Abstract
Background/Objectives
The objective of this study is to propose a definition of intraventricular hemorrhage (IVH) growth and to investigate whether IVH growth is associated with ICH expansion and functional outcome.
Methods
We performed a prospective observational study of ICH patients between July 2011 and March 2017 in a tertiary hospital. Patients were included if they had a baseline CT scan within 6 h after onset of symptoms and a follow-up CT within 36 h. IVH growth was defined as either any newly occurring intraventricular bleeding on follow-up CT scan in patients without baseline IVH or an increase in IVH volume ≥ 1 mL on follow-up CT scan in patients with initial IVH. Poor outcome was defined as modified Rankin Scale score of 3–6 at 90 days. The association between IVH growth and functional outcome was assessed by using multivariable logistic regression analysis.
Results
IVH growth was observed in 59 (19.5%) of 303 patients. Patients with IVH growth had larger baseline hematoma volume, higher NIHSS score and lower GCS score than those without. Of 44 patients who had concurrent IVH growth and hematoma growth, 41 (93.2%) had poor functional outcome at 3-month follow-up. IVH growth (adjusted OR 4.15, 95% CI 1.31–13.20; P = 0.016) was an independent predictor of poor functional outcome (mRS 3–6) at 3 months in multivariable analysis.
Conclusion
IVH growth is not uncommon and independently predicts poor outcome in ICH patients. It may serve as a promising therapeutic target for intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a devastating form of stroke associated with high rates of mortality and unfavorable outcome [1, 2]. Intraventricular hemorrhage (IVH) occurs in up to 50% of patients with ICH and is an independent predictor of 30-day mortality [3,4,5].
Many authors have examined early hematoma expansion after ICH, referring specifically to expansion of the parenchymal component, and have found this is associated with poor outcome [6,7,8,9]. However, few have studied whether the ventricular component (IVH) expands as well. Hematoma expansion has been a hot therapeutic target for intervention in patients with ICH. However, clinical trials aimed at reducing hematoma growth have shown little impact on functional outcome in patients with ICH [10]. Refining therapeutic targets for anti-expansion treatment may have important clinical implications for designing future clinical trials.
In the previous studies, most researchers have investigated the effect of IVH presence alone on functional outcome [4, 11]. Several studies suggested that ventricular extension of ICH itself is a dynamic pathophysiological process [12, 13], and IVH expansion may occur in a process independent of that associated with ICH expansion [14]. In order to evaluate the frequency of IVH growth and the impact (if any) on functional outcome, we performed a prospective observational study of patients with ICH. We examined IVH presence and volume and hypothesized that IVH growth would be relatively common and associated with worst outcome.
Study Design
Adult patients presenting with spontaneous ICH to The First Affiliated Hospital of Chongqing Medical University between July 2011 and March 2017 were included in our ongoing prospective cohort study. This study was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. Patients were included in our study if they had baseline CT scan within 6 h after the onset of symptoms. Follow-up CT scan was performed within 36 h after the baseline CT scan. Patients who were surgically treated before the follow-up CT scan were excluded from our study. Patients with ICH attributable to arteriovenous malformation, head trauma, cerebral aneurysm, brain tumor, hemorrhagic transformation of brain infarction were also excluded from the study. Patients with anticoagulant-associated ICH were also excluded from the study. Relevant demographic and clinical data including age, sex, past medical history and drug use were obtained. The admission blood pressure, time from symptom onset to baseline CT scan, time interval between baseline and follow-up CT scan, Glasgow Coma Scale (GCS) score and the National Institute of Health stroke scale (NIHSS) score were prospectively collected.
Imaging Analysis
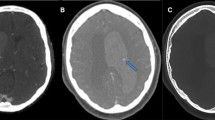
All CT scans were performed using multidetector CT scanner (Siemens SOMATOM Perspective; Siemens, Germany) with axial 5-mm section thickness. The admission and follow-up CT scans were acquired and saved as DICOM format for further review. In our study, we have defined hematoma growth as an increase in hematoma volume of > 33% or > 6 mL at follow-up CT scan according to previous definitions [15,16,17,18]. Two experienced neurologists independently reviewed all images in a blinded manner. The readers assessed the presence of IVH on both baseline and follow-up CT scans. The parenchymal hematoma volumes were measured using abc/2 method. Baseline and follow-up IVH volumes were measured using semiautomated computer-assisted volumetric analysis (AnalyzeDirect medical imaging software, version 11.0; AnalyzeDirect, Inc.). The ventricular hemorrhage was outlined manually on each slice and calculated with AnalyzeDirect software. IVH growth was defined as either any newly occurring intraventricular bleeding on follow-up CT scan in patients without baseline IVH or an increase in IVH volume ≥ 1 mL on follow-up CT scan in patients with initial IVH (Fig. 1).
