Abstract
Background
Timely intensive care unit (ICU) admission for patients with encephalitis is associated with better prognosis. Therefore, our aim was to create a risk score predicting ICU admission in adults with encephalitis, which could aid in optimal management and resource allocation.
Methods
We initially identified variables that would be most predictive of ICU admission among 372 patients with encephalitis from two hospital systems in Houston, Texas (cohort 1), who met the International Encephalitis Consortium (IEC) criteria from 2005 to 2023. Subsequently, we used a binary logistic regression model to create a risk score for ICU admission, which we then validated externally using a separate cohort of patients from two hospitals in Baltimore, Maryland (cohort 2), who met the IEC criteria from 2006 to 2022.
Results
Of 634 patients with encephalitis, 255 (40%) were admitted to the ICU, including 45 of 113 (39.8%) patients with an autoimmune cause, 100 of 272 (36.7%) with an infectious cause, and 110 of 249 (44.1%) with an unknown cause (p = 0.225). After conducting a multivariate analysis in cohort 1, we found that the presence of focal neurological signs, new-onset seizure, a Full Outline of Unresponsiveness score ≤ 14, leukocytosis, and a history of chronic kidney disease at admission were associated with an increased risk of ICU admission. The resultant clinical score for predicting ICU admission had an area under the receiver operating characteristic curve (AUROC) of 0.77 (95% confidence interval [CI] 0.72–0.82, p < 0.001). Patients were classified into three risk categories for ICU admission: low risk (score 0, 12.5%), intermediate risk (scores 1–5, 49.5%), and high risk (scores 6–8, 87.5%). External validation in cohort 2 yielded an AUROC of 0.76 (95% CI 0.69–0.83, p < 0.001).
Conclusions
ICU admission is common in patients with encephalitis, regardless of etiology. Our risk score, encompassing neurologic and systemic factors, may aid physicians in decisions regarding intensity of care for adult patients with encephalitis upon hospital admission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Encephalitis is a severe neurological syndrome characterized by inflammation of the brain parenchyma resulting from infectious and autoimmune etiologies [1, 2]. More than one third of patients need intensive care unit (ICU) admission from a variety of potential causes, including reduction in consciousness, seizures, central hypoventilation, severe movement disorders, and dysautonomia [3,4,5,6]. Delayed admission to the ICU has been found to be associated with unfavorable outcomes in patients with encephalitis [7]. Hence, it is crucial to rapidly identify the need for ICU care to improve outcomes and optimize resource allocation. Our primary objective was to develop and validate a risk assessment tool for predicting the likelihood of ICU admission in adults with new-onset encephalitis. Additionally, we aimed to delineate the clinical characteristics, complications, and outcomes associated with ICU admission in patients with encephalitis compared to those not admitted to the ICU.
Methods
Study Population
Two populations of patients with encephalitis were used in this study.
Cohort 1
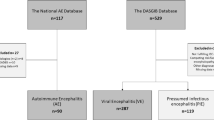
Between February 2005 and February 2023, a total of 1565 adults who were 18 years or older and had an encephalitis-related discharge diagnosis identified with the International Classification of Diseases, Ninth Revision (ICD-9) discharge codes at two hospital systems in Houston, Texas, were screened. Among them, 372 patients were identified as having encephalitis using the 2013 International Encephalitis Consortium (IEC) criteria [1].
Cohort 2
The second group of patients consisted of individuals who were 18 years of age or older and had received a discharge diagnosis related to encephalitis, as identified by the ICD-9 discharge codes, at either the Johns Hopkins Hospital or Johns Hopkins Bayview Medical Center in Baltimore, Maryland, between June 1, 2006, and March 15, 2016. Additional patients were prospectively enrolled from the Johns Hopkins Hospital and outpatient center between January 2016 and December 2022. Overall, 262 patients met the IEC criteria for encephalitis and were included in this cohort.
Data Extraction and Definitions
A multidisciplinary approach was employed to establish study parameters and risk factors for ICU admission in patients with encephalitis. Prestudy meetings involving physicians from diverse specialties, including neuroimmunology, neurocritical care, general neurology, and infectious diseases, resulted in facilitated collaborative discussions to define key parameters. Patient data were collected retrospectively during the initial hospitalization for encephalitis.
