Abstract
Our goal was to perform a systematic review of the literature on the use of ketamine in traumatic brain injury (TBI) and its effects on intracranial pressure (ICP). All articles from MEDLINE, BIOSIS, EMBASE, Global Health, HealthStar, Scopus, Cochrane Library, the International Clinical Trials Registry Platform (inception to November 2013), reference lists of relevant articles, and gray literature were searched. Two reviewers independently identified all manuscripts pertaining to the administration of ketamine in human TBI patients that recorded effects on ICP. Secondary outcomes of effect on cerebral perfusion pressure, mean arterial pressure, patient outcome, and adverse effects were recorded. Two reviewers independently extracted data including population characteristics and treatment characteristics. The strength of evidence was adjudicated using both the Oxford and GRADE methodology. Our search strategy produced a total 371 citations. Seven articles, six manuscripts and one meeting proceeding, were considered for the review with all utilizing ketamine, while documenting ICP in severe TBI patients. All studies were prospective studies. Five and two studies pertained to adults and pediatrics, respectively. Across all studies, of the 101 adult and 55 pediatric patients described, ICP did not increase in any of the studies during ketamine administration. Three studies reported a significant decrease in ICP with ketamine bolus. Cerebral perfusion pressure and mean blood pressure increased in two studies, leading to a decrease in vasopressors in one. No significant adverse events related to ketamine were recorded in any of the studies. Outcome data were poorly documented. There currently exists Oxford level 2b, GRADE C evidence to support that ketamine does not increase ICP in severe TBI patients that are sedated and ventilated, and in fact may lower it in selected cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine’s use as a dissociative anesthetic agent has afforded its application in a variety of instances where the side effect profile of standard anesthetics has negated their use [1, 2]. The quick action and lack of significant hemodynamic derangements with ketamine make it attractive as an agent for procedural sedation and induction [3] in those patients suffering from shock. However, despite the advantages of ketamine, one area of medicine that has yet to widely implement this medication is neurosurgery [4].
Anesthetic texts and literature [5–8] have perpetuated the concern that the use of ketamine can lead to uncontrollable intracranial pressure (ICP). Thus, especially in those patients where there is concern for elevated ICP, the medication has been avoided. The postulated mechanism surrounds large vessel vasodilation from an elevation in \( {\text{p}}_{{{\text{CO}}_{ 2} }} \) in nonventilated patients [4], and the small vessel vasoconstriction effects related to ketamine’s nitric oxide (NO) synthase inhibition [9] leading to a potential increase in cerebral oxygen extraction.
Despite this concern first documented in the 1970s, there has yet to be substantial evidence within the literature to support ICP elevations with ketamine use in traumatic brain injury (TBI) and the most other neurosurgical pathology. Furthermore, there are studies to date that refute claims of ICP issues, when ketamine is used in TBI [10–16]. In addition, recent randomized control trials (RCT) utilizing N-methyl-d-aspartate (NMDA) antagonists as neuroprotective agents have failed to document any issues with ICP control in large cohorts of patients [17–19].
The goal of our study is to perform a systematic review of the literature on the use of ketamine in TBI and its effects on ICP.
Methods
A systematic review using the methodology outlined in the Cochrane Handbook for Systematic Reviewers [20] was conducted. The data were reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [21]. The review questions and search strategy were decided upon by the primary author and supervisor.
Search Question, Population, Inclusion, and Exclusion Criteria
The question posed for systematic review was: In patients with TBI, what is the effect of ketamine on ICP? All studies, prospective and retrospective of any size based on human subjects were included. The reason for an all-inclusive search was based on the small number of studies of any type identified by the primary author during a preliminary search of MEDLINE.
The primary outcome measure was documented effect on ICP post-ketamine administration. Secondary outcome measures were cerebral perfusion pressure (CPP), mean arterial blood pressure (MABP), patient outcome, and adverse effects of ketamine.
