Abstract
Background
External ventricular drain (EVD) usage in patients with intraventricular hemorrhage (IVH) is variable in current practice and in clinical trials, and its impact on outcome remains controversial. The objective of this study was to identify the clinical predictors of EVD utilization, and associated outcome in adults with spontaneous IVH with or without intracerebral hemorrhage (ICH).
Methods
Retrospective review of 183 consecutive IVH patients admitted to a University Hospital between 2003 and 2010. Clinical and radiographic data were analyzed for associations between EVD placement and mortality, poor outcome, and improvement in Glasgow Coma Scale score (GCS) using multivariate logistic regression models.
Results
Average age was 62 ± 15.6 years, and average ICH and IVH volumes were 35.8 ± 40.9 cc and 19.7 ± 25.3 cc, respectively. Independent predictors of EVD placement within first 5 days of admission were GCS ≤ 8 (OR 11.5; P < 0.001), Graeb score >5 (OR 4.6; P = 0.001), and non-lobar ICH ≤ 30 cc (OR 9.7; P < 0.001). Median GCS increased from 5 (IQR 3–7) 48 h post-EVD (P < 0.001). EVD placement was an independent predictor of reduced mortality (OR 0.31; P = 0.04) and modified Rankin score 0–3 (OR 15.7; P = 0.01) at hospital discharge. In patients with hydrocephalus on presentation, EVD was associated with reduced mortality for patients with GCS > 3 after controlling for ICH and IVH severity (OR 0.02; P = 0.01).
Conclusions
Patients with lower GCS, higher IVH severity, and lower ICH volume are more likely to have an EVD placed. EVD placement is associated with reduced mortality and improved short-term outcomes in patients with IVH after adjusting for known severity factors. EVD use should be protocolized in clinical trials of ICH management where IVH is included.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraventricular hemorrhage (IVH) occurs in approximately 40 % of primary intracerebral hemorrhage (ICH) patients, and is a consistent predictor of poor outcome [1–3]. External ventricular drains (EVD) are often placed in patients with IVH as a life-saving measure to manage intracranial pressure (ICP) and may reduce short-term mortality [4–6]. However, EVD use remains variable because there is no evidence that EVD improves functional outcome or prevents development of communicating hydrocephalus [5–10]. The lack of criteria defining which patients benefit from an EVD leaves this decision unprotocolized in many recent randomized trials of therapies for ICH and may both influence and bias outcomes of these studies. The purpose of this study was threefold: (1) To identify demographic and clinical factors that contribute to the decision for EVD placement, (2) to determine the impact of EVD placement in the acute phase on mortality and short-term neurologic outcomes in a single university center, and (3) to identify clinical factors and thresholds associated with benefit and the absence of benefit from EVD placement. To our knowledge, our data represent the largest retrospective study of EVD placement in patients with IVH from a single University Hospital.
Methods
Patient Population
Records of consecutive adult patients with IVH admitted to a University Hospital between 2003 and 2010 were reviewed. All patients with a primary diagnosis of ICH (ICD-9 code 431) were identified, and those with radiographic evidence of any IVH were included. Patients were excluded for the following reasons: craniotomy or craniectomy, aneurismal subarachnoid hemorrhage, or ICH related to trauma or underlying lesions, including aneurysms, brain tumors, and arterio-venous malformations. The Johns Hopkins Institutional Review Board approved the study.
Patients were admitted to a Neuroscience Critical Care Unit (NCCU) with staff experienced in the acute care of patients with IVH and EVDs. Although no protocol exists for EVD placement, in general, EVDs are considered in patients with acute obstructive hydrocephalus and Glasgow Coma Scale (GCS) ≤8. EVDs were placed into the frontal horn of the lateral ventricle and tunneled under the scalp by neurosurgical staff. In the absence of accepted practice guidelines regarding catheter location, the choice of catheter placement ipsilateral or contralateral to dominant lateral ventricular IVH was left up to the neurosurgical team. Intraventricular location of the catheter tip was confirmed by ICP waveform morphology and by computed tomography (CT) scan. Prior to March 2006, prophylactic oxacillin was administered for the duration of EVD. Afterward, a periprocedural single dose of cefazolin was given prior to placement and antibiotic impregnated EVDs became available (large bore antimicrobial EVDs were not available).
