Abstract
In rheumatoid arthritis (RA), immune homeostasis is maintained by T regulatory cells (Tregs) that in an inflammatory milieu can change towards T-helper-like phenotypes (Th-like Tregs). Our aim was to examine the phenotypic and functional characteristics of CD4+CD25+CD127lo/− Tregs, Th-like Tregs and T effector (Teff) cells in the peripheral blood (PB) and synovial fluid (SF) of treatment-naïve early RA, as compared to osteoarthritis (OA) and healthy control (HC) peripheral blood. Frequencies of Tregs, CXCR3, CCR6 expressing Tregs (Th-like Tregs), and Teff cells were analyzed using flow cytometry in RA (n = 80), OA (n = 20), and HC (n = 40). Cytokine concentrations of the respective T cell subsets in plasma and SF were measured using flow cytometric bead array. Tregs sorted from RA and HC PB using magnetic beads were analyzed for functional capacities by CFSE proliferation assay and FOXP3 gene expression using real-time PCR. We observed that the frequencies of Th17 cells in PB and SF were significantly higher in RA when compared to HC, whereas Tregs were lower in PB and high in SF compared to HC and OA respectively. Th1- and Th17-related pro-inflammatory cytokines IL12p70, INF-γ, TNF-α, and IL-6, and IL-17A were significantly higher in the plasma and SF of RA. Tregs expressing CXCR3 (Th1-like Tregs) and CCR6 (Th17-like Treg) were significantly higher in PB and SF of RA compared to controls and was positively associated with seropositivity and disease activity. Treg cells isolated from peripheral blood of RA showed decreased function and reduced FOXP3 gene expression compared to HC. In our study, we have demonstrated higher frequencies of Th1 and Th17 cells and increased circulatory and SF pro-inflammatory cytokines (IL12P70, INF-γ, IL-6, IL-17A, and TNF-α) in RA. This inflammatory milieu might alter total Tregs frequencies and influence conversion of Tregs into Th-like Tregs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disorder characterized by persistent synovitis and progressive destruction of the small and large joints accompanied by functional disability [1]. CD4 + T-helper cells are major players in the initiation and progression of synovitis of RA [2,3,4]. The frequencies of T effector (Teff) Th1, and Th17 cells are increased in both the peripheral blood (PB) and synovial fluid (SF) [5, 6]. Although elevated frequency of T regulatory cells (Tregs) in RA SF has been reported, the frequencies of Treg in RA PB are conflicting [7, 8]. This discrepancy in reports may be due to the heterogeneity in the RA patients studied as well as markers used to define Treg subsets.
Classically, Tregs are defined as either CD4+CD25+ or CD4+CD25+FOXP3+, but it has been shown that conventional T cells (Tconv) also express a low level of FOXP3 upon TCR stimulation [9]. Liu et al. demonstrated that CD127 expression inversely correlates with FOXP3 and suppressive function of human CD4 + Treg cells; thus, CD127 can be an alternate marker to differentiate Tregs from the activated Tconv cells [10]. Therefore, CD3+CD4+CD25hiCD127lo/− can be used as a surrogate Treg phenotypic markers when isolating Tregs for in vitro culture or transcriptome investigations. In recent years, it has been observed that Tregs can develop into effector T-helper-like cells (Th-like Tregs) either Th1-like or Th17-like Tregs primarily producing interferon-γ (IFN-γ) and IL-17 respectively in normal human PB [11, 12] and in PB and intestinal tissue from Crohn’s disease [13]. Th17-like Tregs expressing CCR6 [14] retain suppressive function in some studies. For example, Beriou et al. found that IL-17+/FOXP3+ Treg clones in RA retain their suppressive function depending on the stimulus provided by their ability to either secrete IL-17 or suppress IL-17 [15]. Velmori et al. also demonstrated that suppressive function of Tregs can be retained or lost depending upon the stimulus given [16]. In contrast to findings of Th17-like Tregs in RA, the increased percentages of Th1-like Tregs and their reduced suppressive function in RA have been only recently demonstrated [17]. Dominguez-Villar et al. have shown that Tregs from healthy subjects when exposed to IL-12 acquire Th1-like phenotype with reduced suppressive function [18]. Similarly, McClymont et al. studied the plasticity of Tregs in patients with type 1 diabetes, wherein, upon in vitro stimulation of Tregs with IL-12, the Tregs become IFN gamma–producing cells (surrogate for Th1). However, there is still paucity of published data on Th1-like Tregs in RA [19].
