Abstract
Objective
We want to investigate the effect of aquaporin-4 (AQP4) on cerebral edema induced by ischemic stroke in rats and explore whether inhibiting the expression of AQP4 through acetazolamide (AZA) could attenuate brain edema and protect cerebral function.
Methods
The Sprague Dawley (SD) rats were randomly divided into four groups: sham + saline group, sham + AZA group, AZA intervention group, and nonintervention group. Each group was divided into five subgroups according to the time of cerebral ischemia (6 h, 1 day, 3 days, 5 days, and 7 days). The model of cerebral infarction in rats was adopted by means of the bilateral carotid arteries ligation (2-VO) method. The rats in intervention group were given intraperitoneal injection of AZA (35 mg/kg/day). Hematoxylin–eosin staining was performed for pathological analysis of the infarcted area. The brain water content was calculated to evaluate the degree of brain edema. The messenger RNA (mRNA) and protein expressions of AQP4 in the brain were measured by quantitative real-time polymerase chain reaction and immunohistochemistry, respectively.
Results
Significant cerebral pathological damages were found in ischemic stroke rats. The brain water content, protein, and mRNA expression of AQP4 of the intervention and nonintervention groups were markedly higher than those of the sham groups. By contrast, AZA administration reduced the brain water content, whereas improved cerebral dysfunction was induced by ischemic stroke. Moreover, AZA obviously reduced the protein and mRNA expression of AQP4 after ischemic stroke in rats’ brains.
Conclusions
The expression of AQP4 was closely related to cerebral edema induced by ischemic stroke. Decreasing the expression of AQP4 mRNA by AZA administration can effectively relieve cerebral edema and decrease cerebral pathological damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is caused by a decrease in cerebral blood flow due to occlusion of cerebral arteries. The occurrence and development of it have complex pathological mechanisms. It contributes significantly to mortality and disability. The pathological reaction caused by ischemia lasts for several days to weeks in the infarcted area of the brain [1]. Cerebral edema is a common result of ischemic stroke, which often leads to its aggravation and deterioration. Moreover, malignant brain edema is often the direct cause of death. Malignant brain edema leads to acute increased intracranial pressure, leading to the formation of hernia. Current treatments for cerebral edema after ischemic stroke include hypertonic dehydration, decompressive craniectomy, and hypothermia therapy. However, patients still have a poor prognosis. Therefore, it is valuable to discover new methods to reduce edema.
Aquaporins (AQPs) are a family of transmembrane channel proteins that process high permeability to water molecules. AQPs are reported to be closely associated with cellular edema. Currently, more than ten AQPs have been found in mammals [2]. Aquaporin-4 (AQP4) was found to be the most abundant in the brain and spinal cord, mainly distributed in astrocytes, capillary endothelial cells, and ependymal cells [3]. Researchers have confirmed that AQP4 participates in the pathophysiological processes of ischemic cerebral edema. Manley et al. [4] demonstrated that the swelling of pericapillary astrocytic foot processes in AQP4-deficient mice was evidently decreased with substantially better improvement in survival rate in water intoxication experiment. Researchers found that in brain edema, the increase in AQP4 expression is related to the release of inflammatory factors, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) [5, 6]. In addition, there are more recent studies revealing related pathways about AQP4 and brain edema, such as astrocyte migration, PPAR-γ, K + reuptake and thresholds, MMP activity, etc. [7,8,9,10,11,12].
Acetazolamide (AZA) is a carbonic anhydrase inhibitor that has been clinically used in the treatment of various diseases. However, recent studies have also found that AZA functions as an AQP4 inhibitor, widely throughout the brain [13]. So, we aim to find out whether we can suppress the expression of AQP4 by AZA to reduce brain edema. This study was designed to examine the effect of AZA on AQP4 expression and cerebral edema after ischemic stroke, which could potentially provide therapeutic alternatives for patients with cerebral infarction.
Methods
Reagents
Acetazolamide was purchased from Wuhan Nanjian Biochemical Manufacturing Co. Ltd. The rabbit anti-AQP4 antibody was purchased from Wuhan Boster Biological Engineering Co. Ltd.