Clinical Data Collection
Patients were stratified into two groups: no EVD and EVD. Patients were placed in the EVD group if they had an EVD placed within 5 days of hospital admission. Five days were chosen to balance clinical variability in EVD placement with the aim of assessing early benefit of this intervention. The median GCS score on Day 1 was calculated from the first six GCS score evaluations (every 4 h). We used the median Day 1 GCS score for baseline GCS score, as a significant number of patients’ admission GCS scores fluctuated by 3 or more points in the first 12 h, and the mean time from CT scan to EVD placement was 7 h. Daily cerebrospinal fluid (CSF) drainage and the first ICP reading after EVD placement were collected in the EVD group. All hospital admission and pre-EVD CT scans were reviewed. Side of catheter entry and catheter tip location relative to ICH and IVH and the presence of catheter tract hemorrhage were recorded when post-EVD placement imaging was available. Both Graeb score and bicaudate index were calculated [11, 12]. Bicaudate index was considered abnormal if it was above the age-adjusted 95th percentile [12]. All admission CT scans were reviewed for the presence of obstructive hydrocephalus, sulcal effacement, and loss of hemicisterns. Hydrocephalus was determined by visual analysis of CT scans as described by Phan et al. [7] Computerized volumetrics were used to calculate total IVH and ICH volume, and third ventricle maximum diameter [13].
Bacterial infection was defined as a positive culture or, in the absence of a positive culture, greater than 50 % polymorphonuclear leukocytes on CSF count with a minimum of 50 cells counted or CSF glucose less than 15 mg/100 ml [14]. Clinical herniation was defined as the acute onset of unilateral or bilateral pupillary dilation with loss of reactivity to light, and with associated decline in GCS [15]. Admission GCS is the first GCS score recorded upon admission to the NCCU, as for most patients this represented the initial patient status used for EVD decision making and all patients had GCS score recorded at this time. Intubated patients were assigned one point for the verbal component of the GCS. Improvement in GCS was defined as ≥2-point increase in GCS (from NCCU admission to discharge). Poor outcome was defined as modified Rankin score (mRS) of 4–6 upon hospital discharge [16]. Early withdrawal of care was defined as having care withdrawn within 48 h of admission. ICH volume was dichotomized to <30 and ≥30 cc, because this cut-point has been found to segregate probability of mortality in previous models [17, 18].
Statistical Analysis
All statistical analyses were performed using STATA version 11 (Stata Corp, College Station, TX). Patients who had an EVD placed (EVD group) were compared to those who did not have an EVD placed (non-EVD group) for demographic, clinical, and radiographic characteristics using χ 2, Fisher’s exact test, t test analyses, Wilcoxon signed-rank test, and Kruskal–Wallis one-way analysis of variance as appropriate. Univariate and backwards-stepwise multivariate logistic regression models were used to identify independent predictors of EVD placement (vs. conservative treatment) within the first 5 days of admission and of in-hospital mortality. The Tuhrim Score (predictor of 30 days mortality) was calculated as previously reported [18]. We calculated the ICH Score as previously reported [19]. The Tuhrim Score and ICH Score were calculated to predict the expected mortality. We considered a Tuhrim Score >50 % as predictive of death. We compared discordance between the expected mortality and the observed mortality at hospital discharge, both among all patients and within various subgroups, using the χ 2 test. We defined expected death as a Tuhrim score greater than 0.5 or ICH score of 3 or greater. All results are presented as mean ± standard deviation or median [interquartile range (IQR)], unless otherwise noted. Variables with P ≤ 0.1 in the univariate analysis were included in multivariate models, and variables with P < 0.2 were retained in the stepwise models. P ≤ 0.05 was considered statistically significant.