There is evidence that in RA, T helper subset frequencies can be affected by disease-modifying anti-rheumatic drug (DMARD) therapy, disease activity, and duration of disease [20,21,22]. But studies on the frequencies of Tregs expressing CXCR3 and CCR6 and their functional capacity in treatment-naïve early RA are limited [20, 21]
So in this study, we evaluated the percentages of Teff cells, Tregs, and CXCR3- and CCR6-expressing Tregs and their cytokines in treatment-naïve female patients with early RA and compared them with osteoarthritis (OA) and healthy controls (HC). We also examined the functional capacities of peripheral Tregs isolated from RA and HC.
Methodology
Study participants
This was a cross-sectional study carried out in the Department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, between March 2016 and December 2019. Eighty treatment-naïve premenopausal female patients with active RA and a disease duration < 1 year were enrolled. Patients fulfilled the 2010 American College of Rheumatology (ACR)/European League of Associations for Rheumatology (EULAR) classification criteria for RA [23]. Demographic and baseline characteristics including age at enrolment and disease duration were collected. RA disease activity was assessed by trained rheumatologists. Tender and swollen joint count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and the disease activity score 28 joints (DAS28) were documented. Those patients with a DAS28 score of 3.2 to 5.1 were classified as having moderate disease activity (MDA), whereas high disease activity (HDA) was patients with DAS28 > 5.2. Forty age- and sex-matched healthy volunteers and 20 female patients with knee OA [24] were enrolled as disease controls. Written consent was obtained from all participants after a full explanation of the procedures. The study protocol was approved by the institutional ethics committee (JIPMER Protocol No. JIP/IEC/2014/10/483).
Sample collection and processing
At the time of enrollment, peripheral venous blood was collected from treatment-naïve RA, OA, and HC and processed immediately. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation method and used for flow cytometry analysis [25]. Plasma was separated by centrifugation at 1500 rpm for 15 min. SF was collected from RA patients with large joint involvement (knee > wrist > elbow) and patients with knee OA. Plasma and SF were stored at − 80 ºC until used for cytokine analysis using cytometry bead array (CBA).
Serological assays
Plasma samples obtained from RA patients were analyzed by nephelometry (BN ProSpec System, Siemens, Germany) for rheumatoid factor (RF) and highly sensitive C-reactive protein (hsCRP). RF values > 15.9 IU/ml were considered positive, and hsCRP > 3.02 mg/L were considered high (following manufacturer’s recommendations). Anti-cyclic citrullinated peptide antibodies (ACPA) were determined by ELISA (AESKU ELISA CCP kit, AESKU Diagnostics GmbH & Co. KG, Germany), and values > 25 U/ml were considered positive.
