive pressure ventilation, blood transfusion, and duration of hospital stay.
One patient of surgical group succumbed to death in postoperative period accounting to mortality rate of 4.7 % in this cohort. He was an 80-year-old male patient with radiological stage IIIB disease who had been treated with neo-adjuvant chemotherapy with partial clinical response. He underwent major resection which comprised radical cholecystectomy with right lobe hepatectomy with distal gastrectomy along with D1 excision, sleeve-stapled excision of antimesenteric part of hepatic flexure of colon. He developed ARDS and septicemia in the ICU and expired on the 5th postoperative day.
ICU care was needed in 13 patients; only one required positive pressure ventilation. Median ICU stay was found to be 1 day. The median hospital stay following surgery was calculated to be 8 days with a range of 4 to 45 days. Five patients required blood transfusion with mean requirement of 0.5 units.
Major complications of surgery were entero-cutaneous fistula (1 patient), biliary fistula (1 patient), early bile leak (2 patients), cholangitis (1 patient), and septicemia (1 patient). All patients salvaged with appropriate measures; two cases required re-exploration and repair of biliary enteric anastomosis and ligation of opened up biliary radical in resected liver bed. Minor complications noted were atelectasis (2 cases), pleural effusion (1 patient), surgical site infection (4 cases), and paralytic ileus (4 cases). Seventeen out of 28 operated patients did not have any postoperative complication. Only one patient required re-admission within 1 month of discharge for cholangitis.
Overall surgical morbidity rate among GBC patients (n = 21) who underwent curative resection was 42.8 % (9/21), and mortality rate was 4.7 %. Surgical morbidity rate was higher among those with biliary involvement and elderly.
Long-Term Outcomes of Surgery
Disease-free survival and overall survival rates were used to define long-term outcomes for curative resection. Stage distribution of resected patients was as follows: stage II (3), stage IIIA (9), stage IIIB (8), and stage IVA (1). Minimum follow-up of operated patients was 1 month and maximum follow up was 24 months.
Cumulative disease-free survival curve of all surgical patients (n = 21) was obtained (Fig. 2). As the number of early-stage GBC (stages I and II) was small, comparison for DFS between early-stage GBC and locally advanced GBC was not attempted. DFS at 12 and 18 months was found to be 82.5 and 73.3 %, respectively. Mean DFS was 19.9 months (SE 1.42, 95 % CI).
Kaplan-Meier survival curves (OS) were obtained for curative resection GBC patients (n = 21) and for those who were found to have unresectable and/or metastatic disease intraoperatively (n = 11). Overall survival for operated patients at 12 and 22 months were 87.7 and 66.4 %. Mean OS was 21.1 months (SE 1.36, 95 % CI).
Patients who were unresectable or metastatic on exploration (n = 11) were treated with palliative chemotherapy or best supportive therapy. Overall survival for these patients at 6 and 12 months was 77.8 and 22.2 %. Mean OS was 11.3 months (SE 2.0, 95 % CI).
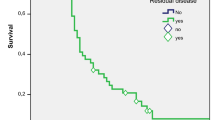
Overall survival for those who could not undergo curative resection (n = 11) was compared with surgically resected patients (n = 21). Mean OS for resected patients was 21 months and that for unresectable patients was 11.3 months only (Fig. 3). Both groups were compared using log rank (Mantel-cox) test and statistically significant difference in OS was observed (p value <0.0001).
Adjuvant Therapy
Till date, there is no consensus on the impact of adjuvant chemotherapy and/or radiotherapy after curative resection of GBC. Adjuvant therapy was offered to those patients with good postoperative recovery and performance status. The nature of adjuvant therapy was decided purely on discretion of medical and radiation oncologist in willing patients. Eleven patients got treated by adjuvant CCRT followed by adjuvant chemotherapy, five patients with adjuvant CCRT, and three patients received only adjuvant chemotherapy. Two patients were unwilling to have any form of adjuvant treatment.