Results
A total of 546 patients were identified with ICH from 2003 to 2010. Of these, 222 (40.4 %) had IVH, of which 183 had medical record and CT data available for analysis and fit the inclusion and exclusion criteria for the study. Demographic characteristics for the 39 patients with IVH not included in the study were not statistically different from the 183-patient cohort [data not shown]. Of 183 patients with IVH, 67 (36.6 %) patients had an EVD placed within 2.8 days of the admission CT scan (meeting the 5 day criterion) and four patients had an EVD placed beyond 5 days after admission (at 7, 10, 15, and 22 days) and were included in the non-EVD group. In the EVD group, average daily CSF drainage was 137.5 ± 76.4 cc (range: 4.3 to 476 cc) and initial ICP was 13.9 ± 10.8 mmHg (range: −7–50 mmHg). Eleven (16.4 %) of 67 patients who had an EVD placed were administered intraventricular (IVR) tissue plasminogen activator (tPA). Admission demographic and clinical characteristics as well as outcome measures at discharge (mRS, GCS, and mortality) were not different between EVD-only patients and patients who were administered IVR tPA. Median discharge GCS scores were 3 and 10 in the EVD-only and EVD plus IVR tPA groups, respectively (P = 0.21). Median discharge mRS was 6 and 5 in the EVD-only and EVD plus IVR tPA groups, respectively (P = 0.13). Thirty-seven of 56 (66.1 %) EVD-only patients died in hospital compared to 4 of 11 (36.4 %) EVD plus IVR tPA patients (P = 0.07). IVR fibrinolysis was not used for maintenance of EVD function. Dual catheters were placed in 5 (7.4 %) of 67 EVD patients. Patients having dual catheters had larger IVH volumes at admission (56.9 cc vs. 29.7 cc, P = 0.03). Patient demographics and admission characteristics are shown in Table 1.
Determinants of EVD Placement
Thirty-eight (56.7 %) of the 67 patients who had an EVD placed presented with obstructive hydrocephalus compared to 42 (36.2 %) of 116 patients who did not have an EVD placed (P = 0.007). Patients who presented with hydrocephalus and did not have an EVD placed were older (72.5 ± 14.0 vs. 57.9 ± 14.5 years, P < 0.001) and had a higher admission GCS (9 (4–14) vs. 4 (3–6), P < 0.001) compared to those with hydrocephalus and an EVD, respectively. Mortality rates were nonsignificantly lower in patients with hydrocephalus but no EVD compared to those with EVD (59.5 % vs. 71.1 %, P = 0.28). Of patients who had an EVD placed, 31 (47.0 %) had an IVH volume ≤20 cc compared to 94 (81.0 %) of patients who did not have an EVD placed (P < 0.001). Twenty (29.9 %) EVD patients had an IVH volume >40 cc compared to 9 (7.8 %) patients in the non-EVD group (P < 0.001). EVDs were placed in patients with GCS scores 3–5, 6–8, 9–11, and 12–14 at the following rates: 64.2, 43.2, 33.3, and 7.5 %, respectively (P < 0.001). Forty-two (23.0 %) patients presented with clinical herniation, of whom 26 (61.9 %) had an EVD placed compared to 41 (29.1 %) of 141 patients without herniation at admission who had an EVD placed (P < 0.001). Of those 26 patients, six (23.1 %) had care withdrawn within 48 h. Eight (50.0 %) of 16 patients with clinical herniation on admission who did not have an EVD placed had care withdrawn early compared to 6 (23.1 %) of 26 patients who had an EVD placed and had care with drawn early (P = 0.07). Ten of 27 (37.0 %) patients who had care withdrawn early had an EVD placed.
On univariate analysis, EVD placement was associated with the following admission characteristics: younger age, African-American ethnicity, lower GCS, lower ICH volume, higher IVH volume, sulcal effacement, thalamic ICH location, non-lobar (caudate/putamen/thalamus) ICH location (vs. lobar), non-lobar ICH ≤ 30 cc, higher Graeb score, presence of clinical herniation, obstructive hydrocephalus, and higher age-adjusted bicaudate index (Tables 1, 2). Independent predictors of EVD placement (Table 2) were admission GCS ≤ 8 (OR 11.50, 95 % CI 3.75–35.5; P < 0.001), Graeb score >5 (OR 4.60, 95 % CI 1.93–10.94; P = 0.001), and non-lobar ICH ≤ 30 cc (OR 9.71, 95 % CI 3.58–26.38; P < 0.001). The AUC from this analysis was 0.88, with 81.0 % correctly classified. Clinical herniation on admission exhibited a marginal positive association with EVD placement (OR 2.63, 95 % CI 098–7.08; P = 0.055).
