Abstract
Background and Purpose
Computed tomography (CT) and CT angiography (CTA) are frequently the initial imaging modalities used in the evaluation of patients with suspected aneurysmal subarachnoid hemorrhage (SAH). It remains unclear whether CTA can provide adequate information to determine best treatment modality (endovascular versus surgical) for ruptured intracranial aneurysms.
Methods
Pertinent clinical and radiological information of consecutive patients with aneurysmal SAH who underwent CTA on a 64-slice multidetector CT (MDCT) scanner were independently reviewed by five endovascular specialists. Subsequently, the interobserver reliability was calculated.
Results
A total of 21 consecutive patients with aneurysmal SAH detected on CTA were reviewed. Of the total of 105 reviews, in 65% a treatment allocation decision was made. Responses were, 26% either treatment; 18% endovascular only; 18% surgical only; and 3% neither treatment. In the remaining 35% it was considered that CTA images were inadequate to make a decision for treatment allocation and more information was requested. Interobserver reliability was poor between endovascular specialists (k = 0.2). The reliability was higher among endovascular/vascular neurosurgeons (k = 0.34) and physicians with >5 years of faculty experience (k = 0.55).
Conclusion
When 64-slice MDCT angiography is used in the evaluation of aneurysmal SAH, the information obtained is adequate to determine treatment modality allocation in two-thirds of the cases. The agreement on best treatment modality varied across primary specialty, practice experience, and site of fellowship completion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In patients with suspected aneurysmal subarachnoid hemorrhage (SAH), computed tomography (CT) followed by CT angiography is frequently used to establish the diagnosis. Once SAH is confirmed, a prompt treatment plan should be initiated. In the decision to whether treat using surgical or endovascular approaches, a detailed understanding of the clinical scenario and aneurysm characteristics are necessary. The morphology, dimensions, and relationship of the aneurysm to adjacent osseous and neurovascular structures are also of importance to the treating physician. Depending on various factors, this information may or may not be acquired with the first noninvasive imaging modalities.
Cerebral digital subtraction angiography (DSA) has been considered the “gold standard” imaging modality for the diagnosis of aneurysmal SAH. Cerebral angiography can evaluate flow dynamics of the parent vessel and also evaluate for potential branches arising from the aneurysm sac. On the other hand, cerebral angiography is invasive, time consuming, carries risk of complications, and requires more resources to accomplish [1–3]. CT angiography is noninvasive and can be performed rapidly. It requires less people to perform, it is more comfortable for the patient, and the images can provide reference of the aneurysm with adjacent structures. The difficulties encountered with CT angiography include bone/foreign body artifact, interference of other vessels (veins), and poor visualization of branches originating from the aneurysm [4].
The sensitivity and specificity of CT angiography and DSA to detect intracranial aneurysms has been estimated to be 89–98 and 90–100%, respectively [5–10]. These estimates have been observed to increase with the use of multidetector scanners [5].
With the present technology, it remains unclear if CT angiography alone can be used in the decision for best treatment modality in ruptured intracranial aneurysms. The goal of our present study is to determine if CT angiography can provide enough information to decide on best treatment modality without the need of conventional cerebral angiography.
Material and Methods
Patients
From September 2006 to August 2007, we collected demographic information and radiological images from consecutive patients admitted to a level I trauma center and a university hospital with the diagnosis of nontraumatic SAH confirmed by noncontrast computerized tomography. Demographic information included age, gender, comorbidities, Hunt and Hess Scale [11, 12], and Fischer grade [13].
Imaging
CTA was performed on a 64-slice multidetector scanner (Siemens Somatom Volume Zoom, Erlangen, Germany). The angiographic source images were reviewed by the facility’s neuroradiologist and then reformatted on a Vitrea (Vital Images Inc., MN, USA) computer workstation into two-dimensional maximum intensity projection views and three-dimensional (3-D) surface rendered and volume rendered reconstructions. At the discretion of the neuroradiologist, few source images and 3-D images were saved into the PACS server. These images were presented to the observers for treatment allocation.
Observers
Three interventional neurologists (AIQ, RAT, VJ) and two endovascular/vascular neurosurgeons (RT, GL) were asked to participate as observers (observers 1–5, respectively). Clinical experience among reviewers varied. One interventional neurologist and one endovascular/vascular neurosurgeon had individual experience greater than 5 years (AIQ and GL). The remaining three reviewers had a combined 3 years of experience as academic faculty.
Each physician reviewed patient information and CTA images independently. For each case, the reviewer was asked to choose the best treatment allocation from one of the five categories: (1) endovascular only, (2) surgical only, (3) either treatment, (4) neither treatment, or (5) imaging information inadequate to make decision.