Multiple variables were collected at the initial encounter in the emergency department (ED). These included demographics, patient-reported symptoms, laboratory test results (serum white blood cell and platelet counts), and vital signs. The cutoff for fever was determined at 38 °C (100.4 °F). Patient medical history at presentation was used to compute the Charlson comorbidity index (CCI). We also identified chronic kidney disease (CKD) and immunosuppression, defined as infection with HIV (regardless of CD4 count), recent chemotherapy, solid organ or bone marrow transplantation, receiving ≥ 20 mg of prednisone or equivalent for > 1 month, receiving other immunosuppressive therapies, or having congenital immunodeficiencies. Level of consciousness at presentation was determined using the Glasgow Coma Scale (GCS) and the Full Outline of Unresponsiveness (FOUR) score, the latter of which has more clinical granularity, particularly for brainstem involvement [8]. Scores were obtained from the chart if available at the initial neurological assessment or derived from the patient’s neurological physical examination. To determine optimal cutoffs for categorical transformations of the FOUR scores in our data set with ICU admission as the outcome, we used the Youden index, which relies on the area under the receiver operating characteristic curve (AUROC) [9].
Other variables were collected during the initial hospitalization as they became available. These include cerebrospinal fluid, electrocardiogram (EKG), magnetic resonance imaging, and electroencephalogram (EEG) studies within 72 h of admission. The Sequential Organ Failure Assessment (SOFA) score was calculated when all components were available [10]. If ABG values needed for SOFA scores were not obtained during hospitalization (which was the case for 309 patients across both cohorts), the PaO2 was estimated using a correlation calculation between SpO2 and PaO2 [11, 12].
Additional variables were collected but were not part of the model development. These included encephalitis etiology and parameters to evaluate the prevalence of complications and outcomes associated with ICU admission, including refractory status epilepticus, requirement for mechanical ventilation, vasopressor usage, Glasgow Outcome Scale (GOS) score, median length of stay, mortality, and discharge disposition. To handle missing data, we performed a complete case analysis [13].
Encephalitis ICU Risk Score Development and Validation
A collaborative effort was made to ensure adherence to the guidelines set forth in the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement checklist [14]. IBM SPSS version 29.0 was used to conduct statistical analysis, and GraphPad Prism version 10 was used to generate figures.
Variable Selection
From cohort 1, we initially performed a univariate analysis to identify clinically relevant variables associated with ICU admission. Categorical variables were analyzed using the χ2 test or Fisher’s exact test, whereas the Mann–Whitney U-test was used to analyze all nonparametric continuous variables. Variables that showed significant association with ICU admission (p < 0.05) were further examined for collinearity using a collinearity matrix. To ensure the independence of the predictors in the multivariate logistic regression model, we omitted one variable from any pair of highly correlated variables.
Model Development
We employed backward selection based on the likelihood ratio to identify a subset of the strongest predictors from the remaining variables. Subsequently, logistic regression was used to model the likelihood of ICU admission based on these selected predictors.
Internal Validation and Calibration
To ensure the robustness of our model, we performed bootstrapping with 1,000 resamples. We assessed the calibration and goodness of fit using the Hosmer–Lemeshow test.
Development of Clinical Scoring System
We then converted the binary logistic regression model into a provisional clinical scoring system by dividing the regression coefficients of each factor by the smallest regression coefficient among the variables and rounding the results to the nearest integer. The resulting risk score classified patients as low, intermediate, or high risk of ICU admission. To determine the cutoff points for our risk levels, we calculated sensitivity, specificity, negative predictive value, and positive predictive value at each score threshold. The low-risk cutoff was determined at the score that maximized sensitivity (≈1), ensuring that nearly all patients who required ICU admission were identified (minimizing false negatives). The high-risk cutoff was determined at the score that maximized specificity (≈1), ensuring that nearly all patients identified as high risk indeed required ICU admission (minimizing false positives).
Model Evaluation and External Validation
To evaluate the model’s discriminative ability, we employed the receiver operating characteristic (ROC). Finally, we externally validated the predictive model for ICU admission by applying it to cohort 2. We assessed the model’s performance on this cohort using ROC analysis and risk stratification, comparing the predicted risk categories to the actual ICU admissions. To evaluate the differences between cohort 1 and cohort 2, we included a univariate analysis comparing both cohorts.
Clinical Characteristics and Outcomes of Patients with Encephalitis Admitted to the ICU
We conducted a univariate analysis on a total of 634 patients from the combined cohorts 1 and 2 to explore clinical characteristics, complications, and outcomes associated with ICU admission in a larger cohort of patients with encephalitis.
Differences in Patients with Encephalitis who Develop New-Onset Seizure, Status Epilepticus, and Refractory Status epilepticus
We similarly combined both cohorts and conducted a univariate analysis to explore differences in patients with new seizure at onset, status epilepticus, and refractory status epilepticus. When comparing differences in categorical and continuous variables between these three groups, we used the Bonferroni test for categorical variables and Dunn’s test with Bonferroni adjustments for continuous variables to account for multiple simultaneous comparisons.