Ethics Statements
Male SD rats weighing 240–300 g were obtained from the Animal Experiment Center of Shanxi Medical University. Animal experiments were performed in accordance with the Guide for Laboratory Animals published by the National Institutes of Health. All rats were fed with standard rat food and water and were kept under 12:12 h light/dark cycles at 22 °C. This study was approved by the Animal Ethics Committee of Shanxi Medical University Animal Center. All animals were randomly assigned into four groups. In each subgroup, six rats were used for brain tissue water content and quantitative real-time polymerase chain reaction (qRT-PCR), and three rats were used for hematoxylin–eosin staining and immunohistochemistry. The animals were numbered randomly after being killed, and the effect evaluator and experimenter did not know the specific animal group.
Construction of the Animal Model of Ischemic Stroke
Animal models were made by bilateral carotid artery ligation. Then they were anesthetized by intraperitoneal injection of 10% chloral hydrate (3 ml/kg). The rat was fixed on the operating table, and the skin was cut off and disinfected with iodophor. The central incision of the neck was taken to expose the bilateral carotid arteries and vagus nerve.
-
1.
Sham + saline and sham + AZA group: only bilateral carotid arteries and the accompanying vagus nerve were separated without ligation
-
2.
Nonintervention group and intervention group: bilateral carotid arteries were ligated by 3–0 nylon thread
After the operation, the rat’s neck wound was sutured carefully, and the appropriate amount of penicillin was used to prevent infection. A rat model of ischemic stroke was successfully established when the rat woke up with drooping eyelids and small fission of eyes. Subsequently, rats in the intervention group and sham + AZA group were injected with AZA (35 mg/kg/day) into the abdominal cavity, whereas rats in the sham + saline group and nonintervention group were intraperitoneally infused with saline [14].
Determination of Brain Water Content
At 6 h, 1 day, 3 days, 5 days, and 7 days after duplicating the mode, rats in each subgroup were decapitated and their brains were removed. The meninges, olfactory bulb, cerebellum, lower brainstem, and other tissues were removed. The liquid on the surface of the brain tissue was absorbed dry with filter paper and immediately placed on a precision electronic analytical balance to weigh the wet weight. Then it was put in a 60 ℃ oven and baked for 3 days, and the dry weight was weighed. The water content of the cerebral hemispheres was calculated according to the following formula: brain water content (BWC) = (wet weight − dry weight)/wet weight × 100%. The spinal cord and liver use the same method.
Hematoxylin–Eosin Staining
Three rats were taken from each subgroup at different time points. After anesthesia, saline and 4% paraformaldehyde were used for heart perfusion experiments successively. Brains were taken when the whole body was just stiff. Then the brains were fixed in 10% formalin for 24 h, embedded in paraffin, and cut into 5-μm slices. The slices were deparaffinized in xylene, rehydrated in gradient ethanol, and then stained with hematoxylin and eosin. Images were captured using a light microscope (OLYMPUS, Tokyo, Japan) with a × 400 magnification.
Immunohistochemistry
Three rats were taken from each subgroup at different time points. The degree and location of AQP4 expression were observed by immunohistochemistry. After being deparaffinized and rehydrated, the slices were subjected to retrieve antigen in antigen retrieval buffer for 10 min. Next, the slices were incubated with 3% H2O2 for 15 min to quench the activity of endogenous peroxidases. Thereafter, the slices were incubated with AQP4 antibody (1:400; Wuhan Boster Bioengineering Co. Ltd.) at 37 °C for 1 h. They were rinsed in phosphate buffered saline (PBS) and incubated with corresponding secondary antibodies (biotin-labeled goat anti-rabbit IgG; 1: 200; Wuhan Boster Bioengineering Co. Ltd) for 30 min at 37 °C later. The slices were rinsed in PBS, incubated with Strept Avidin-Biotin Complex (SABC) for 30 min at 37 °C again. Then diaminobenzidine was used for coloring and counterstaining. Finally, neutral gum was used to mount the slices. The AQP4 was observed under a light microscope. The positive reaction area was brownish yellow, and the nucleus was purple–blue. The negative control replaced the primary antibody with PBS solution, and the rest of the steps were conducted as previously described. Scanscope digital pathology scanning system (American Aperio Company) was used to quantitatively analyze the average gray value of the positive area (five areas for each tissue) and the average value. The gray values were inversely proportional to the extent of positive reaction.