Discussion
With the increasing safety of hepatic and pancreatic surgery, various radical procedures have been advocated to improve the curative outcome for advanced gallbladder cancer. Recent data suggest that aggressive resection may improve long-term survival, even in patients with advanced stage disease. Extent of resection for GBC may include more substantial liver resections, from segmentectomies (4b/5) to right hepatectomies, even trisectionectomy. To completely clear the lymphatics in the porta hepatis, resection of the bile duct may be considered in select patients. Sometimes, a pancreaticoduodenectomy is added to achieve R0 resection status. However, despite adopting this much of surgical aggression, the resectability rate has been reported to range between 30 and 40 %; 5-year survival for cancers of the gallbladder lies between 0 and 10 % in most reported series.
It is indeed despairing to read articles on gallbladder cancer outcomes which always begin with the reference to the disease as one that is associated with a dismal prognosis. Surgery remains the only treatment modality associated with a benefit in terms of survival in GBC. In the last decade, several studies have documented an increase of 5-year survival rates from 5–12 % up to 38 %. It is a well-known fact that palliative chemotherapy or radiotherapy is not very effective for GBC and survival benefit, if any, is limited to months. In this context, an aggressive surgical approach for locally confined disease is justified. However, what is most disconcerting is the lack of consensus across the world on what constitutes an aggressive surgery for a given stage of the disease. Many of the surgical concepts in gallbladder cancer are based on what “we think” as appropriate. Being a relatively uncommon disease around the world, hardly, any of the approaches have been evaluated in evidence based manner.
The presence of icterus in a patient of GBC generally signifies advanced disease due to involvement of extrahepatic biliary tree or due to enlarged periportal lymph nodes. Out of 32 surgically explored GBC patients, 32 % (11/32) had jaundice. Forty-five percent (5/11) of jaundiced patients could not undergo definitive surgery, either because of metastatic disease (n = 3) or because of frozen porta (n = 2). While those without jaundice, only 23.4 % (6/21) were found to have unresectable/metastatic disease on exploration. Overall, morbidity in the form of biliary fistula and paralytic ileus was higher in those who underwent biliary resection. In summary, one can say that invasion of extrahepatic biliary tree in a patient of GBC does not preclude definitive surgery but carries a little higher complication rates.
Borderline resectability for GBC which is usually being considered an indication for staging laparoscopy and neo-adjuvant chemotherapy is not well defined in literature. The presence of extensive liver invasion (>2 cm depth), celiac/portocaval/peripancreatic lymph nodes, radiologically doubtful fixity to those organs that cannot be salvaged such as main portal vessels, and doubtful hepatic nodules are considered borderline resectable GBC [22]. In our view, GBC patients with radiologically doubtful finding of portal vessels invasion, presence of N2 station nodes (AJCC 7th Edition) or suspicious liver/peritoneal nodules, are considered borderline resectable GBC. Our approach in these cases has been to make every attempt to rule out metastatic or unresectable GBC preoperatively to avoid laparotomy. Selective use of MR angiography, PET scan, and diagnostic laparoscopy was found to be helpful to avoid unnecessary laparotomy. But, with the best of our effort to rule out unresectable disease preoperatively, 32 % of radiologically resectable lesions turned out to be unresectable peroperatively and this is attributed to the complex location of GB and biological aggressiveness of gallbladder cancer. Even with the recent radiological advances, we have to confess that preoperative assessment of resectability for GBC is a real challenge and this leads to unnecessary laparotomy and its associated morbidity.
Here, we want to make a note that the commonest cause of unresectability in our series was radiologically occult disseminated disease (7/11) which could have been detected with more liberal use of diagnostic laparoscopy. There are currently growing evidence to suggest routine use of staging laparoscopy for locally advanced GBC to avoid non-therapeutic laparotomy. Agarwal AK et al. in their large prospective series concluded that staging laparoscopy obviated a nontherapeutic laparotomy in 55.9 % of patients with unresectable disease and 23.2 % of overall GBC patients [23]. It had a higher yield in locally advanced tumors than in early-stage tumors, and therefore, its routine application is nowadays recommended especially for those who are at higher risk of disseminated metastases (those with poorly differentiated T3 or higher tumors or margin-positive tumors at cholecystectomy).