Illustration of IVH growth on noncontrast CT. a Baseline CT scan reveals a putaminal hematoma without concurrent intraventricular hemorrhage. b Follow-up CT scan performed 11 h later shows enlarged hematoma and intraventricular extension of parenchymal hemorrhage. c Admission CT scan shows a basal ganglia hemorrhage with ventricular extension of hematoma. d Follow-up CT scan performed 24 h after baseline CT scan reveals the significant increase in ventricular hematoma volume. CT computed tomography, IVH intraventricular hemorrhage
Outcome Assessment
Our primary outcome measure was IVH growth and poor outcome at 90 days. Secondary outcome was IVH growth and ordinal shift analysis of mRS. In-hospital and 30-day mortality rates were recorded. The functional outcome was assessed using modified Rankin Scale (mRS) at 3 months after the onset of symptoms. Poor outcome was defined as 3 month mRS scores ≥ 3 as described previously [7, 19, 20].
Statistical Analysis
Statistical analyses were conducted using SPSS version 21 (Chicago, IL). Discrete variables were presented as count (%). Continuous data are expressed either as median and interquartile ranges (IQR) or mean ± standard (SD) deviations. The demographic, clinical and radiological characteristics were compared between patients with IVH growth and those without by using Fisher’s exact test, or Student’s t test, or Mann–Whitney U test as appropriate. The association of the IVH growth and poor outcome at 90 days was determined by using multivariable logistic regression. We considered ICH location as a variable in the multivariable regression model. We divided the location into three dummy variables: basal ganglia hemorrhage, thalamic hemorrhage and other locations. The basal ganglia hemorrhage was set as a reference variable. We performed multivariate logistic regression analyses including all variables with P ≤ 0.1 in the univariate analysis. A P value < 0.05 was considered statistically significant.
Results
During the study period, 382 patients presented within 6 h after ICH onset and had follow-up CT scans. A total of 79 patients were excluded from the study (supplementary figure 1), leaving 303 patients for the final analysis. Of those, 95 (31.4%) had IVH on admission CT. IVH was observed in 117 of 303 (38.6%) patients on follow-up CT scan. Interestingly, 22 patients with no IVH on initial CT scan developed subsequent IVH on follow-up CT scan. There were 15 patients with history of using antithrombotics use, and 6 patients experienced hematoma expansion and IVH growth. The median time interval between onset of symptom and baseline CT scan was 2.0 h (IQR 1.0–4.0 h), and the median interval between the first CT scan and follow-up CT examination was 18.0 h (IQR 11.5–23.0 h). Of 17 patients who died during their hospital stay, 10 patients had withdrawal of care. The hematomas were located in basal ganglia (n = 170), thalamus (n = 76), cerebral lobes (n = 36), brainstem (n = 9) and cerebellum (n = 12).
IVH growth was observed in 59 (19.5%) of 303 patients. Of those, 22 (37.3%) had delayed IVH and 37 (62.7%) had IVH volume increase. The clinical and radiological characteristics of patients with IVH growth and those without are illustrated in Table 1. The frequency of ICH expansion was significantly higher in patients with IVH growth than those without (74.6% vs 20.1%, P < 0.001). Patients with IVH growth had lower admission GCS score, higher NIHSS score, larger baseline hematoma volume and more frequently thalamic location at baseline. The median time to baseline CT scan was significantly shorter in patients with IVH growth than those without (P < 0.001). Patients with IVH growth had higher in-hospital (23.7% vs 1.2%, P < 0.001) and 30-day mortality (45.8% vs 5.3%, P < 0.001) than those without. IVH growth was also associated with increased risk of death (54.2% vs 9.8%, P < 0.001) and worse clinical outcome (91.5% vs 40.6%, P < 0.001) at 90 days. Interestingly, the median parenchymal ICH increase from admission CT to follow-up CT was significantly greater in patients with IVH growth (median growth of 18.0 mL; IQR 3.6–42.6 mL) than in those without (median growth of 0.7 mL; IQR, − 0.4 to 3.2 mL). Subgroup analysis shows that patients with delayed IVH are more likely to experience hematoma growth than patients with IVH volume increase (100% vs 59.5%; P < 0.001). The median ICH increase from admission CT to follow-up CT was significantly higher in patients with delayed IVH subgroup (median growth of 45.0 mL; IQR 16.8–87.6 mL) than in IVH volume increase subgroup (median growth of 13.0 mL; IQR 0.4–24.5 mL, Table 1). Multivariate analyses demonstrated thalamic hemorrhage (P < 0.001), hematoma expansion (P < 0.001) and time from onset to baseline CT scan (P = 0.007) were independent predictors for IVH growth (Table 2).