Statistical Analysis
We calculated the interobserver reliability among the five specialists using Cohen’s Kappa coefficient, which assesses the likelihood that the agreement between the observers is more than just chance. A Kappa of 0, 0.1–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, 0.81–1.00 is interpreted as no agreement, poor agreement, fair agreement, moderate agreement, substantial agreement, and almost perfect agreement, respectively.
Results
Patient Population
During the period of 12 months, 21 consecutive patients (11 female, 10 male) with 22 aneurysms (one unruptured) were admitted with the diagnosis of aneurysmal SAH (Table 1). The median Hunt and Hess and Fischer grades on presentation were grades 1 (n = 11) and 3 (n = 14), respectively.
Location of Ruptured Aneurysms
Twenty patients had ruptured aneurysms in the anterior circulation (four internal carotid artery, five anterior communicating, two middle cerebral artery, three anterior cerebral artery, and six posterior communicating artery) and one in the posterior circulation (basilar artery).
Interobserver Reliability Results
The reviewers provided a total of 105 responses for the 21 cases. In 64.8% of the reviews a treatment allocation using CTA was made. Responses by modality chosen were the following: either treatment was acceptable in 25.7%, surgery alone in 18.1%, endovascular alone in 18.1%, and no treatment in 2.9% of the cases. In the remaining 35.2% of the reviews, it was considered that CT angiography images were inadequate to make a treatment decision (Table 2). On a case-by-case basis, all five reviewers agreed only in cases 4 and 17. In case 13, four different responses were observed.
There was poor interobserver reliability among all five endovascular specialists (k = 0.2) and the three interventional neurologists (k = 0.15). The agreement between both endovascular/vascular neurosurgeons was fair (k = 0.34). The highest agreement was among observers with over 5 years of faculty experience (k = 0.55).
Discussion
The use of CT angiography in clinical practice for the detection of aneurysms has become an important diagnostic tool to a point that it has been considered to be equivalent to DSA [14, 15], but the impact translating into treatment decision has been unclear. The unique aspect of our study was the use of a high-resolution 64-slice multidetector scanner, which presumably could provide more information than 32-slice scans. We also used a panel of neuroendovascular specialists to determine the applicability of the findings on CTA across physicians. It was in our interest that the treatment modality chosen by the observers would be based on using CTA images alone and for this reason DSA images were not displayed to the observers.
When pooling results from case series (Table 3) allocating treatment in patients with SAH using CTA [5, 9, 10, 16–20], the decision for treatment was variable among these publications (51.9–91.3%). In 8.7–48.1% of the patients a conventional cerebral angiography was performed to decide on best treatment modality. When these patients with SAH (n = 771) are grouped based on treatment decision and treatment modality, in 73.8% of the cases a decision using CTA alone was made (our results 64.8%). Treatment modality results were 65.9, 27.1, 4.6, and 2.4% for surgery, endovascular, either surgery or endovascular, and no treatment, respectively. The need for cerebral angiography occurred in 202 patients (26.2%). Results of case series for unruptured aneurysms [16, 17, 19] show that in 50–78% of the patients a treatment decision was made based on CTA alone. Similarly, when these patients with unruptured aneurysms (n = 220) are grouped, a treatment decision using CTA was possible in 65.9%. The treatment modalities selected were 68.3, 30.3, and 1.4% for surgery, endovascular, and no treatment, respectively.
In a study of patients with SAH, where CT angiography alone was also used for treatment allocation, 26% of the patients were considered eligible for either surgery or endovascular intervention. Surgery (45%), endovascular therapy (21%), need for conventional angiography (16%), and no treatment (2%) were the other outcomes [10].
Another case series of 74 ruptured aneurysms, surgery was recommended in 31.1% (n = 25) and endovascular in 66.2% (n = 49) of cases. When actual treatment took place, the sensitivity, specificity, positive, and negative predictive values of CTA in predicting feasibility of endovascular treatment was 94 (47/50), 92 (22/24), 96 (47/49), and 88% (22/25), respectively [6].
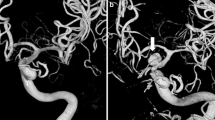
In some circumstances, the location of the ruptured aneurysm by itself leads to preferential selection of surgery or endovascular treatment. For example, in basilar aneurysms, surgery is technically challenging because of the intimate relationship with the skull base, interpeduncular fossa, and perforating vessels. For this reason, endovascular treatment is often favored over surgery. The morbidity and mortality rate of surgery is considered high (28–47.4%) when compared to endovascular therapy [21–26] (4.3–10%). Case #20 illustrates a patient with a previously clipped basilar aneurysm who suffered a re-rupture. CTA images give the impression of a posterior communicating aneurysm (Fig. 1), a possible reason why observers responded either treatment (n = 2), more information is needed (n = 2), and endovascular only (n = 1).