qRT-PCR Analysis
Total RNA in brain tissues was extracted using the Trizol reagent (Takara, Minato-ku, Tokyo, Japan). Complementary DNA synthesis was performed by using the reverse transcription reagent kit according to the instructions (Takara, 78Biotechnology, Tokyo, Japan). qRT-PCR experiments were performed using the SYBR Prellix Ex Taq Real-Time PCR Kit (Takara, Minato-ku, Tokyo, Japan). Relative expression of AQP4 mRNA was calculated using the 2−ΔΔCt method. All samples were run in triplicate. Primer sequences are listed in Table 1.
Statistical Analysis
Statistical Product and Service Solutions 22.0 software (IBM Corporation, Armonk, NY) was used for all statistical analysis. All quantitative data were expressed as mean ± standard deviation of at least three independent experiments. The differences between groups were calculated using Kruskal–Wallis analysis followed by Dunn’s post hoc method for nonparametric comparisons. Statistically significant differences were evaluated by two-way repeated measures analysis of variance and multiple comparisons followed by Bonferroni’s post hoc test for parametric comparisons. P < 0.05 was considered to indicate a statistically significant difference.
Results
AZA Reduces Cerebral Edema through AQP4 but not Other Functions of AZA that Affect Edema
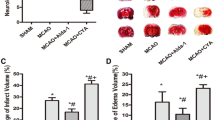
As shown in Fig. 1, we compared the BWC, spinal cord water content, and liver water content of sham + saline and sham + AZA groups. We found that for the liver, there was no significant difference at all time points. For the spinal cord, there was a difference on the 3rd day. For the brain, there was a significant difference on the 7th day, but the AZA group was higher than the saline group. The differences were meaningless. It was speculated that it was caused by sample errors. So, we combined the sham + saline and sham + AZA groups as the sham group in the following analysis.
Results of brain, spinal cord, liver water content of the sham + saline group and the sham + AZA group. a For the brain water content, there was a significant difference on the 7th day (P < 0.05), and the AZA group is higher than the saline group. b For the spinal cord water content, there was a difference on the 3rd day (P < 0.05), and the saline group is higher than the AZA group. c For the liver water content, there was no significant difference at all time points (P > 0.05). AZA acetazolamide
AZA Reduced BWC Increase After Ischemic Stroke
The BWC of the sham operation group did not change significantly at each time point, and the difference was not significant (P > 0.05). The BWC of the nonintervention group and the AZA intervention group began to increase at 6 h after cerebral infarction, reached a peak at 3 days, and then gradually decreased. At each time point, the BWC of the nonintervention group and the intervention group was higher than that of the sham operation group (P < 0.05). In the meantime, the BWC of the AZA intervention group was lower than that of the nonintervention group (P < 0.05; Fig. 2).
Results of brain water content. The figure shows that at each time point the expression of brain water content in the nonintervention group and the intervention group was higher than that of the sham operation group, and the brain water content expression of the intervention group was lower than that of the nonintervention group. Significant differences within the group at each time point (all P < 0.05). The brain water content in the sham group did not change significantly at each time point (P > 0.05). The brain water content of the nonintervention group and the intervention group both began to increase at 6 h, reaching the peak at 3 days and then gradually decreased. Significant differences between groups (P < 0.05)
AZA Attenuated Cerebral Pathological Damage Induced by Ischemic Stroke
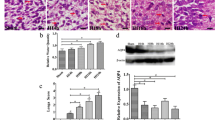
Hematoxylin-Eosin staining was observed under the light microscope. No abnormal pathological changes were observed in rats in the sham operation group. But the nonintervention group and intervention group began to cause pathological changes at 6 h after cerebral infarction, such as neuronal necrosis, widened extravascular space, extracellular space enlargement, organization structure disorder, decreased cytoplasmic staining, etc. The above pathological changes were aggravated at 1 day after cerebral infarction, and the most obvious changes were at 3 days, beginning to show inflammatory cells. The pathology gradually abated from 3 to 7 days, and the reduction in cell swelling was accompanied by the appearance of glial cells. Pathological changes in the intervention group were less severe than those in the nonintervention group (Figs. 3 and 4a). Besides, AQP4 is mainly expressed in astrocytes around capillaries (Fig. 4a).