Inclusion criteria were: All studies including human subjects with TBI whether prospective or retrospective, all study sizes, any age category, the use of ketamine, and documentation of ICP response to ketamine administration. Exclusion criteria were: animal and non-English studies.
Search Strategy
MEDLINE, BIOSIS, EMBASE, Global Health, HealthStar, SCOPUS, and Cochrane Library from inception to November 2013 were searched using individualized search strategies for each database. The search strategy for MEDLINE can be seen in Appendix A of the supplementary material, with a similar search strategy utilized for the other databases. In addition, the World Health Organizations International Clinical Trials Registry Platform was searched looking for studies planned or underway.
As well, meeting proceedings for the last 10 years looking for ongoing and unpublished work based on NMDA antagonists utilized in TBI were examined. The meeting proceedings of the following professional societies were searched: Canadian Neurological Sciences Federation (CNSF), American Association of Neurological Surgeons (AANS), Congress of Neurological Surgeons (CNS), European Neurosurgical Society (ENSS), World Federation of Neurological Surgeons (WFNS), American Neurology Association (ANA), American Academy of Neurology (AAN), European Federation of Neurological Science (EFNS), World Congress of Neurology (WCN), Society of Critical Care Medicine (SCCM), Neurocritical Care Society (NCS), World Federation of Societies of Intensive and Critical Care Medicine (WFSICCM), American Society for Anesthesiologists (ASA), World Federation of Societies of Anesthesiologist (WFSA), Australian Society of Anesthesiologists, International Anesthesia Research Society (IARS), Society of Neurosurgical Anesthesiology and Critical Care (SNACC), Society for Neuroscience in Anesthesiology and Critical Care, and the Japanese Society of Neuroanesthesia and Critical Care (JSNCC).
Finally, reference lists of any review articles or systematic reviews on sedation or ketamine in neurologically ill patients were reviewed for relevant studies on ketamine or NMDA antagonist usage in TBI.
Study Selection
Utilizing two reviewers, a two-step review of all articles returned by our search strategies was performed. First, the reviewers independently screened all titles and abstracts of the returned articles to decide if they met the inclusion criteria. Second, full text of the chosen articles was then assessed to confirm if they met the inclusion criteria and that the primary outcome of ICP response was reported in the study. Any discrepancies between the two reviewers were resolved by discussion.
Data Collection
Data were extracted from the selected articles and stored in an electronic database. Data fields included: patient demographics, type of study (prospective or retrospective), number of patients, dose, and route of ketamine used, timing to administration of drug, duration of drug administration, effect on ICP, effect on CPP, effect on MABP, adverse effects, and patient outcome.
Quality of Evidence Assessment
Assessment of the level of evidence for each included study was conducted by two independent reviewers, utilizing the Oxford criteria [22] and the Grading of Recommendation Assessment Development and Education (GRADE) criteria [23–28] for level of evidence.
The Oxford criteria consist of a 5 level grading system for literature. Level 1 is split into subcategories 1a, 1b, and 1c which represent a systematic review of RCT with homogeneity, individual RCT with narrow confidence interval, and all or none studies, respectively. Oxford level 2 is split into 2a, 2b, and 2c representing systematic review of cohort studies with homogeneity of data, individual cohort study or low quality RCT, and outcomes research, respectively. Oxford level 3 is split into 3a and 3b representing systematic review of case–control studies with homogeneity of data and individual case–control study, respectively. Oxford level 4 represents case-series and poor cohort studies. Finally, Oxford level 5 represents expert opinion.
The GRADE level of evidence is split into four levels: A, B, C, and D. GRADE level A represents high evidence with multiple high-quality studies having consistent results. GRADE level B represents moderate evidence with one high-quality study, or multiple low-quality studies. GRADE level C evidence represents low evidence with one or more studies with severe limitations. Finally, GRADE level D represents very low evidence based on either expert opinion or few studies with severe limitations.