Results
Clinical Characteristics and Outcomes of Patients with Encephalitis Admitted to the ICU
Demographics and Comorbidities
Of the 634 patients in the combined cohort, 255 of 634 (40.2%) were admitted to the ICU (Table 1). The median duration from symptom onset to ICU admission was 5 days (interquartile range [IQR] 2–12 days). There were no differences between ICU-admitted and non-ICU-admitted groups with respect to age and sex, but ICU-admitted patients were more likely to be Hispanic (18.4% in ICU group vs. 13.2% in non-ICU group, p = 0.01).
The ICU group was composed of 17.6% with autoimmune encephalitis (AIE), 39.2% with infectious encephalitis (IE), and 43.1% with an unknown etiology; the non-ICU group had similar proportions (AIE 17.9% [p = 0.924], IE 45.4% [p = 0.124], unknown 36.7% [p = 0.125]). Viewed from the perspective of etiology, 45 of 113 patients (39.8%) with AIE were admitted to the ICU, as compared to 100 of 272 (36.7%) patients with IE (including 77 of 214 [35.9%] with viral encephalitis) and 110 of 249 (44.1%) patients with an unknown etiology (p = 0.225). Notably, patients requiring ICU admission were more likely to be immunocompromised at the time of presentation (26.3% vs. 19.5%; p = 0.045). Additionally, medical history of CKD was more frequently observed in ICU-admitted patients than in patients not admitted to the ICU (9.4% vs. 4.7%; p = 0.018), despite similar CCI scores between the two groups, with 50.2% of patients in the ICU group having a CCI score of 2 or higher, compared to 42.3% in the non-ICU group (p = 0.051).
Symptoms at Presentation
Regarding clinical presentation in the ED, patients admitted to the ICU were more likely to have fever (30.6% vs. 21.6%; p = 0.02), focal neurological deficits (44.7% vs. 34.6%; p = 0.01), new-onset seizure (48% vs. 25%; p < 0.001), altered mental status (93% vs. 66.6%; p < 0.001), and psychiatric symptoms (65.5% vs. 55.8%; p = 0.015).
Laboratory Studies, Neurological Workup, and Clinical Scores
ICU-admitted patients were more likely to have thrombocytopenia (< 150 × 103/μL, SI unit: 150 × 109/L) (20.6% vs. 12.3%; p = 0.005) and leukocytosis (> 11,000 cells/μL) (46.6% vs. 22.8%; p < 0.001) on the complete blood cell count done at ED presentation. Concerning neurological diagnostic testing, no significant differences were observed between the two groups in the cerebrospinal fluid profile. However, patients who required ICU admission were more likely to have abnormalities seen on EEG (86.7% vs. 69%; p < 0.001). In addition, patients who were admitted to the ICU had a significantly higher likelihood of presenting with a SOFA score > 3 when compared to those who were not admitted (62.2% vs. 21.2%; p < 0.001) as well as a higher incidence of a GCS score ≤ 8 (26.8% vs. 3.2%; p < 0.001) and a FOUR score ≤ 14 (45.5% vs. 10.3%; p < 0.001).
Complications and Outcomes
A substantial proportion of patients admitted to the ICU required vasopressors (41%), required mechanical ventilation (75%), and developed status epilepticus (19%) during their hospitalization. The mortality rate was higher among ICU-admitted patients (18.4%) compared to non-ICU-admitted patients (2.9%) (p < 0.001). Additionally, ICU-admitted patients were less likely to be discharged home (32.3% vs. 62.9%; p < 0.001) and more likely to require care in a skilled nursing facility, a rehabilitation center, or hospice care. Furthermore, ICU-admitted patients had a higher likelihood of having unfavorable outcomes, with a median GOS score of 3 (IQR 2–4), compared to a median GOS score of 4 (IQR 3–5) for non-ICU patients (p < 0.001). Lastly, the median length of stay was longer for ICU-admitted patients (20 [IQR 10–37] days vs. 8 [IQR 5–15] days, p < 0.001) (Table 2).
Differences in Patients with Encephalitis who Develop New-Onset Seizure, Status Epilepticus, and Refractory Status Epilepticus
Of the 634 patients in the combined cohort, 413 (65%) experienced no seizures, 164 (26%) presented with new-onset seizures, 45 (7.1%) had status epilepticus, and 12 (1.9%) had refractory status epilepticus. ICU admission rates significantly differed across these groups. Patients without seizures had a 31.2% ICU admission rate (129 of 413), whereas those with seizures at presentation had a 47.6% rate (78 of 164; p < 0.001 compared to no seizures). The ICU admission rate was notably higher for patients with status epilepticus at 80% (36 of 45; p < 0.001 compared to those with no seizures or with seizures at presentation). All patients with refractory status epilepticus (12 of 12) required ICU admission (p < 0.001 compared to other groups) (Supplementary Table 1).