For middle cerebral artery bifurcation aneurysms, surgical treatment is often favored over endovascular treatment. During surgery, these aneurysms can be visualized in the initial dissection planes when the sylvian fissure is exposed. This location provides a wide surgical window resulting in less brain retraction. Also, the morphology of these aneurysms (large neck to dome ratio) is another reason to select surgery. Variable rates of successful coil embolization in middle cerebral artery bifurcation aneurysms have been reported. These rates have been as low as 6% [27], and more recently as high as 62.5–80% [28, 29]. Case #21 (Fig. 2) illustrates a patient with a left middle cerebral artery bifurcation aneurysm. In this case, four observers suggested surgical treatment and one requested more information.
Another circumstance in which a management decision is made despite the quality of the CTA or location of the aneurysms are in patients with a poor Hunt and Hess grade. In Case #15 (Fig. 3), the patient had a Hunt and Hess Grade V. Three observers considered no treatment and two favored endovascular treatments.
Comments from the observers addressing the criteria used to make their final treatment decision included the patient’s clinical condition (age, Hunt and Hess Scale, and comorbidities), the morphology, and location of the aneurysms. Comments from the observers in the reason for choosing “imaging information was inadequate to make decision,” had in common that the 3-D CT angiogram images were of poor quality in some cases. These images did not provide aneurysm morphology details (i.e., neck-dome ratio, anterior/posterior projected aneurysm) and if potential vascular branches could have been arising from the aneurysm sac.
In this study, the observers were able to allocate treatment in 65% of the cases using the latest CTA technology without the need of DSA. In the remaining 35% more information was requested, CT angiography did not provide the information considered necessary for treatment allocation that perhaps a conventional cerebral angiography would provide, as it remains to date the gold standard imaging modality in the evaluation of patients with aneurysmal SAH. It is possible that if the reviewers were presented with the full source images and the ability to reconstruct, format, or improve image windowing, a higher rate of treatment allocation would have been observed.
The treatment modality chosen by each observer represents in their opinion the best therapeutic approach, and this does not necessarily mean that the modality chosen was the correct one since many aneurysms are eligible to be treated by endovascular or surgical modalities, or no treatment at all. The highest kappa was seen between observers #1 and #5. These two observers completed their neuroendovascular fellowship training 1 year apart, in the same institution (institution A), and have been in practice for over 5 years. The second highest kappa value was between observers #4 and #5, who are vascular neurosurgeons with neuroendovascular training in the same institution (institution A) during a different era. Observer #2 (institution B), observer #3 (institution C), and observer #4 (institution A) completed their neuroendovascular fellowships also 1 year apart, but at three different institutions, nearly 6 years after observers #1 and #5. The lowest kappa was seen among observers #1, #2, and #3 who completed training at different institutions during different eras. This proposes that training completion in the same institution correlates with increasing kappa values influenced by practice experience and primary specialty.
Conclusion
Even when aneurysms are detected on CTA using 64-slice multidetector scanners in patients with SAH, the information is considered adequate to make a treatment allocation in two-thirds of the cases. The agreement for best treatment allocation varied upon primary specialty, site of fellowship training, and practice experience.
References
Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003;227:522–8.
Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol. 1994;15:1401–7, discussion 8–11.
Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999;30:317–20.
Goddard AJ, Tan G, Becker J. Computed tomography angiography for the detection and characterization of intra-cranial aneurysms: current status. Clin Radiol. 2005;60:1221–36.
Westerlaan HE, Gravendeel J, Fiore D, et al. Multislice CT angiography in the selection of patients with ruptured intracranial aneurysms suitable for clipping or coiling. Neuroradiology 2007;49:997–1007.
Papke K, Kuhl CK, Fruth M, et al. Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning. Radiology 2007;244:532–40.
Lubicz B, Levivier M, Francois O, et al. Sixty-four-row multisection CT angiography for detection and evaluation of ruptured intracranial aneurysms: interobserver and intertechnique reproducibility. AJNR Am J Neuroradiol. 2007;28:1949–55.
Karamessini MT, Kagadis GC, Petsas T, et al. CT angiography with three-dimensional techniques for the early diagnosis of intracranial aneurysms. Comparison with intra-arterial DSA and the surgical findings. Eur J Radiol. 2004;49:212–23.