Any discrepancies between the grading of the two reviewers were resolved via discussion and a third reviewer, when required.
Statistical Analysis
A meta-analysis was not performed in this study due to the heterogeneity of data within the articles and the presence of a small number of studies/patients involved.
Results
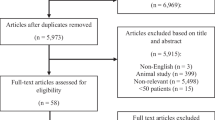
The results of the search strategy across all databases and other sources are summarized in Fig. 1. Overall a total of 371 articles were identified, with 368 from the database search and 3 from the search of published meeting proceedings. By applying the inclusion/exclusion criteria to the title and abstract of the articles, we identified 39 articles that fit these criteria. Of the 39 identified, 36 were from the database search and 3 were from published meeting proceedings. After removing duplicates, there were a total of 22 articles. Applying the inclusion/exclusion criteria to the full text documents, only seven articles were eligible for inclusion in the systematic review, with six from database and one from meeting proceeding sources. The 15 articles that were excluded were done so because they either did not report details around the administration of ketamine in TBI patients, or because they were review articles. Reference sections from these review articles were searched for any other articles missed in the database search, with none being identified.
Of the seven articles included in the review, all seven were original studies [10–16]. All studies were prospective studies, with four prospective randomized trials [12, 13, 15, 16], two prospective single arm trials [11, 14], and one prospective case–control (non-randomized) study [10]. Two studies focused on pediatric patients [10, 11], for a total of 55 children with TBI treated with ketamine.
Across all studies, a total of 156 TBI patients were studied utilizing ketamine continuous sedation [12, 13, 15, 16], intermittent bolus for preventative measures in stimulating events [10, 11] or bolus for attempts at ICP reduction [14]. All patients were classified as severe TBI, with Glasgow Coma Scores (GCS) of 8 or less, and all being ventilated and sedated. Fifty-five patients were pediatric (age range 1–16 years), and 101 were adult (age range 16–75 years). Study demographics and patient characteristics can be seen in Table 1, while treatment characteristics and effect on ICP, CPP, MABP, and adverse events are reported in Table 2.
Ketamine Treatment Characteristics
Within the seven studies [10–16], four utilized continuous infusions of ketamine [12, 13, 15, 16]. Two studies prospectively compared the use of ketamine/midazolam versus sufentanil/midazolam as continuous infusions for sedation in mechanically ventilated TBI patients [12, 13]. One study compared ketamine to sufentanil with a targeted plasma ketamine concentration of 1 mcg/mL, followed by titration to behavioral pain scale [12]. The second study compared ketamine to sufentanil started an initial ketamine dose at 50 mcg/kg/min followed by titration to sedation level, with a mean dose of 82 mcg/kg/min for a mean duration of 6.2 days [13]. A third study utilizing continuous infusions prospectively focused on ketamine/midazolam versus fentanyl/midazolam as sedation for TBI patients [15]. This study utilized a ketamine infusion at 65 mg/kg/day, titrated to sedative effect for a duration of 1–10 days. The final study utilizing a continuous infusion prospectively compared fentanyl/methohexitone versus ketamine/methohexitone for sedation in TBI, with a loading dose of 50 mcg/kg followed by titration to a Ramsay Sedation Score of 6 for a duration of 5 days [16].
The three remaining studies used bolus dosing of ketamine [10, 11, 14]. The first focused on the use of ketamine bolus prospectively in pediatric patients with normal ICP and high ICP during suctioning of their endotracheal tube [10]. The dose used was 1–1.5 mg/kg administered 1–3 min prior to stimulation. The second study focused on ketamine bolus prospectively for a cohort of pediatric patients during stimulation and during episodes of ICP elevations, with a dose of 1–1.5 mg/kg at 1–3 min either prior to stimulation or at ICP elevation [11]. The final study evaluated the ICP effect of three separate bolus doses of ketamine, 1.5/3/5 mg/kg, separated by 6 h [14]. Ketamine treatment characteristics can be seen in Table 2.
