Abstract
Objective
Elevated troponin levels are a common occurrence after ischemic stroke and subarachnoid hemorrhage (SAH), and have been described as a neurogenic form of myocardial injury. The prognostic significance of this event is controversial with numerous studies citing conflicting results. The importance of cardiac stress is of particular relevance in the operative management of intracerebral hemorrhage (ICH). To this end, we investigated whether troponin levels were an independent predictor of in-hospital mortality from all causes in surgically treated ICH patients.
Methods
We performed a retrospective analysis of 110 patients admitted to Columbia Presbyterian hospital between 1999 and 2007 for ICH and subsequent clot evacuation. Those with angina or recent myocardial infarction were excluded. CT scans were reviewed to determine hematoma size, location, presence of intraventricular hemorrhage (IVH) or SAH, hydrocephalus, and midline shift. Hospital records were examined for known demographic and clinical predictors of mortality. Univariate analysis was used to screen for predictive factors (P ≤ 0.20) and these variables were entered into the final multivariable logistic regression model along with gender and age.
Results
Of 110 patients, 10 were excluded due to insufficient records or pre-existing cardiovascular disease. Ninety-five patients had at least one troponin level and 83 had multiple levels. Univariate analysis revealed nine factors that predicted in-hospital mortality (P < 0.20): smoking, volume of hemorrhage, midline shift, IVH, neurological status on admission, admission troponin, post-surgical troponin, warfarin use, and international normalized ratio. Only two factors were significant in the final multi-variate model: admission troponin and volume of hemorrhage. Admission troponin levels were a significant risk factor for in-hospital mortality even after controlling for hemorrhage volume, gender, and age.
Conclusions
Elevated cardiac troponin levels are predictive of mortality in surgically treated ICH patients and should be considered in management decisions. Implementation of cardio-protective strategies may improve outcomes in this patient population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracerebral hemorrhage (ICH) accounts for approximately 10–20% of stroke [3]. Despite best available therapy, ICH continues to cause considerable morbidity and mortality, with estimates of 30-day mortality ranging from 30 to 52%. Those patients who do survive typically have some degree of neurologic disability with only 20% regaining independence by 6 months [7]. Although the majority of the morbidity and mortality is attributed to neurological causes, other evidence has suggested that cardiac damage may also be a contributing factor [4, 5].
Although electrocardiographic abnormalities and myocardial enzyme release is common after ICH, the clinical significance of this finding is unclear [1]. In contrast to true coronary artery disease, angiographic evidence of vascular obstruction is typically not found, and the extent of myocardial enzyme release is below the diagnostic thresholds used at many institutions [8, 13].
This has prompted further efforts to determine the etiology of cardiac damage in neurologic patients and investigate the clinical importance of this finding. At present, the best evidence from histological studies suggests the etiology may be catecholamine-mediated myocardium damage [4, 5, 15, 16].
In subarachnoid hemorrhage (SAH) patients, electrocardiogram (ECG) changes such as QTc prolongation and repolarization abnormalities can be found in 50–100% of patients, but are generally not considered to have prognostic significance [6, 11, 17]. In contrast, small cardiac troponin I elevations are found in 20–40% of SAH patients with some studies showing an associated increased mortality [5, 9, 13]. This relationship is less clearly defined in ICH patients. To this end, we investigated whether troponin (cTI) release could be used as a clinical predictor of mortality in surgically treated ICH patients.
Materials and Methods
Study Subjects
The study was conducted at a large tertiary academic medical center with a dedicated neurologic and neurosurgical intensive care unit (NICU). Patients were admitted to the NICU either from our emergency department or at the discretion of the admitting neurologist or neurosurgeon as transfers from outside hospitals. The NICU team included full-time coverage by neurointensivists; neurologists, neurosurgeons, and nurses with specialty training in neurocritical care.
The neurosurgical service maintains a database in which all admissions are recorded along with the diagnoses, procedures, treatments, and demographic information. Eligible patients were identified by searching the NICU database for those patients who received clot evacuation for ICH between February 1998 and June 2007. The diagnosis of ICH was established by CT scan.