Histopathological changes of rat brain (HE staining, × 400). a Six hours after cerebral infarction in nonintervention group. b One day after cerebral infarction in nonintervention group. c Three days after cerebral infarction in the nonintervention group. d Five days after cerebral infarction in nonintervention group. e Seven days after cerebral infarction in nonintervention group. f–j Pathological changes at the same time point in the intervention group. Arrowhead, neurons; asterisk, capillaries; arrow, neutrophils. HE xxx
a Results of AQP4 immunohistochemistry in brain tissue of rats (× 400). Results of AQP4 immunohistochemistry in brain tissue of rats at the same time point in the intervention group. b Results of AQP4 content in the brain. The figure shows that at each time point, the expression of AQP4 in the nonintervention group and the intervention group was higher than that of the sham operation group, and the AQP4 expression of the intervention group was lower than that of the nonintervention group. Significant differences within the group at each time point (all P < 0.05). The AQP4 content in the sham group did not change significantly at each time point (P > 0.05). The AQP4 content of the nonintervention group and the intervention group began to increase at 6 h, reaching the peak at 3 days, and then gradually decreased. Differences between groups were significant (P < 0.05). APQ4, aquaporin 4
AZA Inhibited the Ischemic Stroke Induced Increase in the mRNA and Protein Expression of AQP4
According to the results of immunohistochemistry, the expression of AQP4 in the brain tissue of the sham group did not change significantly at each time point (P > 0.05). The expression of AQP4 in the brain tissue of the nonintervention group and the AZA intervention group began to increase at 6 h after cerebral infarction, and the expression of AQP4 reached the peak at 3 days and then gradually decreased. The difference was significant (P < 0.05). At each time point, the expression of AQP4 and AQP4 mRNA in brain tissue of the nonintervention group and the intervention group was higher than that of the sham group. It was significant (P < 0.05), and the expression of AQP4 and AQP4 mRNA in the brain tissue of the intervention group was lower than that of the nonintervention group, and the difference was significant (P < 0.05) (Fig. 4b).
For the nonintervention group, the expression of AQP4 mRNA began to increase at 6 h, reached the highest level on the 1st day, then began to decline, reaching the lowest level on the 5th day, and then increased. For the intervention group, the overall trend was the same as that of the intervention group, but it reached the lowest point on the 3rd day and then slightly increased (Fig. 5). Because mRNA changes earlier than protein, combined with protein expression (Fig. 4b), we can infer that the highest level of mRNA should be between 1 and 3 days. These results show that the use of AZA can lead to the arrival of the lowest level of AQP4 mRNA in advance.
Results of relative AQP4 mRNA expression. The chart shows that at each time point the expression of AQP4 mRNA in the nonintervention group and the intervention group was higher than that of the sham operation group, and the AQP4 mRNA expression of the intervention group was lower than that of the nonintervention group. Significant differences within the group at each time point (all P < 0.05). The AQP4 mRNA in the sham group did not change significantly (P > 0.05). The AQP4 mRNA of the nonintervention group began to increase at 6 h, reaching the peak at 1 day, and then gradually decreased to the lowest level at 5 days. As for the intervention group, the AQP4 mRNA began to increase at 6 h, reaching the peak at 1 day, and then gradually decreased to the lowest level at 3 days. Differences between groups were significant (P < 0.05). APQ4, aquaporin, mRNA, messenger RNA
Discussion
In this study, we mainly focused on the effect of AQP4 on cerebral edema after ischemic stroke. Our results demonstrated that significant cerebral edema occurred in rats after ischemic stroke, which were confirmed by pathological section and BWC. The mRNA and protein expression of AQP4 in the brain were markedly increased in cerebral edema rats. AZA could significantly inhibit AQP4 protein and mRNA expression in the brain. Although AZA might not reverse cerebral edema, it could attenuate cerebral edema and protect brain function to a certain degree, especially if the peak of cerebral edema can be significantly reduced.