T-helper subsets (Th1, Th2, Th17, Treg, Th1-like Treg, and Th17-like Treg) analysis by flow cytometry
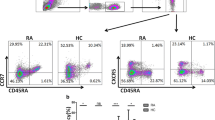
Single-cell suspension of cells was prepared from PBMCs and stained with the following antibodies: CD3-PerCP-Cy™5.5 (#340,949), CD4–APC (#340,672), CXCR3-PE (#557,185), CCR6-BB515 (#564,479), CRTH2-BV421 (#562,992), CD25-BB515 (#564,467), CD127-BV421 (#562,436), FOXP3-PE (#560,046) obtained from Becton, Dickinson and Company, NJ, USA. Samples were processed for phenotype and frequency using FACS Aria III flow cytometer. CD4+ T cells were gated from lymphocytes, then Th1, Th2, Th17, and Treg cells were gated from CD4+ cells. Th-like Tregs were gated from Treg cells. The various subsets were defined as following: Th1 cells as CD3+CD4+CXCR3+CCR6−, Th2 cells as CD3+CD4+CXCR3−CCR6−CRTH2+, Th17 cells as CD3+CD4+CXCR3−CCR6+, Treg cells as CD3+CD4+CD25hiCD127Lo/− [25]. As CD127 expression inversely correlates with FOXP3 [10, 26], only a subset of Treg cells was further assessed for FOXP3 intracellular expression using PE-FOXP3 and Human FOXP3 Buffer Set (Becton, Dickinson and Company, NJ, USA, 560,098) according to the manufacturer’s protocol. Treg cells were sub-phenotyped as Th1-like Treg: CD3+CD4+CD25hiCD127Lo/−CXCR3+, Th17-like Treg: CD3+CD4+CD25hiCD127Lo/−CCR6+ [17] (Supplementary Fig. 1). Fluorescent minus one (FMO) controls were used to gate the different subsets (Supplementary Fig. 2). Data were analyzed using FlowJo v 8.0.2 (Tristar, Palo Alto, CA, USA) and FCS express v 7 (De Novo Software, Pasadena, CA) software.
Quantification of cytokines using cytokine bead array
Cytokines (IL-4, IL-5, IL-6, IL-10, IL12p70, IL-17A, interferon gamma (IFN-γ), and tumor necrosis factor (TNF-α)) were measured in plasma of RA and HC and SF of RA and OA using a custom-designed CBA array (Becton, Dickinson and Company, NJ, USA, 551,811) and analyzed on a FACS Aria III flow cytometer. Standard curves were determined for each cytokine from a range of 1–5000 pg/mL. The quantity (pg/mL) of respective cytokine was calculated using FCAP Array™ Software Version 3.0 (Becton, Dickinson and Company, NJ, USA). Plasma of HCs and SF of OA was used as controls for RA plasma and SF respectively.
FOXP3 gene expression by real-time PCR
Total RNA was extracted from MACS (magnetic-activated cell sorting) sorted Tregs from PBMCs using Qiagen RNeasy mini kit (Qiagen, Hilden, Germany) and reverse transcribed using Takara PrimeScript RT Reagent kit (Takara Bio, Japan). The reaction was carried out at 42 °C for 45 min, followed by denaturation at 95 °C for 10 min and cooling at 4 °C. A SYBR green intercalation quantitative PCR (qPCR) was run in ABI StepOnePlus real-time PCR system (Applied Biosystems, Foster city, CA, USA) using FOXP3 primer pair (Forward primer: 5′-CAGCACATTCCCAGAGTTCCTC-3'; reverse primer: 5′-GCGTGTGAACCAGTGGTAGATC-3′). Thirty nanograms of cDNA was amplified in a 25-µl reaction containing 12.5 µl of 2 × SYBR green master mix and 2 µl of primer mix (2.5 μM). The following PCR cycling conditions were used: 95 °C for 10 min, 40 cycles at 95 °C for 15 s, followed by 60 °C for 30 s each and melt curve at 95 °C for 15 s, 60 °C for 20 s, and 95 °C for 15 s. All reactions were run in duplicates. Primers specific to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward primer: 5′-AGCCGAGCCACATCGCT-3′; reverse primer: 5′-CAGCCCTGGTGACCAGGC-3′) were used as an endogenous control, and FOXP3 transcript levels were normalized to GAPDH levels. Relative gene expression was calculated using 2−∆CT and was expressed as fold change compared to controls.