Patients without seizures had a median GOS score of 4 (IQR 3–5), whereas those with seizures at presentation had a median GOS score of 3 (IQR 3–4, p < 0.001). For patients with status epilepticus, the median GOS score was 3 (IQR 3–3, p < 0.001 compared to no seizures), and for those with refractory status epilepticus, the median GOS score was 2 (IQR 1–3, p = 0.002 compared to no seizures, and p = 0.03 compared to seizures but not status epilepticus at presentation).
Mortality rates at discharge were 9.3% (38 of 410) for patients without seizures, 6.7% (11 of 164) for those with seizures but not status epilepticus at presentation, 11.1% (5 of 45) for those with status epilepticus, and 33.3% (4 of 12) for those with refractory status epilepticus. The mortality rate for patients with refractory status epilepticus was significantly higher compared to those for patients without seizures (p = 0.04) and those with seizures but not status epilepticus at presentation (p = 0.01). There were no significant differences between refractory status epilepticus and status epilepticus in terms of any of the parameters examined (Supplementary Table 1).
Derivation of ICU Risk Score
The derivation cohort encompassed the 372 patients from cohort 1. Upon examination of the variables available at the time of presentation, CCI score, CKD, fever, focal neurological deficits, new-onset seizure, headache, thrombocytopenia, white blood cell (WBC) count > 11,000 cells/μL, SOFA score > 3, GCS score < 8, and FOUR score ≤ 14 were observed to be associated with ICU admission on the univariate analysis (p < 0.05) (Supplementary Table 2). Because of high collinearity with multiple variables, CCI and SOFA scores were excluded from the subsequent backward regression analysis. Similarly, the GCS score was excluded because of its high correlation with the FOUR score, and headache was excluded because of its high correlation with fever.
Stepwise backward logistic regression was then performed on the remaining variables. This analysis identified a serum WBC count > 11,000 cells/µL, focal neurological deficits, new-onset seizures, CKD, and a FOUR score ≤ 14 as significant predictors of ICU admission (Hosmer–Lemeshow χ2 = 5.19, p = 0.52). The internal validity of the model was verified using bootstrapping with 1000 resamples. All model components remained significant with minimal bootstrap bias (Supplementary Table 3).
A weighted score was calculated for each variable based on their regression coefficients (Table 3). The variables included in the risk score were the following: FOUR score ≤ 14 (score of 3 points), medical history of CKD (2 points), focal neurological deficits (1 point), new-onset seizure (1 point), and serum WBC count > 11,000 cells/μL (1 point). All patients were scored accordingly. Of the 372 patients, 2 were excluded from cohort 1 because we could not identify their FOUR scores. The ROC curve analysis showed good diagnostic accuracy, with an AUROC of 0.77 (95% confidence interval [CI] 0.72–0.82; Fig. 1). Subsequently, the model was validated externally using 208 patients from cohort 2. Fifty-four patients were excluded from the validation cohort because they were missing at least one of the risk score variables. Despite being a geographically and demographically distinct population of patients (see Supplementary Table 4), the ICU risk score again showed good performance with cohort 2 (AUROC 0.76 [95% CI 0.7–0.83]; Fig. 1).
Model performance. Receiver operating characteristics (ROC) curve for intensive care unit (ICU) risk according to our model in cohort 1 (a) (area under the ROC [AUROC] 0.77, 95% confidence interval [CI] 0.72–0.82) and cohort 2 (b) (AUROC 0.76, 95% CI 0.69–0.83). AUROC values for cohort 1 (a): ICU risk score 0.77 (95%CI: 0.72–0.82). Focal neurological deficit 0.57 (95%CI: 0.51–0.64). Four Score ≤ 14 0.69 (95%CI: 0.63–0.75). Chronic Kidney disease 0.54 (95%CI: 0.48–0.6). New-Onset Seizure 0.58 (95%CI: 0.52–0.64) Leukocytosis 0.62 (95%CI: 0.56–0.68). AUROC values for cohort 2 (b): ICU risk score 0.76 (95%CI: 0.69–0.83). Focal neurological deficit 0.55 (95%CI: 0.47–0.63). Four score ≤ 14 0.69 (95%CI: 0.63–9.75). Chronic Kidney disease 0.5 (95%CI: 0.42–0.58). New-Onset Seizure 0.63 (95%CI: 0.54–0.71) Leukocytosis 0.66 (95%CI: 0.58–0.74). FOUR full outline of unresponsiveness.