A total of 153 (50.5%) patients in our cohort had poor functional outcome (mRS 3–6) at 3-month follow-up. The sensitivity, specificity and overall accuracy of different cut-off values for IVH volume increase are illustrated in Supplementary Table 1. IVH growth of 1 mL is associated with the highest accuracy for predicting poor outcome. Univariate and multivariate logistic regression analysis was performed to predict the poor outcome (mRS 3–6) at 3 months (Table 3). Further analysis of the 90-day ordinal mRS scores demonstrates that IVH growth was associated with a significant shift toward poor outcomes and increased mortality (Fig. 2). After controlling for age, admission GCS score, admission NIHSS score, baseline hematoma volume, IVH at baseline CT, time from onset to CT, final IVH volume, ICH location and hematoma expansion, IVH growth (adjusted OR 4.15, 95% CI 1.31–13.20; P = 0.016) was an independent predictor of poor functional outcome (mRS 3–6) at 3 months (Table 3).
Distribution of modified Rankin scale in patients with or without IVH growth. The ordinal analysis showed a significant unfavorable shift in the distribution of scores on the modified Rankin scale IVH growth (pooled odds ratio for shift to higher modified Rankin score, 10.69; 95% CI 6.00 to 19.04; P < 0.001) (Fig. 2)
Discussion
In our study, we demonstrated that IVH growth is an independent predictor of death and poor functional outcome in patients with ICH. The association between IVH growth and poor outcome remained significant even with adjustment for potential confounding factors.
Prior authors have examined either the development of delayed IVH, or increase in IVH volume, individually. In a study of 216 ICH patients, Maas et al. [21] found that delayed IVH occurred in 21%, higher than our rate of 10% but similar to our overall frequency of 18% (including IVH growth). Their study did also find that delayed IVH was an independent predictor of poor outcome. In another study of 282 patients, Witsch et al. [22] reported 19 (14.5%) of 131 patients without initial IVH developed delayed IVH. However, no association with poor outcome was found. In a pooled analysis of the INTERACT trials, Moullaali et al. [23] demonstrated that delayed IVH was independently associated with poor outcome in small to moderate ICH. In a study of 374 patients, Steiner et al. [24] reported that 12% of patients with initial IVH may have a subsequent volume increase > 2 mL. In a recent study, Dowlatshahi et al. [25] reported that (6.1%) of patients without hematoma expansion had ≥ 2 mL IVH expansion and is associated with poor outcome. Overall, these results are generally consistent with ours, suggesting that new or expanding IVH after ICH is common and high risk. In a recent analysis of 5345 patients, Al-Shahi Salman et al. [26] found that time to baseline CT was a reliable predictor of hematoma growth. We are interested to observe that time from onset to baseline CT scan was also an independent predictors for IVH growth. Therefore, a repeat CT scan is warranted in patients presented early since they are at higher risk of both hematoma growth and IVH growth.
Our findings have important clinical implications. First, IVH growth is strongly associated with early hematoma growth. In previous reports, many researchers have focused on early hematoma growth and presence or absence of IVH on baseline CT scan [5,6,7,8,9,10,11, 27]. The ICH score is a well-established and widely used tool for outcome prediction [28, 29]. Our findings suggest that a significant proportion of patients without initial IVH will develop delayed IVH subsequently. We are interested to observe that patients with “delayed IVH” experience more severe hematoma growth compared to patients with IVH growth; a possible explanation is that delayed IVH might be a complication of severe hemorrhage expansion. In these patients, early use of the ICH score to predict outcome may be inaccurate. In addition, patients who had hematoma extension into the ventricles may not meet the criteria for early hematoma growth. Therefore, patients with IVH growth but no parenchymal hematoma growth are traditionally classified as nonexpanders. However, such patients may have just as poor (or worse) prognosis because they underwent active bleeding into the ventricular space. Since IVH growth is fairly common in patients with ICH and was an independent predictor of poor outcome, it is important to report the status of IVH growth together with parenchymal hematoma growth in studies of ICH.
Our study has several limitations. First, the sample size of our study is relatively small. Second, we only assessed the admission and 36-h follow-up CT scan. As an observational study, CT scans were not performed at study-defined time points, and the natural history of hematoma growth after 36 h is still unknown. Third, as a single-center study, it may be that the natural history of ICH in this population (or aspects of clinical care) does not translate into other populations. Future studies with serial imaging follow-up are needed to further clarify the natural history and time course of IVH growth. Fourth, since the number of patients receiving EVD treatment is relatively small, our conclusions may be limited to untreated IVH.