Assessment of Treg function by CFSE cell proliferation assay
Functional capacities of Tregs isolated from PB of RA and HC were measured using carboxyfluorescein succinimidyl ester (CFSE) proliferation assay (Becton, Dickinson and Company, NJ, USA, 565,082). Briefly, Tregs (CD4+CD25hiCD127lo/−) and Teff cells (CD4+CD25−) were sorted from PBMCs of HC and RA by using EasySep Human CD4+ CD127lo CD25+ Regulatory T Cell Isolation Kit (STEMCELL technologies, USA, no. 18063). The MACS-sorted Teff cells were resuspended in PBS + 0.1% BSA at concentration of 2–3 × 106 cells/ml and labeled with CFSE. The CFSE labeled Teff cell concentration was adjusted to 5 × 105 cells/ml with RPMI medium and co-cultured with Treg cells at 1:1 ratio for 72 h. The percentage of proliferating Teff cells was analyzed by measuring the mean intensity of CFSE using BD FACS Aria III flow cytometer, 8.0.2 (Tristar, Palo Alto, CA, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism v8.0.2. Normality of data was tested using Kolmogorov–Smirnov test. Categorical variables were expressed as n [percentage (%)] and continuous variables with a normal distribution as mean ± SD. From the total number of CD4 + cells, the percentage of Th1, Th2, Th17, and Treg cells was estimated. From the total number of Treg cells, the percentage of CXCR3 and CCR6-expressing Tregs was calculated. Percentage of different T cell subsets was represented as mean ± SD. The percentage of Th1, Th2, Th17, Tregs, Th1-like Tregs, and Th17-like Tregs was compared between RA, OA, and HC using one-way ANOVA (Tukey’s post hoc test) with 95% confidence interval or Kruskal–Wallis (Dunn’s post hoc test). The difference in ACPA + vs ACPA − and MDA vs HDA was compared using unpaired t test or Mann Whitney test depending on normality of data. Correlation between Th like Tregs and their respective cytokines was tested using Pearson’s correlation analysis; p < 0.05 was considered significant.
Results
Demographic, biochemical, and clinical characteristic of study participants
All the RA patients were women with a mean age of 36 years. Other baseline characteristics including disease activity [22], seropositivity for RF and ACPA, and hsCRP are summarized in Table 1.
Frequencies of CD4 + T cell subsets
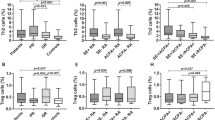
CD4+ T helper subtype frequencies were analyzed in PB and SF of RA and OA patients and PB of healthy controls. The frequency (mean ± SD) of Th1 in RA, OA, and HC PB was 26.65 ± 5.62, 25.95 ± 7.23, and 24.23 ± 5.67; Th2 was 5.10 ± 1.43, 5.137 ± 1.30, and 5.32 ± 1.1; Th17 was 14.05 ± 3.27, 10.87 ± 2.33, and 10.82 ± 2.20; Treg was 10.68 ± 2.48, 12.16 ± 1.99, and 13.23 ± 2.10 respectively.
In PB, the frequency of Th17 cells was higher in RA compared to OA and HC. On the other hand, Tregs were decreased in RA compared to HC and similar to OA. However, Th1 and Th2 frequency was similar across all groups (Fig. 1a).
Frequency of Th1, Th2, Th17, and Treg cells among CD4+ T-helper cells in peripheral blood (PB) and synovial fluid (SF) of patients with rheumatoid arthritis (RA) and osteoarthritis (OA) and in peripheral blood of healthy controls (HC). The frequencies of Th1, Th2, Th17, and Treg subsets among the CD4+ T helper cells in a PB of RA OA and HC, b SF of RA and OA, and c RA PB and SF; d in PB and e SF of RA based on ACPA status; f in PB and g SF of RA according to disease activity. The frequencies are represented as mean ± SD. The difference between RA, OA, and HC was compared using one-way ANOVA (Tukey’s post hoc test) for normal data or Kruskal–Wallis test (Dunn’s post hoc test) for non-normal data. The difference in ACPA + vs ACPA − and MDA vs HDA was compared using the unpaired t test or Mann–Whitney test depending on normality of data. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. RAPB, rheumatoid arthritis peripheral blood (n = 80); OAPB, osteoarthritis peripheral blood (n = 20); HCPB, healthy control peripheral blood (n = 40); RASF, rheumatoid arthritis synovial fluid (n = 20); OASF, osteoarthritis synovial fluid (n = 20); ACPA, anti-citrullinated peptide antibodies; MDA, moderate disease activity; HDA, high disease activity
Similarly, the frequency of Th1 in RA and OA SF was 35.03 ± 1.34, and 26.45 ± 1.87; Th2 was 16.29 ± 1.76 and 1.44 ± 0.10; Th17 was 21.27 ± 2.09 and 2.43 ± 0.25; Treg was 33.64 ± 2.15 and 33.45 ± 2.56. In RA SF, the frequencies of Th1, Th2, and Th17 cells were higher than in OA SF (Fig. 1b), whereas the frequency of Treg cell in RA and OA SF was comparable. The frequency of all T-helper subsets increased significantly in SF when compared to RA PB (Fig. 1c). There was no correlation in T helper cell subsets between RA PB and RA SF.