Gonzalez-Darder JM, Pesudo-Martinez JV, Feliu-Tatay RA. Microsurgical management of cerebral aneurysms based in CT angiography with three-dimensional reconstruction (3D-CTA) and without preoperative cerebral angiography. Acta Neurochir (Wien). 2001;143:673–9.
Dehdashti AR, Rufenacht DA, Delavelle J, Reverdin A, de Tribolet N. Therapeutic decision and management of aneurysmal subarachnoid haemorrhage based on computed tomographic angiography. Br J Neurosurg. 2003;17:46–53.
Hunt WE, Meagher JN, Hess RM. Intracranial aneurysm. A nine-year study. Ohio State Med J. 1966;62:1168–71.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980;6:1–9.
Zouaoui A, Sahel M, Marro B, et al. Three-dimensional computed tomographic angiography in detection of cerebral aneurysms in acute subarachnoid hemorrhage. Neurosurgery 1997;41:125–30.
Tipper G, U-King-Im JM, Price SJ, et al. Detection and evaluation of intracranial aneurysms with 16-row multislice CT angiography. Clin Radiol. 2005;60:565–72.
Pechlivanis I, Schmieder K, Scholz M, Konig M, Heuser L, Harders A. 3-Dimensional computed tomographic angiography for use of surgery planning in patients with intracranial aneurysms. Acta Neurochir (Wien). 2005;147:1045–53, discussion 53.
Hoh BL, Cheung AC, Rabinov JD, Pryor JC, Carter BS, Ogilvy CS. Results of a prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnostic and pretreatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery 2004;54:1329–40, discussion 40–2.
Anderson GB, Steinke DE, Petruk KC, Ashforth R, Findlay JM. Computed tomographic angiography versus digital subtraction angiography for the diagnosis and early treatment of ruptured intracranial aneurysms. Neurosurgery 1999;45:1315–20, discussion 20–2.
Amagasaki K, Sato T, Kakizawa T, Shimizu T. Treatment of ruptured anterior circulation aneurysm based on computerized tomography angiography: surgical results and indications for additional digital subtraction angiography. J Clin Neurosci. 2002;9:22–9.
Agid R, Lee SK, Willinsky RA, Farb RI, terBrugge KG. Acute subarachnoid hemorrhage: using 64-slice multidetector CT angiography to “triage” patients’ treatment. Neuroradiology 2006;48:787–94.
Vallee JN, Aymard A, Vicaut E, Reis M, Merland JJ. Endovascular treatment of basilar tip aneurysms with Guglielmi detachable coils: predictors of immediate and long-term results with multivariate analysis 6-year experience. Radiology 2003;226:867–79.
Tateshima S, Murayama Y, Gobin YP, Duckwiler GR, Guglielmi G, Vinuela F. Endovascular treatment of basilar tip aneurysms using Guglielmi detachable coils: anatomic and clinical outcomes in 73 patients from a single institution. Neurosurgery 2000;47:1332–9, discussion 9–42.
Redekop G, Willinsky R, Montanera W, TerBrugge K, Tymianski M, Wallace MC. Endovascular occlusion of basilar bifurcation aneurysms with electrolytically detachable coils. Can J Neurol Sci. 1999;26:172–81.
Pierot L, Boulin A, Castaings L, Rey A, Moret J. Selective occlusion of basilar artery aneurysms using controlled detachable coils: report of 35 cases. Neurosurgery 1996;38:948–53, discussion 53–4.
Mordasini P, Schroth G, Guzman R, Barth A, Seiler RW, Remonda L. Endovascular treatment of posterior circulation cerebral aneurysms by using Guglielmi detachable coils: a 10-year single-center experience with special regard to technical development. AJNR Am J Neuroradiol. 2005;26:1732–8.
Bavinzski G, Killer M, Gruber A, Reinprecht A, Gross CE, Richling B. Treatment of basilar artery bifurcation aneurysms by using Guglielmi detachable coils: a 6-year experience. J Neurosurg. 1999;90:843–52.
Regli L, Dehdashti AR, Uske A, de Tribolet N. Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir Suppl. 2002;82:41–6.
Horowitz M, Gupta R, Gologorsky Y, et al. Clinical and anatomic outcomes after endovascular coiling of middle cerebral artery aneurysms: report on 30 treated aneurysms and review of the literature. Surg Neurol. 2006;66:167–71, discussion 71.
Doerfler A, Wanke I, Goericke SL, et al. Endovascular treatment of middle cerebral artery aneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol. 2006;27:513–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miley, J.T., Taylor, R.A., Janardhan, V. et al. The Value of Computed Tomography Angiography in Determining Treatment Allocation for Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 9, 300–306 (2008). https://doi.org/10.1007/s12028-008-9109-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9109-4