Outcomes Associated with EVD Placement
In-hospital mortality occurred in 91/183 (49.7 %) patients. Crude discharge mortality rates (and expected 30 days mortality rates based on the median ICH score and Tuhrim score) in the no EVD and EVD groups were 43.1 % (26.0, 16.4 %), and 61.2 % (72.0, 87.2 %), respectively. All patients who had early withdrawal of care (n = 27) died in hospital and did not have statistically different demographic and admission characteristics compared to 64 patients who died, but did not have early withdrawal of care.
Patients in the EVD group did not experience a significant improvement between median pre-EVD GCS (5 (3–7)) and median discharge GCS (3 (3–11)) (P = 0.27). However, in EVD-treated patients, median GCS increased from 5 (3–7) pre-EVD to 6 (4–9) 48 h post-EVD (P < 0.001). Of the 57 patients who had an EVD placed and did not have care withdrawn early, median GCS increased from 5 (4–7) pre-EVD to 7 (4–9) at 48 h post-EVD (P < 0.001). Of the 26 EVD-treated patients alive at hospital discharge, the median pre-EVD GCS improved from 7 (5–7) to 11 (10–14) at hospital discharge (P < 0.001). The 66 patients alive at discharge, who did not have an EVD placed, experienced an increase in median GCS from admission (14 (12–15)) to discharge (15 (14–15)) (P < 0.001), respectively. Median length of ICU and hospital length of stay (LOS) in the EVD group was 10 (3–18) and 12 days (3–25), compared to 3 (1–5) (P < 0.001) and 6 (2–13) (P = 0.003) days in the non-EVD group, respectively.
Using multivariate logistic stepwise regression (Table 3), clinical and radiographic factors associated with in-hospital mortality were age (OR 1.05; P = 0.01), lower median day 1 GCS score (OR 0.58; P < 0.001), higher ICH volume (per 10 cc) (OR 1.18; P = 0.03), higher IVH volume (per 10 cc) (OR 1.25; P = 0.03), and EVD (OR 0.31; P = 0.04). The AUC from this analysis was 0.92, with 83 % correctly classified. IVH volume was a superior predictor to Graeb score or other radiological indicators of increased ICP. The multivariate regression evaluating factors associated with Rankin score 0-3 at hospital discharge showed all factors remaining significant including EVD use (OR 15.7; P = 0.01). The AUC from this analysis was 0.98 with 93.8 % correctly classified. After excluding patients from the analyses who were administered IVR rtPA, all factors remained significant; EVD use was an independent predictor of discharge Rankin 0–3 (OR 16.3, P = 0.01) and near significantly associated with reduced mortality (OR 0.32, P = 0.075).
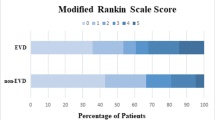
We performed a similar analysis for in-hospital mortality on the subgroup of patients presenting with obstructive hydrocephalus (n = 80). In this analysis, EVD placement in patients with GCS score >3 was significantly associated with lower mortality (OR 0.02; P = 0.01), while again lower median day 1 GCS score (OR 0.55; P < 0.001), higher ICH volume (per 10 cc) (OR 1.4; P = 0.034), and higher IVH volume (OR 1.44; P = 0.032) predicted higher odds of mortality (AUC = 0.95, 90.0 % correctly classified). Age was not associated with mortality in patients with obstructive hydrocephalus. No patients with obstructive hydrocephalus who had an EVD placed (37 patients) were discharged with a modified Rankin score of 0–3, compared to 6 (15.0 %) of 40 patients without an EVD placed (P = 0.01). We were unable to perform a multivariate analysis on this subpopulation due to EVD placement being a perfect predictor of outcome.