Cardiac Testing
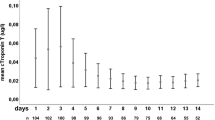
All patients had an ECG on admission, 95% had at least one cTI level (revised Dimension RxL cTnI method, Dade Behring, Chicago, IL) and 83% had ≥1 cTI measurement. In addition, ECGs obtained from transferring hospitals were reviewed. According to our management protocol, cTI was measured in all patients with an abnormal ECG (Q waves, QTc prolongation, ST-segment abnormalities, or T-wave inversion) or clinical signs/symptoms of potential cardiovascular dysfunction [pulmonary edema, hypertension, or hypotension (systolic blood pressure >160 or <100 mmHg, respectively), cardiac dysrhythmia or chest pain]. When the first cTI level was abnormal (>0.3 μg/l in our laboratory), daily serial measurements were obtained as clinically indicated. In our laboratory, cTI levels >2.0 μg/l are considered diagnostic of myocardial ischemia. The first troponin level drawn before surgery was considered the “admission troponin level.” When >1 cTI measurement was obtained in the 1–2 days following surgery, the highest level was recorded as “post-surgery troponin level.”
Admission Clinical and Radiographic Variables
Demographic data, past medical and social histories, and clinical features on admission were obtained through patient and surrogate interviews, as well as medical chart review. Prior cardiac disease was defined as a history of angina, myocardial infarction, arrhythmia, heart failure, or valvular heart disease. Initial cranial CT scans were evaluated in order to determine hemorrhage location and volume; extension into the ventricles, subdural, or subarachnoid space; presence and extent of hydrocephalus; and degree of midline shift. Hemorrhage volume was estimated using the ABC/2 rule where A, B, and C represent the respective diameters [2]. Neurological status was determined from the admission neurological exam, and was coded using a 4-tiered system following the Glasgow outcome scale: intact, focal neurological deficit, lethargic, or unresponsive.
Statistical Analysis
Statistical analysis of the data was performed using commercially available software (JMP 7.0, SAS Institute Inc., Cary, NC). A univariate analysis was performed to screen for those variables with P < 0.20 to be entered into the full model. This full model was then successively reduced by removing the least significant covariate from the model, until all covariates had a P-value of <0.10. These variables then constituted the final multivariate model. In the final model, covariates were considered to have a significant effect at a value of P ≤ 0.05. Covariates with P-values between 0.05 and 0.10 were considered not to have a significant effect on the outcome but being of sufficient importance for the model fit to be kept in the final model.
Results
The baseline clinical and radiographic characteristics as well as discharge location of patients are summarized in Tables 1 and 2. In total, there were ten patients in whom past medical history was incomplete, there was a history of cardiac disease, or the patient had already undergone prior evacuation.
Troponin levels were ≥0.01 μg/l in 38 patients on admission and 37 patients post-surgery. Troponin levels were not related to the side of hemorrhage. Overall, 15 patients (15%) had admission troponin levels ≥0.40 μg/l of which 47% died compared to a 14% mortality rate among those with an admission troponin level <0.40 μg/l (P = 0.004). Twenty-one patients (21%) had a post-surgery troponin level ≥0.40 μg/l of which 33% died compared to a 14% mortality rate of those with post-surgery troponin levels <0.40 μg/l (P = 0.04).
The univariate analysis identified nine factors that were predictive of in-hospital mortality: smoking, volume of hemorrhage, midline shift, intraventricular hemorrhage (IVH), neurological status on admission, admission troponin, post-surgical troponin, history of warfarin use, and international normalized ratio (INR) on admission (Table 3).
Sequentially removing the least significant predictive variable until every variable had a P-value of <0.10 while still retaining gender and age led to a final multivariate model with only two significant predictors: volume of hemorrhage and admission troponin level. The parameter estimates, standard errors’ odds ratios, and P-values for these variables are summarized in Table 4.
Discussion
Prior investigations have described an association between neurological injury and the presence of electrographic and biochemical markers of cardiac damage [7]. Although studies have supported this phenomenon to be primarily related to excess catecholamine release [16], the clinical significance of these findings remains unclear. In this study involving 100 ICH patients undergoing clot evacuation, we demonstrate that admission troponin measurements carry prognostic significance. Even after controlling for other known predictors of mortality, troponin level on admission was still significantly associated with in-hospital mortality in this population. These results suggest that admission troponin levels should be routinely measured in ICH patients and may be used to more accurately determine prognosis and guide management decisions.
Our univariate analysis identified nine factors that were predictive of in-hospital mortality: smoking, volume of hemorrhage, midline shift, IVH, neurological status on admission, admission troponin, post-surgical troponin, warfarin use, and INR. After controlling for volume of hemorrhage, however, all of the factors except for admission troponin become statistically insignificant. This may be explained by the possibility that many of the factors (e.g., midline shift, IVH, neurological status on admission, INR, and warfarin use) may be surrogate markers for initial hemorrhage size. In contrast, troponin levels may be a marker for recuperative potential and thus remain a significant predictor even after controlling for factors that are linked to the magnitude of initial insult. As expected, admission and post-surgical troponin levels were highly correlated and thus post-surgical troponins became non-significant in the multi-variate analysis.