Patients were stratified into three levels of risk for ICU admission based on their scores. The low-risk cutoff was determined at 0 points, with a sensitivity of 1, and 12.5% of these patients (11 of 88) were admitted to the ICU. The intermediate-risk category, ranging from 1 to 5 points, included 49.3% of patients (131 of 266) who were admitted to the ICU. The high-risk cutoff was determined at ≥ 6 points, with a specificity of 1, and 87.5% of these patients (14 of 16) were admitted to the ICU (Supplementary Table 5). Stratification was similar across both cohorts (Fig. 2).
Factors Associated with CKD
Given the importance of CKD in our risk score, we next examined associated factors. Patients with CKD were more likely to be older (58 [IQR 45.8–67.3] years) compared to patients without CKD (46 [IQR 32–61.8] years) (p = 0.005) and were more likely to have higher rates of comorbidities as indicated by a CCI score > 2 (CKD 86% vs. non-CKD 38.7%; p < 0.001). Immunocompromised status was also more common among patients with CKD (44% vs. 23.8%; p = 0.01).
During hospitalization, acute kidney injury (AKI) occurred with a higher incidence in patients with CKD (52.9%) compared to patients without CKD (23.5%) (p < 0.001), and 47.2% of patients with CKD required hemodialysis. EKG abnormalities were also more prevalent in patients with CKD (42.9% vs. non-CKD 20.8%; p = 0.003) (Supplementary Table 6).
Regarding antimicrobial use, there was no significant difference in the administration of vancomycin and acyclovir between those with and without CKD. Patients with CKD had a worse outcome at discharge, as noted by the median GOS score, which was lower in patients with CKD (3, IQR 3–4) compared to patients without CKD (4, IQR 3–5) (p = 0.02) (Supplementary Table 6).
Discussion
In this study of more than 600 patients with encephalitis from two separate health systems, we found that (1) 40% of patients required ICU care, underscoring the serious nature of acute encephalitis; (2) neurologic factors, including seizures and altered mental status, were strong drivers of ICU admission that distinguish encephalitis from other systemic emergencies; (3) a score based on five factors, including neurologic and systemic manifestations, can identify those at low, medium, or high risk for ICU care; and (4) ICU-admitted patients had an 18% mortality rate and poorer prognosis on multiple outcome measures, comparable to findings from other studies [4, 15,16,17].
The importance of the development and validation of an ICU risk score is underscored by data that early admission to the ICU improves prognoses of patients with encephalitis, likely because of the more timely identification and management of complications [7]. Moreover, the need for prompt identification and triage of patients who require ICU care in general has been highlighted by the recent COVID-19 pandemic and is highly relevant to otherwise resource-limited settings [18]. The performance of our model was validated on a distinct cohort of patients with encephalitis from another geographical location. Despite these variations, the model still showed good diagnostic accuracy, with similar risk stratification of patients for ICU admission. Thus, this score has the potential to provide valuable assistance to physicians in the acute care setting, enabling them to identify patients with encephalitis who are likely to require ICU admission. Furthermore, in scenarios in which the availability of ICU beds is constrained, the employment of the score could expedite the transfer of patients to specialized health care facilities that possess the necessary resources for ICU care.
It is noteworthy that, despite age and the CCI score being widely acknowledged as factors in predicting deterioration in various clinical conditions [19,20,21], they were not associated with need for ICU admission in our patients with encephalitis. This highlights the critical impact of neurological worsening associated with encephalitis in driving the need for ICU admission as compared to other systemic comorbidities. Our study found that new-onset seizures, status epilepticus, and measures of altered consciousness, such as the GCS and FOUR scores, are strong predictors of ICU admission in patients with encephalitis. These findings are consistent with several other studies, which have also identified new-onset seizures and severe alteration in mental status requiring intubation as contributors to ICU admission in patients with encephalitis [22,23,24].
Our model included a FOUR score ≤ 14 as a major contributor to the overall risk score. A previous study identified the same FOUR score cutoff as the optimal predictor of poor hospital outcomes, as measured by GOS at discharge, for patients admitted to the neurosurgery service with altered mental status [25]. We found that both the FOUR score and the GCS score were linked to ICU admission. However, we elected to use the FOUR score because it provides a more accurate assessment of the severity of altered mental status by reflecting brainstem function and respiratory drive, which play an important role in determining the depth of alteration in mental status and its effects on the need for mechanical ventilation [26].