We next compared ICH Score and Tuhrim Score predicted 30 days mortality rates to observed mortality at hospital discharge by EVD group (Table 4). Using the ICH Score for prediction of mortality, the proportion of discordant outcomes (expected does not agree with observed) was not statistically different regardless of treatment group or subpopulation. All patients with ICH scores greater than three died, regardless of treatment group. However, the Tuhrim score revealed differences in expected and observed mortality among all patients and within the subpopulations of patients without herniation at admission or without hydrocephalus at admission. The difference in expected and observed mortality in patients with hydrocephalus approached statistical significance. In patients with hydrocephalus, 6 (15.8 %) patients in the EVD group who were predicted to die survived at discharge compared to one patient (2.4 %) in the non-EVD group; conversely, 2 (5.3 %) and 6 (14.3 %) patients in the EVD and non-EVD groups, respectively, who were predicted to survive (P = 0.056) died in hospital. In patients without hydrocephalus, 11/29 (37.9 %) and 5/74 (6.8 %) patients in the EVD and non-EVD groups, respectively, with expected mortality survived (P < 0.001). In patients without herniation at admission, 12/41 (29.3 %) and 6/100 (6.0 %) patients in the EVD and non-EVD group, respectively, with expected mortality survived (P = 0.001).
Twenty-three (34.3 %) of 67 patients who had an EVD placed presented with clinical signs of herniation, of which four (17.4 %) patients had a clinical reversal of herniation after EVD placement. Of the 41 EVD patients who did not present with herniation, 6 (14.6 %) patients had new-onset herniation after EVD placement.
Four (80 %) of 5 patients who had dual catheters placed died. One patient improved their admission GCS from 8 at admission to 11 at discharge.
Complications Associated with EVD Placement
Eighty-two EVDs (67 patients with one EVD, 5 patients with dual EVDs, and 10 replacement EVDs) were placed in 72 patients during the entire hospital stay, of which three (3.7 %) resulted in a bacterial CSF infection. No patients with a bacterial CSF infection died or had poor outcomes, but their LOS was non-significantly longer than patients without CSF infection (33.3 ± 6.7 vs. 16.5 ± 17.1 days; P = 0.10). Six (9.0 %) of 67 patients in the EVD group had a permanent ventriculoperitoneal (VP) shunt placed. None of the 11 patients administered IVR rtPA required a VP shunt (P = 0.58).
Fourteen of 72 (19.4 %) initial EVDs became obstructed and 10 (13.9 %) were replaced. Patients who had EVD obstruction had larger IVH volumes (49.2 ± 37.8 vs. 27.2 ± 21.6 cc; P = 0.01) compared to those with no obstruction. In 62 EVD patients with adequate radiographic data catheters were placed in the following positions relative to the largest IVH clot: 33 (53.2 %) contralateral, 20 (32.3 %) ipsilateral, 6 (9.7 %) in the 3rd ventricle, and 3 (4.8 %) had symmetric IVH clots. Nine (27.3 %) of 33 contralaterally placed catheters clotted compared to 2 (10.0 %) of 20 ipsilaterally placed catheters (P = 0.13). Three of 62 (4.8 %) catheters with available CT data after EVD placement demonstrated catheter tract hemorrhage, two of whom died (unrelated to EVD associated hemorrhage). Median length of EVD duration was 7 (3–12) days.
Discussion
The main findings of this study are that EVD placement in patients with spontaneous IVH is an independent predictor of reduced mortality and improved discharge mRS after adjusting for known predictors of ICH/IVH outcomes. The rate of EVD placement in patients with spontaneous IVH in this single center study was 37 %. While this rate is lower than the proportion of patients presenting with obstructive hydrocephalus (44.1 %), it is higher than reported rates of EVD use in recent clinical trials. In both the International Surgical Trial in Intracerebral Hemorrhage (STICH) study [20] and the recombinant activated factor VII for acute intracerebral hemorrhage (NovoSeven) trial, [3] EVDs were placed in ≤10 % of patients with IVH. This may be due to the lack of definitive guidelines for EVD use in spontaneous ICH which currently state unknown utility of ICP management in patients with ICH [21].