In summary, we have demonstrated that IVH growth independently predicts poor outcome in patients with ICH. IVH growth appears to be associated with early hematoma growth in most cases. It may be a promising potential therapeutic target for intervention. Future studies are needed to determine the effect of anti-expansion treatment in preventing hematoma growth and IVH growth.
References
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60.
Tsai HH, Kim JS, Jouvent E, Gurol ME. Updates on prevention of hemorrhagic and lacunar strokes. J Stroke. 2018;20:167–79.
Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40:1533–58.
Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–21.
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93.
Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–81.
Delcourt C, Huang Y, Arima H, et al. Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 study. Neurology. 2012;79:314–9.
Boulouis G, Morotti A, Brouwers HB, et al. Noncontrast computed tomography hypodensities predict poor outcome in intracerebral hemorrhage patients. Stroke. 2016;47:2511–6.
Li Q, Yang WS, Shen YQ, et al. Benign intracerebral hemorrhage: a population at low risk for hematoma growth and poor outcome. J Am Heart Assoc. 2019;16(8):e011892.
Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37.
Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology. 1990;40:616–9.
Yogendrakumar V, Ramsay T, Fergusson D, et al. New and expanding ventricular hemorrhage predicts poor outcome in acute intracerebral hemorrhage. Neurology. 2019;93:e879–88.
Roeder SS, Sprugel MI, Sembill JA, et al. Influence of the extent of intraventricular hemorrhage on functional outcome and mortality in intracerebral hemorrhage. Cerebrovasc Dis. 2019;47:245–52.
Trifan G, Arshi B, Testai FD. Intraventricular hemorrhage severity as a predictor of outcome in intracerebral hemorrhage. Front Neurol. 2019;12(10):217.
Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–14.
Dowlatshahi D, Demchuk AM, Flaherty ML, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–44.
Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71:158–64.
Morotti A, Boulouis G, Romero JM, et al. Blood pressure reduction and noncontrast CT markers of intracerebral hemorrhage expansion. Neurology. 2017;89:548–54.
Anderson CS, Huang Y, Wang JG, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–9.
Wang X, Arima H, Al-Shahi Salman R, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–65.
Maas MB, Nemeth AJ, Rosenberg NF, et al. Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology. 2013;80:1295–9.
Witsch J, Bruce E, Meyers E, et al. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. 2015;84:989–94.
Moullaali TJ, Sato S, Wang X, et al. Prognostic significance of delayed intraventricular haemorrhage in the INTERACT studies. J Neurol Neurosurg Psychiatry. 2017;88:19–24.
Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–73 (discussion 773–774).
Dowlatshahi D, Deshpande A, Aviv RI, Rodriguez-Luna D, Molina CA, Blas YS, et al. Do Intracerebral hemorrhage nonexpanders actually expand into the ventricular space? Stroke. 2018;49:201–3.
Al-Shahi Salman R, Frantzias J, Lee RJ, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17(10):885–94.
Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–70.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7.
Kim JY, Bae HJ. Spontaneous intracerebral hemorrhage: management. J Stroke. 2017;19:28–39.
Acknowledgements
Dr. Goldstein has received consulting and research contracts from CSL Behring and Boehringer Ingelheim. No other disclosures were reported.
Author information
Authors and Affiliations
Contributions
Dr. QL took part in study concept and design. Drs. QL, RL, L-BZ, X-MY, W-SY, LD, X-NL, MW, Y-NZ, F-JL involved in acquisition of data. W-SY participated in statistical analysis. All authors participated in analysis and interpretation of data. Dr. QL. involved in drafting of the manuscript. QL, RL, L-BZ, JG and PX took part in critical revision of the manuscript for important intellectual content. Dr. QL. obtained funding. Drs. QL, X-CS and PX participated in study supervision.
Corresponding authors
Ethics declarations
Sources of Funding
This study was supported by grants from the National Key R&D Program of China (Nos. 2018YFC1312200, 2018YFC1312203) and Chongqing High-end Young Investigator Project (No. 2019GDRC005).
Conflicts of interest
The authors declare no competing interests.
Informed consent
Written informed consent was obtained from all participants or their legal representatives.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Li and Rui Li are co-first authors.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Q., Li, R., Zhao, LB. et al. Intraventricular Hemorrhage Growth: Definition, Prevalence and Association with Hematoma Expansion and Prognosis. Neurocrit Care 33, 732–739 (2020). https://doi.org/10.1007/s12028-020-00958-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-00958-8