Furthermore, the frequencies of CD4+ T-helper subsets were compared between the RA patient subgroups based on ACPA status and disease activity. The frequency of Th1, Th2, Th17, and Treg in PB of ACPA + was 28.32 ± 5.04, 5.01 ± 1.46, 14.93 ± 2.78, and 10.79 ± 2.61, whereas in ACPA − they were 22.75 ± 5.04, 5.11 ± 1.40, 11.96 ± 3.43, and 10.43 ± 2.2. Comparison between ACPA + and ACPA − RA subgroups showed higher frequencies of Th1 and Th17 in ACPA + RA. However, there were no significant differences in Th2 and Treg frequency between ACPA + and ACPA − (Fig. 1d). In the SF, only Th17 cells were higher in ACPA + group (Fig. 1e). Similarly, in PB of RA with moderate disease activity (MDA), the Th1, Th2, Th17, and Treg percentages were 25.35 ± 6.24, 5.06 ± 1.37, 12.42 ± 3.09, and 10.08 ± 1.9 respectively. In RA with high disease activity (HDA), they were 27.52 ± 5.05, 5.13 ± 1.47, 15.27 ± 2.86, and 11.10 ± 2.73. Th1 and Th17 subset frequencies were higher in RA with HDA than MDA (Fig. 1f), while Tregs were not different. In SF, only Th17 cells were higher in RA with HDA compared to MDA (Fig. 1g).
Next, the differences in ratios of Th1/Treg and Th17/Treg were assessed and compared between the groups. Overall, ratio of Th1/Treg and Th17/Treg was significantly higher in PB and SF of RA as compared to OA and PB of HC (Supplementary Fig. 3a & b). Upon comparison between RA ACPA + and ACPA − subgroups, the Th1/Treg and Th17/Treg ratio was higher in ACPA + RA in PB and SF (Supplementary Fig. 3c & d). Similar comparison between RA MDA vs. HDA showed that there was increased Th1/Treg and Th17/Treg ratio in RA patients with HDA in PB, but in SF, only Th17/Treg ratio was increased in the HDA group (Supplementary Fig. 3e & f).
Th-like Treg frequencies among Treg in RA, OA, and HC
Furthermore, we sub-phenotyped Treg cells as CXCR3 expressing Treg (Th1-like Treg) and CCR6 expressing Treg (Th17- like Treg) (Supplementary Fig. 1). The percentage of Th1-like Tregs in RA PB and SF was 13.42 ± 1.17 and 39.35 ± 1.75, respectively, in OA PB, OA SF, and PB HC was 3.29 ± 0.5, 3.79 ± 0.11, and 0.20 ± 0.05, respectively. Similarly, the Th17-like Tregs in RA PB and SF were 6.77 ± 1.0 and 23.63 ± 1.45, respectively, and in OA PB, OA SF, and PB HC were 0.7 ± 0.11, 0.51 ± 0.10, and 0.11 ± 0.02, respectively. In PB and in SF, both Th1-like Treg and Th17-like Treg percentages were higher in RA compared to OA (Fig. 2a and b).