Though, occasional long-term survival has been reported with surgical resection even when paraaortic lymph node metastasis and/or liver metastasis is present, surgical indications in advanced disease should be determined on an individual basis, based on clinical status. In the absence of strong data to support such an aggression, presence of liver nodule (even if it is resectable), histologically proven N2 nodes, and invasion of major vessels in hepatoduodenal ligament are considered incurable disease and curative intent surgery is not attempted in this series.
The complex location of gallbladder, coupled by morbid anatomy due to cancer, poses a great difficulty to assess resectability even intraoperatively. Before taking any irreversible step, one must be very sure that R0 resection can be achieved with an acceptable morbidity. In our experience, encased hepatic artery or portal vein (and their left branches) or fixity of hepatoduodenal ligament are considered contraindication for resection. In the present series, we have not proceeded with any non-therapeutic resection for such an advanced disease considering very high morbidity and mortality, besides oncologic concern of margin positive resection.
The armamentarium of surgical procedures mainly comprised liver resection, common bile duct resection, and lymph node dissection in the hepatoduodenal ligament and especially practiced in Japan—concomitant pancreatoduodenectomy or lymph dissection of the interaortocaval compartment.
Overall curative resection rates for GBC at presentation have been only 10 to 30 % [24]. In the present series, the resectability rate was 19.8 % (21/106). Here, we would like to mention that presence of N2 nodes and vascular invasion at porta have been considered unresectable disease in our series based upon the literature review which showed poor survival and greater morbidity and mortality with N2 nodal dissection (retroportal, peripancreatic, common hepatic artery, celiac, superior mesenteric, and interaorticocaval lymph nodes) and vascular resection with reconstruction [14, 25, 26]. But few recent Japanese studies have reported occasional long-term survival in patients with N2 lymphadenectomy in proven metastatic nodes in this region [27].
At our institute, we are performing standard regional lymphadenectomy which includes lymph nodes around the cystic duct and pericholedochal and hepatoduodenal ligaments. In this regard, attempts should perhaps be made to define a minimum number of lymph nodes (from specific locations) necessary for optimal staging (and perhaps prognostication) of GBC. Shirai Y et al. [27] in a large retrospective study concluded that aggressive lymphadenectomy which includes first-echelon and second-echelon nodes can achieve an acceptable rate of long-term survival even in patients with nodal metastasis, provided that a potentially curative (R0) resection is feasible. They also suggested that a minimum of three lymph nodes needs to be dissected out from adequate staging. In our series, the median yield of lymphadenectomy was eight lymph nodes.
Another controversial issue in management of GBC is the extent of liver resection adequate for each stage. Most of us would agree that for T1a tumor, a simple cholecystectomy constitute an adequate surgery. However, for T1b tumor, the extent of surgery is not so clear. Some authors have suggested that a simple cholecystectomy is sufficient in such patients but in view of high incidence of lymph node metastasis (even up to 35 %) [28]; a lymphadenectomy with excision of at least a wedge of liver tissue from segments 4b and 5 seems to be prudent for T1b tumors.
For T2-T4 tumors, aggressive resection is justified which includes a cholecystectomy with an en bloc resection of the liver, with a lymphadenectomy with or without a radical resection of the bile duct or other adjacent invaded structures. The extent of liver resection continues to be a matter of debate which ranges from non-anatomic wedge resection to formal segment IVb and V resection and even right hepatic lobectomy. However, most of us now agree that major hepatic resections, including major hepatectomy and CBD excision, are appropriate when necessary to clear disease but are not mandatory in all cases [29]. In our series, all patients who underwent surgery were stage T2 or above. We routinely perform non-anatomical wedge resection except in few select situations where right hepatic lobectomy had been performed.