In recent years, the incidence of ischemic cerebrovascular disease has been getting higher. The main pathological change is cerebral edema after infarction, which is the root cause of disability and death of patients. Reducing the damage caused by cerebral edema is an important therapeutic target to improve the prognosis of cerebral ischemic infarction. Current treatment strategies are limited to decompressive craniectomy and intravascular administration of hypertonic drugs, which were introduced 70 years ago. So new treatments need to be developed. According to our experiments, we found that AZA can play a therapeutic role in the whole course of cerebral edema, especially in the peak period of edema. In human brain infarction, brain edema reaches its peak in the first 3–5 days of infarction, and then the edema slowly decreases. However, sometimes patients have to be implemented decompressive craniectomy because of high intracranial pressure and decreased consciousness before reaching the remission period of edema. This study suggests that the use of AZA in patients with cerebral infarction can reduce the peak of cerebral edema and reach the remission period of edema earlier. Besides, many potential treatments have now been discovered, such as the use of mesenchymal stem cells, 60% normobaric oxygen, hypothermia treatment, and so on. In the future, the combination of AZA and other methods to reduce cerebral edema may have a more obvious effect on inhibiting cerebral edema.
AQPs are a type of cell membrane transport protein that is selective for a water molecule. AQPs cannot only pass through water but can also form a pore in the center of the tetramer, which may promote the flow of water, cations, and gases. They are widely distributed in animals, plants, and microorganisms. Thirteen kinds of AQPs (AQP0–AQP12) have been found so far [15], of which AQP4 is the most widely distributed AQP in the brain [16]. It is mainly expressed on astrocytes and perivascular, areas such as arachnoid, pia mater, and capillaries [3], indicating that AQP4 plays an important role in regulating water metabolism in the brain. The immunohistochemistry in our experiment showed that AQP4 is most abundantly expressed on the astrocyte membranes near the capillaries and around the arachnoid, but its expression is not seen in neurons, which is consistent with previous reports. Arno Vandebroek et al. [17] found that the activity of AQP4 increased in epilepsy, edema and glioblastoma, and other central nervous system diseases. Manley et al. [4] revealed that cerebral edema after ischemic stroke is closely related to AQP4, and there is a positive correlation between them. The results of this experiment showed that the BWC began to increase at 6 h after cerebral ischemia, reached the peak of edema at 3 days, and then decreased. The results of immunohistochemistry suggested that the expression of AQP4 also showed the same trend. More importantly, the BWC and AQP4 protein expression in the intervention and nonintervention groups at each time point were higher than those in the sham operation group. It proved that AQP4 participates in the progression of cerebral edema after ischemic stroke with an evident time correlation.