ICP Response
Continuous Infusions
Among the four studies looking at continuous ketamine infusion [12, 13, 15, 16] all failed to show clinically significant elevations in ICP during the administration of ketamine. When comparing infusions of ketamine/midazolam versus fentanyl/midazolam, there was no difference in ICP between groups within the two prospective randomized studies [12, 13]. Prospectively comparing ketamine/midazolam versus fentanyl/midazolam infusions, there was a statistically significant (at days 8 and 10) yet not clinically significant 2 mmHg elevation of ICP in the ketamine group [15]. Finally, prospectively comparing ketamine/methohexitone versus fentanyl/methohexitone as continuous infusions, there was no difference in ICP values between groups.
Bolus Dosing
Within the three studies focused on bolus dosing of ketamine in severe TBI patients [10, 11, 14], all failed to demonstrate elevations in ICP, and all trended toward reduction of ICP. Two of the three bolus dose studies were conducted on pediatric patients [10, 11]. Both studies demonstrated a reduction in ICP of 32 % [10] and 33 % [11], respectively, in patients with elevated ICP. One study demonstrated a reduction of ICP by a mean of 7.3 mmHg in those patients undergoing stimulation, and this reduction was sustained throughout the duration of the event [11]. Finally, the last study on bolus dosing prospectively evaluated severe TBI patients on propofol sedation receiving three separate boluses of ketamine (1.5, 3, and 5 mg/kg) 6 h apart. In this study, there was a statistically significant but nonsustained decrease in ICP after ketamine administration.
CPP and MABP Response
Continuous Infusions
In those four studies utilizing continuous infusions of ketamine, one study failed to document effect of CPP and MABP [13]. Two studies documented an increase in CPP and MABP [15, 16], with one documenting decreased vasopressor requirement compared to the fentanyl group [16] and the other documenting a statistically significant increase in CPP by 8 mmHg compared to the fentanyl group [15]. The fourth study failed to document a difference in CPP and MABP comparing ketamine to sufentanil [12].
Bolus Dosing
Within the three studies utilizing bolus dosing of ketamine, two failed to document the effect of CPP and MABP [10, 14]. The third study documented a statistically significant increase in CPP by a mean of 4 mmHg, with a decrease of MABP of 4 mmHg [11].
Adverse Effects of Ketamine
Only two patients in all of the studies reviewed displayed adverse events related to ketamine. These patients displayed nonclinically significant tachycardia related to ketamine [13]. This data were acquired directly from the manuscripts when reported.
Outcome
Patient outcome was reported sparingly and heterogeneously in most studies, and any conclusions on the use of ketamine in TBI patients as it pertains to outcome cannot be made.
Outcome was recorded as Glasgow Outcome Scale (GOS) scores in only three studies [13, 15, 16], two at six months [13, 15] and one at discharge from the ICU [16]. Outcome at discharge from ICU was reported as the same with GOS as 2.6 versus 2.0 in fentanyl versus ketamine groups [16]. Similarly, at six months, GOS was reported as “comparable” [15] between fentanyl versus ketamine groups, and “favorable” in four of the ketamine patients versus six of the sufentanil patients [13]. Generalizations across all studies on outcome using ketamine in TBI patients could not be made.
Level of Evidence
Based on two independent reviewers, there were a total of seven studies reviewed. Six studies were level 2b [10–13, 15, 16], and one was level 4 [14] evidence against ICP elevation with the administration of ketamine in severe TBI.
Similarly, two studies were GRADE B [12, 13], four were GRADE C [10, 11, 15, 16], and one was GRADE D [14] level of evidence against the elevation of ICP with the administration of ketamine in severe TBI.
Overall, we can recommend Oxford 2b, GRADE C level of evidence against the elevation of ICP with the administration of ketamine in severe TBI patients that are intubated and sedated. A summary of all levels of evidence for individual articles can be seen in Table 3.