Despite neurological complications being highly associated with ICU admission, we also found that some systemic factors increased the risk of ICU admission in our cohort. These included thrombocytopenia, leukocytosis, and CKD. Thrombocytopenia is the most frequent hematological abnormality in ICU patients and has previously been linked to worse outcomes among patients with encephalitis [16, 27,28,29]. Serum leukocytosis was also associated with an increased likelihood of ICU admission in our study. Given the possible involvement of systemic inflammation in the development of encephalitis [30,31,32], the role of serological inflammatory indicators in predicting the severity, prognosis, and treatment response in AIE has been examined. In a retrospective study involving a cohort of 146 patients with AIE, several indicators of systemic inflammation, grouped together as the systemic immune-inflammation index, were associated with a higher likelihood of ICU admission and a reduced response to immunotherapy [33]. Notably, the upsurge in peripheral inflammation caused by status epilepticus [34] or a disseminated infection may also contribute to the association between leukocytosis and ICU admission in encephalitis. In our study, 9.4% of patients with encephalitis admitted to the ICU had CKD. Patients with CKD are more prone to develop AKI [35]. This could be particularly problematic in patients with encephalitis because they already face a risk of developing AKI due to multiple factors, including exposure to nephrotoxic drugs (such as acyclovir) and hemodynamic instability secondary to dysautonomia [36,37,38]. It has been noted that the early mortality for critically ill patients with CKD is lower than that for those with AKI in the ICU, suggesting that outcomes among patients with CKD are driven by the propensity to develop AKI or other complications rather than a lower baseline in kidney function [39]. To better understand the factors that contribute to the strong association between CKD and ICU admission in our cohort, we conducted a subanalysis comparing patients with CKD to those without CKD. We found that patients with CKD had more comorbidities and were twice as likely to develop AKI during their hospital stay (52.9% vs. non-CKD 23.5%; p < 0.001) and to have abnormalities on EKG (42.9% vs. non-CKD 20.8%; p = 0.003). These factors could have contributed to their increased propensity of being admitted to the ICU and experiencing worse outcomes at discharge, as indicated by the median GOS score.
Finally, our data suggest that the need for ICU care may not stratify by whether the etiology of encephalitis is infectious or autoimmune. Of patients with definite AIE, 40% required ICU admission, consistent with another study conducted on 60 patients with severe AIE, in which 40.6% were admitted to the ICU [40]. This proportion was similar to that of our patients with IE (37% admitted to the ICU) and those with unknown encephalitis (44% admitted to the ICU). In addition, the proportion of patients with viral encephalitis in our study who required ICU admission (36%) was similar to that for the rest of the cohort and also to the results of another study conducted in China on more than 400 patients with viral encephalitis, in which 34% were admitted to the ICU [6].
Our sample size is noteworthy, considering the rarity of the condition. Additionally, this study introduces an easily implementable risk score using variables available at presentation to predict ICU admission in patients with suspected encephalitis, irrespective of the underlying etiology. The optimal combination of variables for predicting admission to the ICU was selected from a comprehensive list of readily available predictors. The risk score was externally validated and had a high and similar diagnostic accuracy in a second cohort of patients with encephalitis with different characteristics. Sharing the results of our ICU risk score can promote collaboration and constructive feedback. Comparative analysis of the results can help refine and optimize the model, laying the groundwork for future implementation efforts. Additionally, clinicians can gain valuable insights into the factors influencing ICU admission even before the model is implemented.
We observed that 12.5% of patients initially categorized as low risk ultimately required admission to the ICU. This could be due to the presence of factors not considered during development of our model or due to the emergence of clinical manifestations not evident at the time of presentation. After a patients is initially classified as low risk, the decision to admit them to the floor instead of the ICU may depend on various factors, including the patient’s specific presentation, the hospital’s resources, and ease of accessibility to the ICU in case of unforeseen complications. Nonetheless, our risk score effectively identifies patients with a high risk of requiring ICU, as 87.5% of patients in the high-risk group were ultimately admitted to the ICU during their initial hospitalization. It is important to note that our study has some limitations due to its retrospective design. This may have resulted in some variables not being considered and its observational nature with inherent risk of confounding. However, we have made an effort to address these limitations in our analysis and interpretation of results. We also encountered some missing data, but we addressed this by using a complete case analysis. Furthermore, although the two cohorts are from distinct geographical locations, they are both located within the United States, which may limit generalizability.
Despite the limitations, the study’s large sample size for a relatively uncommon condition, coupled with the external validation of our risk score, contributes to the validity and generalizability of our findings.
Conclusions
Two fifths of patients with encephalitis require admission to the ICU, and ICU admission is independently associated with worse outcomes and an increased burden of the disease. Our model includes five readily available variables at the time of presentation that take into account neurological and systemic factors and may help clinicians decide which patients require ICU care upon presentation, thus potentially improving triage and prognosis.