Independent predictors of EVD placement in this study were admission GCS ≤ 8, Graeb score >5, and non-lobar ICH ≤ 30 cc. These findings are consistent with the fact that non-lobar deep ICH usually has larger associated IVH volume than lobar ICH. The decision to place EVDs in spontaneous IVH is multifactorial. We found that EVD placement is most likely to occur in patients with lower GCS, high IVH volume, and small non-lobar ICH. After adjustment for these factors, the presence of hydrocephalus was not an independent predictor. EVD placement was associated with reduced odds of mortality. Patients who presented with obstructive hydrocephalus with GCS > 3 also benefited from EVD placement. Although 15 % of patients had care withdrawn within the first 48 h of admission, patients in whom an EVD was placed and care was not withdrawn early had a significant increase in GCS 48 h post procedure.
Clinical evidence suggests that early aggressive neurointensivist-directed care in an ICU can decrease mortality and improve functional outcomes in patients with ICH [22, 23]. However, patient selection for EVD placement is variable in patients with IVH, and the reported impact of EVD placement on outcome has been less than favorable. Adams study of 22 patients with spontaneous ICH and hydrocephalus suggested that EVD drainage reduces ventricular volume but does not change the level of consciousness [4]. Shapiro et al. [24] concluded that ventriculostomy to treat hemorrhagic dilation of the 4th ventricle does not improve outcome. Nieuwkamp et al. [5] found in their meta-analysis of seven studies that the case fatality rate for IVH caused by either ICH or subarachnoid hemorrhage was only minimally improved with EVD drainage (relative risk vs. conservative treatment, 0.74; 95 %CI 0.55–0.99). However, the poor outcome rate with EVD alone vs. conservative treatment was no different (89 vs. 90 %, respectively; RR 0.98). They concluded that EVD alone does not reduce poor outcomes or significantly reduce mortality. Staykov et al. [25] reported a mortality rate of 53 % (n = 133) for EVD alone, compared to 71 % (n = 91) in conservatively treated patients. The latter study is most consistent with our data.
Although such data are provocative, there is no prospective and randomized controlled trial addressing the effect of EVD in spontaneous ICH on clinical outcome. Adjusting for known severity factors in ICH/IVH, our analysis shows that EVD use is associated with a 69 % reduction in the odds of in-hospital patient death. Among patients presenting with obstructive hydrocephalus with a GCS score above 3 on admission, after adjustment, EVD was associated with a 98 % reduction in the odds of in-hospital patient death. This result is consistent with the known impact of hydrocephalus on ICH-related morbidity and mortality [1, 26]. In the international surgical trial of intracerebral hemorrhage (STICH) trial of early hematoma evacuation, 377/902 (42 %) patients with follow up data had IVH, 208 of these had hydrocephalus (23 % of all patients, 55 % of those with IVH) and hydrocephalus predicted poor outcome [27]. These analyses suggest that patient selection may be important, and that patients presenting with obstructive hydrocephalus and GCS > 3 should have an EVD placed regardless of other severity factors. Alternatively, patients presenting with poor GCS of 3 or clinical signs of herniation may have little to gain from EVD placement. Because EVD placement is often considered a component of brain code protocols for acute management of clinical herniation, aborting this procedure may not be acceptable.
Differences between observed and predicted mortality by the Tuhrim scale suggest that EVD may be effective both with hydrocephalus and clinical herniation. Patients with hydrocephalus had significantly larger IVH volume than those without (32.4 ± 31.7 cc vs. 9.5 ± 11.9 cc; P < 0.001). IVH volume and hydrocephalus are components of the Tuhrim score calculation, whereas ICH location and EVD use are not and in fact, relatively few patients were treated with EVDs in the derivation of the Tuhrim score. Therefore, it is possible that EVD use explains some of the lower observed versus predicted mortality. Other explanations are that ICU care has improved since the Tuhrim score was developed and that our analysis overestimated the prediction of mortality because we assigned a predicted mortality to any patient with a Tuhrim score >50 %. A major question is whether outcomes with EVD use are better because of CSF drainage, relief of hydrocephalus, or notification of elevated ICP requiring treatment by other methods. A major factor supporting the view that control of hydrocephalus on its own is insufficient to improve overall outcome in IVH is that EVD alone does not accelerate the resolution of the IVH clot volume [28]. There is evidence that elevated ICP in severe IVH is associated with worse mortality [29].