We further analyzed the distribution of these Th-like Tregs among various subgroups of RA patients. In PB of ACPA + RA, Th1-like Tregs and Th17 − like Tregs were 13.73 ± 0.92 and 7.03 ± 0.81 and in SF were 39.34 ± 1.71 and 23.64 ± 1.14. Similarly, in PB of ACPA − RA, Th1-like Tregs and Th17-like Tregs were 12.70 ± 1.14 and 6.17 ± 1.01 and in SF were 38.87 ± 1.70 and 23.62 ± 1.72. Percentage of Th1-like Tregs and Th17-like Tregs was higher in ACPA + RA compared to ACPA − RA in PB but not in SF (Fig. 2 c and d). In PB of RA with MDA, Th1-like Tregs and Th17-like Tregs were 12.93 ± 1.15 and 6.41 ± 0.89 and in SF were 39.58 ± 1.82 and 23.54 ± 1.45. In PB of HDA Th1-like Tregs and Th17-like Tregs were 13.74 ± 1.10 and 7.01 ± 1.87 and in SF were 39.01 ± 1.70 and 23.80 ± 1.47. The percentage of Th1-like Tregs and Th17- like Tregs was significantly higher in PB of RA with HDA, but the not in SF (Fig. 2 e and f). The percentage of Th1-like Tregs and Th17-like Tregs was significantly higher in RA SF compared to RA PB (Fig. 2g). Next, the relative percentage of Th-like Tregs among the Tregs was analyzed. The percentages were as follows: RA PB 20% (Th1-like Treg 13% and Th17-like Treg 7%) and SF 63% (Th1-like Treg 39% and Th17-like Treg 24%); patients with OA PB 3.6% (Th1-like Treg 3.2% and Th17-like Treg 0.6%) and SF 6.3% (Th1-like Treg 3.8% and Th17-like Treg 2.5%); HC PB was 0.5% (Th1-like Treg 0.3% and Th17-like Treg 0.2%) (Fig. 2h). Cumulative frequencies of Th-like Tregs are significantly higher in PB and SF of patients with RA compared to OA and HC (Fig. 2i).
Comparison of frequency of Th-like Tregs among Treg cells in peripheral blood (PB) and synovial fluid (SF) of rheumatoid arthritis (RA) and osteoarthritis (OA) and in PB of healthy controls (HC). Frequencies of Th1-like and Th17-like Treg among Treg subsets in a PB of RA, OA, and HC; b SF of RA and OA; and in c PB and d SF of ACPA-positive and ACPA-negative RA; in e PB and f SF of RA according to disease activity; g PB and SF of RA; h cumulative frequencies of Th-like Treg populations in PB of HC, RA, and OA and in SF of RA and OA; i bar diagram showing percentage of Th-like Treg populations in HCPB, RAPB, RASF, OAPB, and OASF. The frequencies were represented as mean ± SD. The Th1/Treg and Th17/Treg ratios were represented as median with interquartile range. The difference between RA, OA, and HC was compared using one-way ANOVA (Tukey’s post hoc test) or Kruskal–Wallis test (Dunn’s post hoc test). The difference in ACPA + vs ACPA − and MDA vs HDA was compared using unpaired t test or Mann–Whitney test depending on normality of data *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. RAPB, rheumatoid arthritis peripheral blood (n = 80); OAPB, osteoarthritis peripheral blood (n = 20); HCPB, healthy control peripheral blood (n = 40); RASF, rheumatoid arthritis synovial fluid (n = 20); OASF, osteoarthritis synovial fluid (n = 20). ACPA, anti-citrullinated peptide antibodies; MDA, moderate disease activity; HDA, high disease activity
Circulating cytokine levels
We quantified eight different cytokines in plasma of RA and HC and SF of RA and OA patients. As expected in PB, the levels of pro-inflammatory cytokines IL-12p70, IL-17A (Th17), TNF-α (Th1), IFN-γ, and IL-6 were significantly higher in RA whereas anti-inflammatory cytokines IL-4, IL-5 (Th2), and IL-10 (Treg) were not different compared to HC (Fig. 3a). In contrast, all of the cytokines were elevated in RA SF compared to OA SF (Fig. 3b). Likewise, all the cytokines were higher in RA SF when compared to their levels corresponding to PB (Fig. 3c). There was no correlation in the cytokine levels between RA PB and RA SF.