References
Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al.; International Encephalitis Consortium. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114–28.
Bloch KC, Glaser C, Gaston D, Venkatesan A. State of the art: acute encephalitis. Clin Infect Dis. 2023;77(5):e14-33.
Venkatesan A, Habis R, Geocadin RG. Approach to acute encephalitis in the intensive care unit. Curr Opin Crit Care. 2023;29(2):89–98.
Sonneville R, Gault N, De Montmollin E, Klein IF, Mariotte E, Chemam S, et al. Clinical spectrum and outcomes of patients with encephalitis requiring intensive care. Eur J Neurol. 2015;22(1):6–16.
Ali F, Wijdicks EF. Treatment of movement disorder emergencies in autoimmune encephalitis in the neurosciences ICU. Neurocrit Care. 2020;32(1):286–94.
Feng G, Zhou L, Li F, Hu Y, Wang X, Tian X. Predictors of outcome in clinically diagnosed viral encephalitis patients: a 5-year prospective study. BioMed Res Int. 2020;2020:2832418.
Sonneville R, de Montmollin E, Contou D, Ferrer R, Gurjar M, Klouche K, et al.; EURECA Investigator Study Group. Clinical features, etiologies, and outcomes in adult patients with meningoencephalitis requiring intensive care (EURECA): an international prospective multicenter cohort study. Intensive Care Med. 2023;49(5):517–29.
Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58(4):585–93.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47(4):458–72.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensiv Care Med. 1996;22(7):707–10.
Grissom CK, Brown SM, Kuttler KG, Boltax JP, Jones J, Jephson AR, et al. A modified sequential organ failure assessment score for critical care triage. Disaster Med Public Health Prep. 2010;4(4):277–84.
Madan A. Correlation between the levels of SpO2and PaO2. Lung India. 2017;34(3):307–8.
Groenwold RH, Donders AR, Roes KC, Harrell FE Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175(3):210–7.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 2015;13(1):1.
Fillatre P, Mailles A, Stahl JP, Tattevin P, Abgrall S, Argaud L, et al.; Scientific Committee and Investigators Group. Characteristics, management, and outcomes of patients with infectious encephalitis requiring intensive care: a prospective multicentre observational study. J Crit Care. 2023;77:154300.
Thakur KT, Motta M, Asemota AO, Kirsch HL, Benavides DR, Schneider EB, et al. Predictors of outcome in acute encephalitis. Neurology. 2013;81(9):793–800.
Cohen J, Sotoca J, Gandhi S, Yeshokumar AK, Gordon-Lipkin E, Geocadin RG, et al. Autoimmune encephalitis: a costly condition. Neurology. 2019;92(9):e964–72.
Cardona M, Dobler CC, Koreshe E, Heyland DK, Nguyen RH, Sim JP, et al. A catalogue of tools and variables from crisis and routine care to support decision-making about allocation of intensive care beds and ventilator treatment during pandemics: scoping review. J Crit Care. 2021;66:33–43.
St-Louis E, Iqbal S, Feldman LS, Sudarshan M, Deckelbaum DL, Razek TS, et al. Using the age-adjusted Charlson comorbidity index to predict outcomes in emergency general surgery. J Trauma Acute Care Surg. 2015;78(2):318–23.
Zhan YF, Li F, Wu LC, Li JM, Zhu CY, Han MS, et al. Role of Charlson comorbidity index in predicting the ICU admission in patients with thoracic aortic aneurysm undergoing surgery. J Orthop Surg Res. 2023;18(1):870.
Argun Barış S, Boyacı H, Akhan S, Mutlu B, Deniz M, Başyiğit İ. Charlson comorbidity index in predicting poor clinical outcomes and mortality in patients with COVID-19. Turk Thorac J. 2022;23(2):145–53.
Harutyunyan G, Hauer L, Dünser MW, Karamyan A, Moser T, Pikija S, et al. Autoimmune encephalitis at the neurological intensive care unit: etiologies, reasons for admission and survival. Neurocrit Care. 2017;27(1):82–9.
Diaz-Arias LA, Pardo CA, Probasco JC. Autoimmune encephalitis in the intensive care unit. In: Nelson S, Nyquist P, editors. Neurointensive care unit. Cham: Springer; 2020. p. 249–63.
Sonneville R, Jaquet P, Vellieux G, de Montmollin E, Visseaux B. Intensive care management of patients with viral encephalitis. Rev Neurol (Paris). 2022;178(1–2):48–56.