Many studies have shown that the inflammatory response, oxidative stress, ion abnormalities, and other damage mechanisms in the brain tissue after ischemia can regulate the expression of the AQP4 and AQP4 genes. A low oxygen environment in the brain can trigger inflammatory response, which AQP4 can be upregulated through the release of TNF-α and IL-6 from microglia [18]. Puerarin can reduce the content of AQP4 by inhibiting the phosphorylation of NF-κB and MAPK pathways [19]. Transcriptional factor nuclear factor erythroid 2 can endogenously resist brain stress and inflammation by regulating the content of AQP4 [20]. Oxidative stress acts on the inflammatory factor leukotrienes to induce the expression of AQP4. Studies [21] have shown that the P38 and ERK signaling pathways can activate the cysteinyl leukotrienes 2 receptor for cysteine leukotrienes to upregulate the expression of AQP4. Besides, the phosphorylation pathway can also regulate the AQP4’s expression [17]. After the AQP4 gene was knocked out, the water content of the brain tissue of the mice was lower than that of the control group, and the degree of cell swelling was much lower than that of the control group. Katada et al. [22] created a global cerebral ischemia mouse model and found that AQP4−/− mice had greatly reduced brain tissue and cell swelling and significantly improved survival rate. Hirt et al. [23] have also confirmed that AQP4-lacking mice are less severe than wild-type mice in terms of lesion volume, neuroinflammatory response, and nerve cell necrosis. Yao et al. [24] also confirmed that cerebral edema and infarct degree in AQP4-deficient mice were reduced after focal cerebral ischemia. Some studies have shown that in the edema caused by cerebral ischemia, the increase in AQP4 expression is related to the release of inflammatory factors, such as IL-1β, IL-6, and TNF-α [5, 6]. This is consistent with our research results. As shown in Fig. 2, a large number of inflammatory cells appeared on the third day of the nonintervention group. At present, there is little research that reveals the mechanism of AQP4 and brain edema, and further experimental evidence is still required for verification. Zhao et al. [8] found that AQP4 deletion reduced inflammatory responses due to the upregulation of PPAR-γ expression. Researchers have found that AQP4 can modulate water and potassium ions fluxes associated with neuronal activity, participating in the reuptake of potassium ions, and can change the threshold of it [9, 10]. Steiner et al. [11] found brain infarction can lead to loss of astrocyte polarity. AQP4 redistributes from the terminal foot of astrocytes around the capillaries to the membrane area outside the terminal area, and this effect was affected by Matrix Metallopeptidase 3. Thrane et al. [12] provided evidence that brain edema can trigger Ca2+ signaling in astrocytes and that deletion of the AQP4 gene markedly interferes with this event.
Our experiment also found that the level of AQP4 mRNA in the intervention group was higher than that in the sham operation group at all time points and lower than that in the nonintervention group. Because mRNA changes earlier than protein, combining Figs. 4 and 5, we can infer that the highest level of mRNA should be between 1 and 3 days. These results prove that the use of AZA can make the lowest level of AQP4 mRNA come earlier in rats with cerebral infarction. But we found an interesting point. The mRNA expression will rebound slightly after reaching the lowest level. We speculate that this is caused by other factors that affect AQP4 mRNA and is related to the regression of cerebral edema. Therefore, it is tempting to speculate that AZA reduces the level of AQP4 increase by reducing the expression of AQP4 mRNA and acts indirectly on the AQP4 protein. When brain edema occurs, by taking measures to reduce the expression of the AQP4 gene, the increased content of AQP4 in the brain can be effectively reduced, thereby reducing the degree of cerebral edema.
AZA can act on carbonic anhydrase in renal tubular epithelial cells. In recent years, with AZA’s wider application in kidney diseases, it has an increasingly important role in the body water balance. However, more and more researchers have found that the nondiuretic effect of AZA can be used to treat other types of diseases. Studies have found that AZA has a certain role in the treatment and prevention of glaucoma [25, 26]. AZA worsens skeletal muscle fatigue in animals and humans, and taking it before climbing can prevent acute mountain sickness [27, 28]. AZA is a recommended first-line drug treatment for acute mountain diseases. It can reduce the lack of ventilation at high altitudes [29]. AZA may be a useful therapy medicine for some patients with respiratory failure and metabolic disorders [30]. AZA may help improve ventilatory instability in Obstructive Sleep Apnea [31]. However, there are few international studies on the relationship between AZA and ischemic cerebral edema to date. This experiment explored the nondiuretic effect of AZA in the treatment of cerebral edema and its influence on brain tissue for the first time in the literature, providing evidence for clinical treatment of cerebral edema in patients with ischemic stroke. We can see that the change of cerebral edema overtime after cerebral infarction is regular. After using AZA, we found that the cerebral edema did not reverse but decreased to a certain extent, and the trend of change over time was the same as that of the nonintervention group. In this experiment, the dosage of AZA was 35 mg/kg/day. This dosage is more suitable for rats, but for human use, we still need to find the appropriate concentration. Now in vitro experiments show that the concentration of AZA to reduce the water permeability of rat AQP4 by 50% is 1.25 mm [32], and the half maximal inhibitory concentration (IC50) of human AQP4 is 0.9 µM [33]. These show that AZA not only reduces the water permeability of AQP4 but also reduces the expression of AQP4. More experiments are needed to explore the optimal concentration for human application to avoid possible off-target or confusion.