Discussion
Ketamine is an interesting medication with a variety of potential applications in the neurologically ill. Centrally, ketamine functions via NMDA receptor antagonism, inhibiting glutamate activation [1]. This inhibition leads to suppression of the sensory cortex, limbic system, and thalamus [1], providing a dissociative anesthesia. Ketamine and other NMDA antagonists have also been postulated to provide a neuroprotective effect in TBI [17–19]. In addition, ketamine works peripherally at NMDA receptors, leading to pain modulation. Finally, ketamine also inhibits NO synthase inhibition, leading to pain modulation and vasoconstriction [9]. This vasoconstrictive property of ketamine provides hemodynamic stability in higher doses, in contrast to the usual hypotensive effects of other induction agents. Thus, with all of these benefits, ketamine has the potential for multiple uses within the neurological patient.
The cerebral hemodynamic effects of ketamine have been of concern for decades. Reported elevations in ICP have emerged, thought initially to be related to increase CBF from vasoconstriction and increased cerebral oxygen consumption [1]. However, upon further analysis it seems that in those patients spontaneously breathing, once given ketamine, have an increase in \( {\text{p}}_{{{\text{CO}}_{ 2} }} \) likely causing the ICP elevation [4]. Thus, based on this presumed mechanism ketamine is theoretically safe in a sedated ventilated patient [1, 4].
Despite this, the controversy surrounding the use of ketamine in the neurologically ill has existed since the 1970s. Throughout the majority of literature sources in neurosurgery and neuroanesthesia, the same articles are quoted when referring to the ICP elevation secondary to ketamine administration [5–8]. All of these studies focused on the use of ketamine as a dissociative anesthetic for elective neurosurgical procedures,the most of which were shunt revisions. In the few patients described [5–8] the ICP, as measured via transduction from a ventricular catheter or lumbar catheter, elevated post-ketamine administration. Despite the small number of patients in these four studies, the fear over ketamine use has perpetuated since. This fear has particularly been prominent in the TBI population, where concerns over ICP crisis have led to almost complete avoidance of the medication.
The goal of our study was to perform a systematic review of the literature on the effect of ketamine use on ICP in patients with TBI. In addition, our secondary interest was the impact on CPP, MABP, and any adverse effects. Our review identified seven prospective studies that met the inclusion and exclusion criteria. Within these 7 articles, a total of 156 patients (101 adults, 55 pediatric) with severe TBI were treated with varying doses of ketamine. Four articles focused on comparing ketamine versus opiates infusions (with either background benzodiazepine or methohexitone in both groups) [12, 13, 15, 16]. All four demonstrated no difference in ICP control, no ICP fluctuations with ketamine administration, and finally equal sedative properties. The remaining three studies focused on bolus dosing of ketamine to prevent ICP elevation during stimulus [10, 11], or as a means to reduce ICP during an episode of acute elevation [11, 14]. In all of these studies, the trend was toward a reduction in ICP with ketamine bolus, and a sustained effect when used preemptively for stimulating procedures. CPP and MABP were identical compared to control in all but two studies, which displayed higher mean CPP and lower vasopressor requirements [15, 16]. No significant adverse effects of ketamine were identified. Overall, we can make an Oxford 2b, GRADE C recommendation that ketamine does not lead to an elevation in ICP in severe TBI, in the setting of an intubated and sedated patient. In addition, bolus dose ketamine may provide a means of acute ICP reduction in these patients under intravenous sedation.