Many Japanese surgeons routinely performed resection of the common bile duct in the course of a curative resection for advanced gallbladder cancer because the lymphatics surrounding the duct are a main route of tumor spread [26, 27, 30]; however, no improvement in long-term survival has been reported as the result of this resection. Moreover, numerous studies have highlighted the increased morbidity associated with routine excision of the duct [31]. However, there are specific indications where the extrahepatic duct may have to be excised and these include a positive cystic duct margin, presence of an anomalous bile duct junction, and synchronous malignancy in the extrahepatic bile duct, as well as to aid lymph nodal clearance when there are large lymph nodes, the clearance of which may be associated with a risk of devascularizing the common bile duct. We, at this institute, perform CBD resection only for the abovementioned indications. In the present series, extra hepatic biliary radical excision was done in eight patients either because of direct invasion or to perform adequate lymphadenectomy.
Another controversial issue pertinent to incidental GBC is management of port-site metastases. While Giuliante et al. [32] recommended routine “complete” excisions of the port sites, surgically, this may not always be feasible. More importantly, there is no evidence to date to indicate that routine excision of the port sites improves overall survival.
The operative mortality and morbidity with this radical approach varies considerably in different series. The large review of 724 patients by the French Surgical Association showed an overall mortality rate of 22 % [2], whereas others have observed rates as low as 0.9 % [33]. In another series by Tsukada et al. [34], the morbidity rate of 34 % was reported after extended procedure. Michel D’Angelica et al. [29] in their series reported high morbidity (53 %) and mortality (5 %). In our study, one of our elderly male patient with major surgical resection succumbed to death in postoperative period accounting to mortality rate of 4.7 % in this cohort. The morbidity rate in patients with radical resection was 42.8 % (9/21). Surgical morbidity rate was higher among those with biliary involvement and elderly. Thus, our hospital mortality and morbidity rates are comparable to rates reported by other studies with aggressive surgical approach. In a study by Hideki Nishio et al. [20] for stage IV GBC, male sex and portal vein resection were associated with increased mortality.
Most of our surgical patients belonged to advanced stage GBC (stages III and IV) with only three patients of stage II. The initial results of this study are encouraging with acceptable morbidity and excellent survival with curative intent radical resection. With the follow-up of 1 to 24 months, the mean disease-free survival (DFS) of 19.9 months and mean overall survival of 21 months were calculated. There was a significant difference in OS between surgically resected patients and those who could not undergo curative resection (p value <0.0001). Longer follow-up data is needed to compare our survival results with other contemporary series. Overall survival for operated patients at 12 and 22 months was 87.7 and 66.4 %. The 3-year survival has ranged from 0 to 63 % for T3/T4 tumors in different series [33]. These survival data are extremely heterogeneous due to the changing definition of TMN staging and individualized approach of surgery.
In conclusion, we should realize that GBC is a rare malignancy worldwide with dismal prognosis. Thus, instead of retrospectively analyzing individual institutional data, high-volume institutions with the necessary expertise for treating gallbladder cancer should collaborate with a view to generating strong evidence to support the different surgical strategies—a move that may provide us with the evidence-based surgical guidelines we are looking for to better enable us to tackle this dreadful disease.
References
Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet. 1978;147:929–42.
Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder: results of the French Surgical Association Survey. Ann Surg. 1994;219:275–80.
Wilkinson DS. Carcinoma of the gall-bladder: an experience and review of the literature. Aust N Z J Surg. 1995;65:724–7.
Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18.
Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406.
Taylor M, Forster J, Langer B, et al. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg. 1997;173:467–71.
Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer: implications for staging and management. Ann Surg. 1996;224:639–46.
Kondo S, Nimura Y, Hayakawa N, et al. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418–22.
Kondo S, Nimura Y, Hayakawa N, et al. Extensive surgery for carcinoma of the gallbladder. Br J Surg. 2002;89:179–84.
Kondo S, Nimura Y, Kamiya J, et al. Mode of tumor spread and surgical strategy in gallbladder carcinoma. Langenbecks Arch Surg. 2002;387:222–8.
Kondo S, Nimura Y, Kamiya J, et al. Five-year survivors after aggressive surgery for stage IV gallbladder cancer. J Hepatobiliary Pancreat Surg. 2001;8:511–7.