AQP4 plays an important role in the formation and resolution of edema. The use of AZA inhibits the increase of AQP4, which reduces the rate of edema resolution to a certain extent, but the more important role is to reduce the degree of brain edema peak. This is very important in clinical applications. AZA could increase the patient’s treatment time window and greatly improve the survival rate of patients. In addition, when AZA enters the brain tissue through the blood–brain barrier, its efficiency is relatively low. Improving the passage efficiency can increase its ability to reduce cerebral edema, which is also worth studying. Although this article provides a new treatment direction, further investigations will be required to demonstrate its effectiveness of the clinical application.
Conclusions
The increased expression of AQP4 in brain was closely related to cerebral edema after ischemic stroke. Inhibiting the expression of AQP4 mRNA by AZA could reduce the increasing of AQP4 and then attenuate cerebral edema and pathological damage. Therefore, the targeted therapy of AQP4 might be a potential treatment strategy for cerebral edema. The limitation of the current study was the lack of research on the cellular mechanism. Thus, further research is still needed to investigate the potential therapeutic target of AQP4 in brain in vitro.
References
Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–7.
Takata K, Matsuzaki T, Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem. 2004;39(1):1–83.
Nielsen S, Nagelhus EA, Amiry MM, et al. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17(1):1171–80.
Manley GT, Fujimura M, Ma T, et al. Aquaporin- 4 deletion in mice reduces brain edema after actue water intoxication and Ischemic stroke. Nat Med. 2000;6(2):159–63.
Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13(4):259–77.
Tang G, Yang GY. Aquaporin-4: a potential therapeutic target for cerebral edema. Int J Mol Sci. 2016;17(10):1413.
Hiroaki Y, Tani K, Kamegawa A, et al. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006;355(4):628–39.
Zhao F, Deng J, Xu X, et al. Aquaporin-4 deletion ameliorates hypoglycemia-induced BBB permeability by inhibiting inflammatory responses. J Neuroinflammation. 2018;15(1):157.
Binder DK, Yao X, Zador Z, et al. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53(6):631–6.
Amiry-Moghaddam M, Williamson A, Palomba M, et al. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A. 2003;100(23):13615–20.
Steiner E, Enzmann GU, Lin S, et al. Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia. 2012;60(11):1646–59.
Thrane AS, Rappold PM, Fujita T, et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A. 2011;108(2):846–51.
Sripathirathan K, Brown JR, Neafsey EJ, et al. Linking binge alcohol-induced neurodamage to brain edema and potential aquaporin-4 upregulation: evidence in rat organotypic brain slice cultures and in vivo. J Neurotrauma. 2009;26(2):261–73.
Duan L, Di Q. Acetazolamide suppresses multi-drug resistance-related protein 1 and P-glycoprotein expression by inhibiting aquaporins expression in a mesial temporal epilepsy rat model. Med Sci Monit. 2017;23:5818–25.
Itoh T, Rai T, Kuwahara M, et al. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem Biophys Res Commun. 2005;330(3):832–8.
Xu M, Xiao M, Li S, et al. Aquaporins in nervous system. Adv Exp Med Biol. 2017;969:81–103.
Vandebroek A, Yasui M. Regulation of AQP4 in the central nervous system. Int J Mol Sci. 2020;21(5):1603.
Song TT, Bi YH, Gao YQ, et al. Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J Neuroinflammation. 2016;13(1):63.
Wang C, Yan M, Jiang H, et al. Mechanism of aquaporin 4 (AQP 4) up-regulation in rat cerebral edema under hypobaric hypoxia and the preventative effect of puerarin. Life Sci. 2018;193:270–81.
Ampawong S, Luplertlop N. Experimental scedosporiosis induces cerebral oedema associated with abscess regarding aquaporin-4 and Nrf-2 depletions. Biomed Res Int. 2019;59:6076571.