Our review has shed light on some important aspects of ketamine use in severe TBI. First, when utilized as an infusion, it has equal effectiveness to opiate based infusions in terms of sedation effect. We however, cannot comment on the effectiveness of ketamine in TBI when used in isolation, since all studies utilized background low-dose infusions of other sedative compounds. Second, when used in an infusion, ketamine does not lead to ICP elevations or fluctuation, and in fact causes an increase in CPP and decrease in vasopressor usage when compared to opiates. This may have impact on future use of ketamine as a second sedative infusion for severe TBI, as the deleterious effects of high-dose vasopressors should be avoided when possible. Third, when utilized in bolus dosing, ketamine seems to provide a dramatic decrease in ICP, whether at baseline or during an episode of ICP elevation. This further highlights the lack of an uncontrolled ICP increase with ketamine, on contrary to previous thoughts. However, again these patients were on background sedatives, and the effect of ketamine bolus in isolation is unknown. It could be postulated that if \( {\text{p}}_{{{\text{CO}}_{ 2} }} \) was controlled by mechanical ventilation, that bolus ketamine in isolation would be well tolerated without ICP fluctuations. Finally, the use of ketamine in severe TBI patients that are sedated and ventilated has little serious adverse effects, as demonstrated by the lack of complications identified in our review.
Despite what we have learned about ketamine in TBI through this review, there are several important limitations to this study. First, there were a small number of studies with small patient numbers, making the conclusions of this review difficult to generalize to all TBI patients. Furthermore, all patients in the studies identified had severe TBI, thus comments on the use of ketamine as described cannot be extrapolated to other groups of TBI patients at this time. Second, within the studies identified, there was a significant heterogeneity in study design thus negating the ability to perform a meaningful meta-analysis. Third, the use of background sedative compounds, in addition to ketamine and the control medications, makes it difficult to make comments on the use/safety of ketamine in isolation for TBI patients. Fourth, ketamine has been known to induce vasoconstriction [9], however, in all of the studies identified that there were no reports of a negative impact on cerebral blood flow. Similarly, to our knowledge, there are no papers utilizing cerebral microdialysis, brain tissue oxygen monitoring or regional perfusions monitors during ketamine administration in humans. Such further research may shed light on ketamine’s potential for central vasoconstriction, and its impact on cerebral blood flow and metabolism. Finally, there may be a significant publication bias with only those studies utilizing ketamine in TBI patients with good results making it to publication within the literature.
We believe that through this review, the lack of deleterious ICP effect of ketamine in severe TBI has been identified. The potential benefits of NMDA receptor antagonists in TBI warrants further investigation into the safety and utilization of ketamine. Further prospective trials need to be conducted to confirm the efficacy of ketamine bolus dosing for ICP reduction in TBI, potentially during planned noxious stimulus or for directed therapy in acute ICP elevations, and the effectiveness of ketamine as a primary sedative infusion.
Conclusions
There currently exists Oxford level 2b, GRADE C evidence to support that ketamine does not increase ICP in severe TBI patients that are sedated and ventilated, and in fact may lower it in selected cases. Further prospective study of ketamine in TBI is warranted.
References
Aroni F, Iacovidou N, Dontas I, Pourzitaki C, Xanthos T. Pharmacological aspects and potential new clinical applications of ketamine: reevaluation of an old drug. J Clin Pharmacol. 2009;49:957–64.
Roberts DJ, Hall RI, Kramer AH, Roberston HL, Gallagher CN, Zygun DA. Sedation for critically ill adults with severe traumatic brain injury: a systematic review of randomized controlled trials. Crit Care Med. 2011;39(12):2743–51.
Sedev RS, Symmons DAD, Kindl K. Ketamine for rapid sequence induction in patients with head injury in the emergency department. Emerg Med Australas. 2006;18:37–44.
Himmelseher S, Durieux ME. Revising a dogma: ketamine for patients with neurological injury? Anesth Analg. 2005;101:524–34.
Wyte SR, Shapiro HM, Turner P, Harris AB. Ketamine-induced intracranial hypertension. Anesthesiology. 1972;36(2):174–6.
Shapiro HM, Wyte SR, Harris AB. Ketamine anesthesia in patients with intracranial pathology. Br J Anaesth. 1972;44:1200–4.