Shirai Y, Ohtani T, Tsukada K, et al. Radical surgery is justified for locally advanced gallbladder carcinoma if complete resection is feasible. Am J Gastroenterol. 1997;92:181–2.
Shirai Y, Ohtani T, Tsukada K, et al. Pancreaticoduodenectomy for gallbladder cancer with peripancreatic nodal metastases. Hepatogastroenterology. 1997;44:376–7.
Shirai Y, Ohtani T, Tsukada K, et al. Combined pancreaticoduodenectomy and hepatectomy for patients with locally advanced gallbladder carcinoma: long term results. Cancer. 1997;80:1904–9.
Shirai Y, Yoshida K, Tsukada K, et al. Radical surgery for gallbladder carcinoma: long-term results. Ann Surg. 1992;216:565–8.
Todoroki T, Kawamoto T, Takahashi H, et al. Treatment of gallbladder cancer by radical resection. Br J Surg. 1999;86:622–7.
Todoroki T, Takahashi H, Koike N, et al. Outcomes of aggressive treatment of stage IV gallbladder cancer and predictors of survival. Hepatogastroenterology. 1999;46:2114–21.
Doty JR, Cameron JL, Yeo CJ, et al. Cholecystectomy, liver resection, and pylorus-preserving pancreaticoduodenectomy for gallbladder cancer: report of five cases. J Gastrointest Surg. 2002;6:776–80.
Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–69.
Nishio H, Nagino M, Ebata T, Yokoyama Y, Tsuyoshi I, Yuji N. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg. 2007;14:351–7.
Yokomizo H, Yamane T, Hirata T, et al. Surgical treatment of pT2 gallbladder carcinoma: a reevaluation of the therapeutic effect of hepatectomy and extrahepatic bile duct resection based on the long-term outcome. Ann Surg Oncol. 2007;14:1366–73.
Nair CK, Kothari KC. Role of diagnostic laparoscopy in assessing operability in borderline resectable gastrointestinal cancers. J Min Access Surg. 2012;8:45–9.
Agarwal AK, Kalayarasan R, Javed A, et al. The role of staging laparoscopy in primary gall bladder cancer—an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg. 2013;258(2):318–23.
Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–76.
Tsukada K, Kurosaki I, Uchida K, et al. Lymph node spread from carcinoma of the gallbladder. Cancer. 1997;80:661–7.
Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997;79:892–9.
Yoshio S, Toshifumi W, Jun S, Katsuyoshi H. Regional lymphadenectomy for gallbladder cancer: rational extent, technical details, and patient outcomes. World J Gastroenterol. 2012;18(22):2775–83. June 14.
Shukla PJ, Barreto G, Kakade A, Shrikhande SV. Revision surgery for incidental gallbladder cancer: factors influencing operability and further evidence for T1b tumors. HPB (Oxford). 2008;10:43–7.
D’Angelica M, Dalal KM, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16(4):806–16.
Shirai Y, Ohtani T, Tsukada K, Hatakeyama K. Combined pancreaticoduodenectomy and hepatectomy for patients with locally advanced gallbladder carcinoma. Long Term Results Cancer. 1997;80:1904–9.
Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, et al. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136:1012–7.
Giuliante F, Ardito F, Vellone M, Clemente G, Nuzzo G. Port-sites excision for gallbladder cancer incidentally found after laparoscopic cholecystectomy. Am J Surg. 2006;191:114–6.
DeVita, Hellman and Rosenberg. Principles and practice of oncology.9th Edition. Lippincott Williams & Wilkins 2011: Table 85.17.
Tsukada K, Hatakamaya K, Kurosaki I, et al. Outcome of radical surgery for carcinoma of the gallbladder according to TNM stage. Surgery. 1996;120:816–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was gathered in accordance with the Declaration of Helsinki and approved by the institute’s ethics committee.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Singh, S.K., Talwar, R., Kannan, N. et al. Aggressive Surgical Approach for Gallbladder Cancer: a Single-Center Experience from Northern India. J Gastrointest Canc 46, 399–407 (2015). https://doi.org/10.1007/s12029-015-9766-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-015-9766-4