Our retrospective study has inherent limitations. A future prospective trial would be useful in further validating the prognostic value of these markers as well as the role of cardioprotective strategies, both prophylactic and interventional. Additionally, our cohort was not large enough to stratify by individual causes of death, specifically those cardiac in nature (n = 3). Finally, we did not obtain cTI measurements in all ICH patients and did not perform cTI measurements serially according to a prospective protocol. Therefore, our dataset may be subject to selective biases. Finally, it is possible that over the relatively long-recruitment period (1999–2007), there may have been advances in intensive care management. However, any significantly effective advance in management over this time period would bias toward the null hypothesis.
Despite these limitations, the findings of this study support the clinical significance of cTI elevations following ICH and advocate for its routine measurement upon admission. Since publication of the STICH trial, [12] operative management of ICH has been controversial, with surgical therapy reserved for young patients with superficial lesions and deteriorating neurological exams. Standard troponin values may provide additional information to allow peri-operative cardiac risk assessment and justify aggressive intervention in those select patients with a favorable prognosis.
Of particular interest is the issue of anesthetic risk during surgical evacuation. Recent myocardial injury and congestive cardiac failure have been consistently identified as risk factors for perioperative cardiac events in patients presenting for noncardiac surgery [10]. Measures to minimize intraoperative cardiac risk such as the use of a pulmonary artery catheter, avoidance of myocardial depressant agents, and maintenance of optimal myocardial oxygen supply and demand may prove beneficial in patients with elevated cTI [14].
In summary, our findings indicate that cTI elevations after ICH are predictive of in-hospital mortality. Larger prospective studies are needed to confirm these results and evaluate the utility of intensive care management strategies based on cTI risk stratification.
References
Baroldi G. Anatomy and quantification of myocardial cell death. Methods Achiev Exp Pathol. 1988;13:87–113.
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93.
Caplan LR. Intracerebral haemorrhage. Lancet. 1992;339:656–8.
Connor RC. Myocardial damage secondary to brain lesions. Am Heart J. 1969;78:145–8.
Doshi R, Neil-Dwyer G. Hypothalamic and myocardial lesions after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1977;40:821–6.
Doshi R, Neil-Dwyer G. A clinicopathological study of patients following a subarachnoid hemorrhage. J Neurosurg. 1980;52:295–301.
Hays A, Diringer MN. Elevated troponin levels are associated with higher mortality following intracerebral hemorrhage. Neurology. 2006;66:1330–4.
Kaye MP, McDonald RH, Randall WC. Systolic hypertension and subendocardial hemorrhages produced by electrical stimulation of the stellate ganglion. Circ Res. 1961;9:1164–70.
Kono T, Morita H, Kuroiwa T, Onaka H, Takatsuka H, Fujiwara A. Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol. 1994;24:636–40.
Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153–84.
Mayer SA, LiMandri G, Sherman D, Lennihan L, Fink ME, Solomon RA, et al. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg. 1995;83:889–96.
Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97.
Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–6.
Parekh N, Venkatesh B, Cross D, Leditschke A, Atherton J, Miles W, et al. Cardiac troponin I predicts myocardial dysfunction in aneurysmal subarachnoid hemorrhage. J Am Coll Cardiol. 2000;36:1328–35.
Samuels MA. Neurogenic heart disease: a unifying hypothesis. Am J Cardiol. 1987;60:15J–9J.
Todd GL, Baroldi G, Pieper GM, Clayton FC, Eliot RS. Experimental catecholamine-induced myocardial necrosis. I. Morphology, quantification and regional distribution of acute contraction band lesions. J Mol Cell Cardiol. 1985;17:317–38.
Zaroff JG, Rordorf GA, Ogilvy CS, Picard MH. Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: evidence for neurally mediated cardiac injury. J Am Soc Echocardiogr. 2000;13:774–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garrett, M.C., Komotar, R.J., Starke, R.M. et al. Elevated Troponin Levels are Predictive of Mortality in Surgical Intracerebral Hemorrhage Patients. Neurocrit Care 12, 199–203 (2010). https://doi.org/10.1007/s12028-009-9245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-009-9245-5