Akavipat P, Sookplung P, Kaewsingha P, Maunsaiyat P. Prediction of discharge outcome with the full outline of unresponsiveness (FOUR) score in neurosurgical patients. Acta Med Okayama. 2011;65(3):205–10.
Iyer VN, Mandrekar JN, Danielson RD, Zubkov AY, Elmer JL, Wijdicks EF. Validity of the FOUR score coma scale in the medical intensive care unit. Mayo Clin Proc. 2009;84(8):694–701.
Chakraverty R, Davidson S, Peggs K, Stross P, Garrard C, Littlewood TJ. The incidence and cause of coagulopathies in an intensive care population. Br J Haematol. 1996;93(2):460–3.
Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology. 2015;84(4):359–66.
Hansen MA, Samannodi MS, Hasbun R. Predicting inpatient mortality among encephalitis patients: a novel admission risk score. Open Forum Infect Dis. 2020;7(11):ofaa471.
Sonar SA, Lal G. Blood-brain barrier and its function during inflammation and autoimmunity. J Leukoc Biol. 2018;103(5):839–53.
Wesselingh R, Butzkueven H, Buzzard K, Tarlinton D, O’Brien TJ, Monif M. Innate immunity in the central nervous system: a missing piece of the autoimmune encephalitis puzzle? Front Immunol. 2019;10:2066.
Michael BD, Griffiths MJ, Granerod J, Brown D, Davies NW, Borrow R, et al. Characteristic cytokine and chemokine profiles in encephalitis of infectious, immune-mediated, and unknown aetiology. PLoS ONE. 2016;11(1):e0146288.
Mei Y, Yang J, Yuan Y, Liu Y, Liu X, Li M, et al. Systemic inflammation index values are associated with worsened disease severity and poor response to autoimmune encephalitis treatment. Front Neurol. 2021;12:709553.
Terrone G, Frigerio F, Balosso S, Ravizza T, Vezzani A. Inflammation and reactive oxygen species in status epilepticus: biomarkers and implications for therapy. Epilepsy Behav. 2019;101(Pt B):106275.
Hodgson LE, Sarnowski A, Roderick PJ, Dimitrov BD, Venn RM, Forni LG. Systematic review of prognostic prediction models for acute kidney injury (AKI) in general hospital populations. BMJ Open. 2017;7(9):e016591.
Carod-Artal FJ. Infectious diseases causing autonomic dysfunction. Clin Auton Res. 2018;28(1):67–81.
Halperin JJ. Diagnosis and management of acute encephalitis. Handb Clin Neurol. 2017;140:337–47.
He J, Lian Y. Clinical study of autonomic dysfunction in patients with autoimmune encephalitis. Immunobiology. 2023;228(5):152711.
Fidalgo P, Bagshaw SM. Chronic kidney disease in the intensive care unit. In: Arici M, editor. Management of chronic kidney disease. Berlin: Springer; 2014. p. 417–38.
Wang B, Wang C, Feng J, Hao M, Guo S. Clinical features, treatment, and prognostic factors in neuronal surface antibody-mediated severe autoimmune encephalitis. Front Immunol. 2022;13:890656.
Acknowledgements
We thank Melissa Canales for collection of some data.
Funding
The study was conducted without institutional or external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. Data collection and material and method development were conducted mainly by Ralph Habis, Ashley Heck, and Paris Bean. Statistical analysis was performed by Ralph Habis. The first draft of the manuscript was primarily written by Ralph Habis, and all additional authors provided critical feedback and edits to the manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Rodrigo Hasbun receives research support and personal fees from BioFire Diagnostics. John Probasco receives personal compensation for activity as Editor-in-Chief of The New England Journal of Medicine Journal Watch Neurology and serves as site investigator for a treatment trial for autoimmune encephalitis sponsored by Genentech. Ralph Habis serves as study coordinator for a treatment trial for autoimmune encephalitis sponsored by Genentech. The other authors declare no conflicts of interest.
Ethical Approval/Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki and was approved by the University of Texas and the Johns Hopkins School of Medicine Institutional Review Boards (IRB) (“Aseptic Meningoencephalitis in adults and children,” HSC-MS-08-0417, approval 05/11/2023; “Establishment of an encephalitis and seizure repository and database” IRB00083718, approval 11/30/2015; “Infectious and autoimmune encephalitis: a chart review” IRB00214450, approval 10/26/2021). The need for patient consent was waived by both IRBs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habis, R., Heck, A., Bean, P. et al. Development and Validation of a Risk Score for Predicting ICU Admission in Adults with New-Onset Encephalitis. Neurocrit Care (2024). https://doi.org/10.1007/s12028-024-02063-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12028-024-02063-6