While we controlled for severity of illness in the multivariate analyses, it is possible that the results are biased, as sicker patients might not have been as aggressively treated. However, our results indicate that patients with the lowest GCS scores (3–5) and highest IVH volumes (>40 cc) had the highest percentages of EVDs placed, showing that, in fact, physicians did not give up on patients with low GCS, high IVH volume, or clinical herniation. Our data do not suggest that patients without EVD were sicker. In fact, it appears that with the exception of older age and higher ICH volume in the non-EVD group, patients with EVD had clinically worse neurologic presentations.
The incidence of CSF infection associated with EVD placement was low (3.4 %) compared to a 6.9 % (467/6,787) incidence reported in a meta-analyses by Lo et al. [8]. In the present study, CSF infection did not result in a longer LOS or higher mortality. A significant proportion of EVDs clotted off, obstructing CSF drainage, and patients with larger IVH volumes or caudate hemorrhages were more likely to experience EVD obstruction. A small portion of EVD patients experienced a catheter tract hemorrhage.
All EVDs in this study were placed blindly by neurosurgeons in the ICU setting. Although there is no consensus regarding optimal position of external ventricular drain (EVD) with regard to clearance of IVH, EVD position is likely important and may influence hematoma removal rates, especially when intraventricular thrombolysis is used [30]. Neuroendoscopic techniques may have a role to play in both optimizing EVD position and potentially for therapeutic benefit such as aspiration of IVH. Limited data suggest that, while neuroendoscopic evacuation of severe IVH using a flexible endoscope with a “freehand” technique does not improve mRS outcomes or mortality compared to EVD alone, this technique is associated with lower rates of VP shunt placement [31, 32]. A meta-analysis by Li et al. [32] involving 680 patients also suggests that neuroendoscopy in combination with EVD may outperform EVD plus intraventricular thrombolysis in both hematoma evacuation and clinical outcomes.
Our study is limited in the following ways: (1) Early withdrawal of care perfectly predicted mortality and was not included in multivariate models. The possibility of early withdrawal of care did not, however, seem to impact the decision about whether to place an early EVD (which occurred in 10/27 early withdrawal patients), and inclusion of early withdrawal of care in the multivariate model for mortality model did not significantly change the results of the study. (2) Mortality was assessed at 11 ± 13 days [range: 1–80] rather than at 30 days as was done in the ICH score and Tuhrim 30 days mortality models. It is unlikely this significantly affected the differences between observed and predicted mortality. (3) This is a retrospective study that cannot evaluate unmeasurable factors that contribute to the decision to place EVDs in patients with IVH. In addition, long-term outcome data were not available and short-term outcomes were performed retrospectively.
Conclusions
EVD placement in patients with IVH in the ICU and in clinical trials is variable. There may be advantages to protocolizing EVD use in clinical trials to avoid inconsistencies across centers, which may bias results and adversely impact optimization of outcomes from specific therapies. The present study suggests that EVD placement reduces in-hospital mortality and improves short-term outcomes when applied to a population using ASA/AHA guidelines, [21] such as the population in this study. IVH patients most likely to benefit from EVD placement are those presenting with hydrocephalus and a GCS greater than 3. Although a randomized clinical trial evaluating the use of EVD may be a necessary driving force for some practitioners, this is unlikely to be performed in academic centers providing state of the art neurocritical care. It remains to be determined whether the benefit of EVDs is due to ICP control, alleviation of ventricular dilatation, or to removal of blood products from the ventricular system.
References
Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29:1352–7.
Tuhrim S, Dambrosia JM, Price TR, et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–63.
Steiner T, Schneider D, Mayer S, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor vii. Neurosurgery. 2006;59:767–73.
Adams RE, Diringer MN. Response to external ventricular drainage in spontaneous intracerebral hemorrhage with hydrocephalus. Neurology. 1998;50:519–23.
Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247:117–21.
Engelhard HH, Andrews CO, Slavin KV, Charbel FT. Current management of intraventricular hemorrhage. Surg Neurol. 2003;60:15–21.