Hsu Y, Tran M, Linninger AA, et al. Dynamic regulation of aquaporin-4 water channels in neurological disorder. Croat Med J. 2015;56(5):401–21.
Katada R, Akdemir G, Asavapanumas N, et al. Greatly improved survival and neuroprotection in aquaporin-4-knockout mice following global cerebral ischemia. FASEB J. 2014;28(2):705–14.
Hirt L, Fukuda AM, Ambadipudi K, et al. Improved long-term outcome after transient cerebral ischemia in aquaporin-4 Knockout mice. J Cereb Blood Flow Metab. 2017;37(1):277–90.
Yao X, Derugin N, Manley GT, et al. Reduced brain edema and infarct volume in aquaporin-4 deficient mice after transient focal cerebral ischemia. Neurosci Lett. 2015;584:368–72.
Ghorai S, Pulya S, Ghosh K, et al. Structure-activity relationship of human carbonic anhydrase-II inhibitors: detailed insight for future development as anti-glaucoma agents. Bioorg Chem. 2020;95:103557.
Hayashi K, Yoshida M, Manabe SI, et al. Prophylactic effect of oral acetazolamide against intraocular pressure elevation after cataract surgery in eyes with glaucoma. Ophthalmology. 2017;124(5):701–8.
Dominelli PB, McNeil CJ, Vermeulen TD, et al. Effect of acetazolamide and methazolamide on diaphragm and dorsiflexor fatigue: a randomized controlled trial. J Appl Physiol (1985). 2018;125(3):770–9.
Lipman GS, Pomeranz D, Burns P, et al. Budesonide versus acetazolamide for prevention of acute mountain sickness. Am J Med. 2018;131(2):200.
Richalet JP, Rivera M, Bouchet P, et al. Acetazolamide: a treatment for chronic mountain sickness. Am J Respir Crit Care Med. 2005;172(11):1427–33.
Gulsvik R, Skjørten I, Undhjem K, et al. Acetazolamide improves oxygenation in patients with respiratory failure and metabolic alkalosis. Clin Respir J. 2013;7(4):390–6.
Edwards BA, Connolly JG, Campana LM, et al. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep. 2013;36(2):281–5.
Tanimura Y, Hiroaki Y, Fujiyoshi Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J Struct Biol. 2009;166(1):16–21.
Huber VJ, Tsujita M, Yamazaki M, et al. Identification of arylsulfonamides as aquaporin 4 inhibitors. Bioorg Med Chem Lett. 2007;17(5):1270–3.
Funding
This work was supported by the National Natural Science Foundation of Youth Fund (30600637) and China Postdoctoral Science Foundation Grant (2019T120195).
Author information
Authors and Affiliations
Contributions
Jia-Qi Hao, Xing-Yue He, Xin Yang, and You-Chao Xiao contributed to the entire project, from experimental design to conducting experiments, collecting data, and finally writing articles. Huan Wang, Jia-Ying Shi, and Xiao-Lin Zhu participated in the polishing and revision of the manuscript. Yu Zhang, Hao Bai, and Sheng-Qiang Duan participated in the collection of some experimental data. You-Chao Xiao and Zhuang-Zhuang Wang participated in the process of statistical analysis and drawing figures. Hu-Bin Duan and Chun-Yan Hao were responsible for supervising and providing financial support. The final manuscript was approved by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that (1) no support, financial or otherwise, has been received from any organization that may have an interest in the submitted work and (2) there are no other relationships or activities that could appear to have influenced the submitted work.
Human and Animal Rights
We confirm that we have complied with animal ethical guidelines, and this study was approved by the Animal Ethics Committee of Shanxi Medical University Animal Center.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hao, JQ., He, XY., Yang, X. et al. Acetazolamide Alleviate Cerebral Edema Induced by Ischemic Stroke Through Inhibiting the Expression of AQP4 mRNA. Neurocrit Care 36, 97–105 (2022). https://doi.org/10.1007/s12028-021-01261-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01261-w