Gardner AE, Olson BE, Lichtiger M. Cerebrospinal-fluid pressure during dissociative anesthesia with ketamine. Anesthesiology. 1971;35(2):226–8.
List WF, Crumrine RS, Cascorbi HF, Weiss MH. Increased cerebrospinal fluid pressure after ketamine. Anesthesiology. 1972;36(1):93–4.
Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006;60:341–8.
Bar-Joseph G, Guilburd Y, Guilburd J. Ketamine effectively prevents intracranial pressure elevations during endotracheal suctioning and other distressing interventions in patients with severe traumatic brain injury. Crit Care Med. 2009;37(12):A402.
Bar-Joseph G, Guilburd Y, Tamir A, Guilburd J. Effectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. J Neurosurg Pediatr. 2009;4:40–6.
Boirgoin A, Albanese J, Leone M, Sampol-Manos E, Viviand X, Martin C. Effects of sufentanil or ketamine administered in target-controlled infusion on the cerebral hemodynamics of severely brain injured patients. Crit Care Med. 2005;33(5):1109–13.
Boirgoin A, Albanese J, Wereszczynski N, Charbit M, Vialet R, Martin C. Safety of sedation with ketamine in severe head injury patients: comparison with fentanyl. Crit Care Med. 2003;31(3):711–7.
Albanese J, Arnaud S, Rey M, Thomachot L, Alliez B, Martin C. Ketamine decreases intracranial pressure and electroencephalographic activity in traumatic brain injury patients during propofol sedation. Anesthesiology. 1997;87:1328–34.
Kolenda H, Gremmelt A, Rading S, Braun U, Markakis E. Ketamine for analgosedative therapy in intensive care treatment of head-injured patients. Acta Neurochir (Wien). 1996;138:1193–9.
Schmittner MD, Vajkoczy SL, Horn P, Bertsch T, Quintel M, Vajkoczy P, et al. Effect of fentanyl and S(+)-ketamine on cerebral hemodynamics, gastrointestinal motility, and need of vasopressors in patients with intracranial pathologies a pilot study. J Neurosurg Anesthesiol. 2007;19:257–62.
Yurkewicz L, Weaver J, Bullock MR, Marshall MF. The effect of elective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma. 2005;22(12):1428–43.
Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, Marshall LF, et al. Failure of the competitive N-methyl-d-aspartate antagonist Selfotel (CGS 19755) in treatment of severe head injury: results of two phase III trials. J Neurosurg. 1999;91:737–43.
Stewart L, Bullock R, Teasdale GM, Wagstaff A. First observations of the safety and tolerability of a competitive antagonist to the glutamate NMDA receptor (CGS 19755) in patients with severe head injury. J Neurotrauma. 1999;16(9):843–50.
Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions Version 5.1.0. http://handbook.cochrane.org/. Accessed 25 October 2013.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Phillips B, Ball C, Sackett D, Straus S, Haynes B, Dawes M. Oxford Centre for Evidence-Based Medicine Levels of Evidence Version 2009. http://www.cebm.net/?o=1025. Accessed October 2013.
Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ, et al. Rating quality of evidence and strength of recommendations: what is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–8.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106–10.
Guyatt GH, Oxman AD, Kunz R, Jaeschke R, Helfand M, Liberati A, et al. Rating quality of evidence and strength of recommendations: incorporating considerations of resources use into grading recommendations. BMJ. 2008;336(7654):1170–3.
Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Rating quality of evidence and strength of recommendations: going from evidence to recommendations. BMJ. 2008;336(7652):1049–51.
Jaeschke R, Guyatt GH, Dellinger P, Schünemann H, Levy MM, Kunz R, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeiler, F.A., Teitelbaum, J., West, M. et al. The Ketamine Effect on ICP in Traumatic Brain Injury. Neurocrit Care 21, 163–173 (2014). https://doi.org/10.1007/s12028-013-9950-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-013-9950-y