Phan TG, Koh M, Vierkant RA, Wijdicks EF. Hydrocephalus is a determinant of early mortality in putaminal hemorrhage. Stroke. 2000;31(9):2157–62.
Lo CH, Spelman D, Bailey M, Cooper DJ, Rosenfeld JV, Brecknell JE. External ventricular drain infections are independent of drain duration: an argument against elective revision. J Neurosurg. 2007;106:378–83.
Chan K, Mann KS. Prolonged therapeutic external ventricular drainage: a prospective study. Neurosurgery. 1988;23:436–8.
Ruscalleda J, Peiro A. Prognostic factors in intraparenchymatous hematoma with ventricular hemorrhage. Neuroradiology. 1986;28:34–7.
Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143:91–6.
van Gijn J, Hijdra A, Wijdicks EF, Vermeulen M, van Crevel H. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1985;63:355–62.
Steiner L, Bergvall U, Zwetnow N. Quantitative estimation of intracerebral and intraventricular hematoma by computer tomography. Acta Radiol Suppl. 1975;346:143–54.
Holloway KL, Barnes T, Choi S, et al. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85:419–24.
Koenig MA, Bryan M, Lewin JL III, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023–9.
Rankin J. Cerebral vascular accidents in patients over the age of 60: prognosis. Scott Med J. 1957;2:200–15.
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93.
Tuhrim S, Dambrosia JM, Price TR, et al. Intracerebral hemorrhage: external validation and extension of a model for prediction of 30 days survival. Ann Neurol. 1991;29:658–63.
Hemphill JC III, Bonovich DC, Besmertis L, Manley GT, Johnston SC, Tuhrim S. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7.
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97.
Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professional from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–29.
Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56:766–72.
Mirski MA, Chang CW, Cowan R. Impact of a neuroscience intensive care unit on neurosurgical patient outcomes and cost of care: evidence-based support for an intensivist-directed specialty ICU model of care. J Neurosurg Anesthesiol. 2001;13:83–92.
Shapiro SA, Campbell RL, Scully T. Hemorrhagic dilation of the fourth ventricle: an ominous predictor. J Neurosurg. 1994;80:805–9.
Staykov D, Bardutzky J, Buttner HB, Schwab S. Intraventricular Fibrinolysis for Intracerebral Hemorrhage with Severe Ventricular Involvement. Neurocrit Care. 2011;15:194–209.
Zahuranec DB, Gonzales NR, Brown DL, et al. Presentation of intracerebral haemorrhage in a community. J Neurol Neurosurg Psychiatry. 2006;77:340–4.
Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. STICH Investigators. hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–8.
Naff NJ, Williams MA, Rigamonti D, Keyl PM, Hanley DF. Blood clot resolution in human cerebrospinal fluid: evidence of first-order kinetics. Neurosurgery. 2001;49:614–9.
Ziai WC, Melnychuk E, Thompson CB, Awad I, Lane K, Hanley DF. Occurrence and impact of intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Critical Care Med. 2012;40(5):1601–8.
Jaffe J, Melnychuk E, Muschelli J, Ziai W, Morgan T, Hanley DF, Awad IA. Ventricular catheter location and the clearance of intraventricular hemorrhage. Neurosurgery. 2012;70(5):1258–63 (Discussion 1263–1264).
Basaldella L, Marton E, Fiorindi A, Scarpa B, Badreddine H, Longatti P. External ventricular drainage alone versus endoscopic surgery for severe intraventricular hemorrhage: a comparative retrospective analysis on outcome and shunt dependency. Neurosurg Focus. 2012;32(4):E4.
Li Y, Zhang H, Wang X, et al. Neuroendoscopic surgery versus external ventricular drainage alone or with intraventricular fibrinolysis for intraventricular hemorrhage secondary to spontaneous supratentorial hemorrhage: a systematic review and meta-analysis. PLoS One. 2013;8(11):e80599.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrick, D.B., Ullman, N., Nekoovaght-Tak, S. et al. Determinants of External Ventricular Drain Placement and Associated Outcomes in Patients with Spontaneous Intraventricular Hemorrhage. Neurocrit Care 21, 426–434 (2014). https://doi.org/10.1007/s12028-014-9959-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-9959-x