Cytokine levels in rheumatoid arthritis (RA) peripheral blood (PB) plasma and synovial fluid (SF), in osteoarthritis (OA) SF and healthy control (HC) plasma. IL12p70, IL-17A, TNF-α, IFN-γ, IL-6, IL-4, IL-5, and IL-10 were measured by cytokine bead array in HCPB (n = 20), RAPB (n = 20), RASF (n = 20), and OASF (n = 20). a Comparison between HCPB and RAPB. b Comparison between RASF and OASF. c Comparison between RAPB and RASF. Values are expressed as median ± interquartile range; the differences between two groups RAPB vs HCPB, RASF vs OASF, and RAPB vs RASF were assessed by the Mann–Whitney test; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 considered as significant. RAPB, rheumatoid arthritis peripheral blood (n = 20); HCPB, healthy control peripheral blood (n = 20); RASF, rheumatoid arthritis synovial fluid (n = 20); OASF, osteoarthritis synovial fluid (n = 20)
Correlation between Th-like Tregs, Teff, and inflammatory cytokines
Next, we performed correlation analysis between frequencies of Th-like Tregs and Teff and inflammatory cytokines. The percentages of Th1-like Tregs and Th17-like Treg in both PB and SF showed positive correlation with Th1 and Th17 frequency respectively (Fig. 4 a and b). Similarly, PB and SF Th1-like Tregs showed a positive correlation with IL-12p70 and INF-γ, and Th17-like Treg showed positive correlation with IL-6 and IL-17A (Fig. 4c and 4d). However, there was no correlation between Th2 and its corresponding cytokines.
Correlation between Th-like Treg and Teff cells and cytokines in RA peripheral blood (PB) and synovial fluid (SF). Correlation of Th1 with Th1-like Treg and Th17 with Th17-like Treg in a RA peripheral blood and b RA synovial fluid. Correlation of Th1-like Treg with and IL-12p70, INF-γ, Th17-like Treg with IL-6 and IL-17A in c RA peripheral blood and d RA synovial fluid. The correlation between the parameters was assessed using Pearson’s correlation coefficient. p < 0.05 is considered as significant. RAPB, rheumatoid arthritis peripheral blood (n = 20); RASF, rheumatoid arthritis synovial fluid (n = 20)
FOXP3 mRNA expression and suppression function of peripheral Treg cells
We further investigated FOXP3 gene expression and the function of MACS-sorted CD3+CD4+CD25hiCD127lo/− Tregs from PB of RA and HC. Suppression assay was carried out by co-culturing autologous CFSE-labeled Teff and Treg cells in vitro. Average data from five separate experiments were used to calculate the suppression capacity and were represented as a percentage of suppression. The percentage proliferation of autologous Teff cells was higher in RA compared to HC (33.4% vs 10.4%) in the presence of their respective Tregs (Fig. 5a). RA Tregs showed a significantly lower percentage of suppression of autologous Teff cell proliferation when compared to the HC Treg (56.88% vs 86.33%, p = 0.0005) (Fig. 5b). Relative FOXP3 gene expression was significantly lower in Treg cells isolated from RA PB compared to Treg from HC PB (p = 0.005) (Fig. 5c).
mRNA expression level of FOXP3 and functional assessment of Treg cells from peripheral blood of healthy controls (HC) and rheumatoid arthritis (RA). a Functional capacities of Tregs isolated from peripheral blood of HC and RA were assessed using CFSE proliferation assay. CFSE-labeled autologous Teff cells were stimulated with CD3/CD28 beads and cultured with RA or HC Tregs from PB (pTregs) in a ratio of 1:1 (Teff to Treg) for 72 h. The percentage (%) proliferation of Teff cells was determined by flow cytometry. b Treg suppression capacity calculated using averaged data from five independent experiments expressed as % suppression. The % suppression was calculated as follows: 100 − (% of proliferating Teff cells alone / % of proliferating Teff in the presence of Tregs) × 100. c FOXP3 mRNA expression was analyzed in RA and HC pTreg using the 2.−∆Ct method. Fold change in gene expression was compared between RA and HC. ***p < 0.001; ****p < 0.0001 considered as significant. Treg, T regulatory cells; Teff, T effector cells; RA-pTreg, Treg cells isolated from RA peripheral blood (n = 5); HC-pTreg, Treg cells isolated from HC peripheral blood (n = 5)
Discussion
In this study, we found that the frequencies of Th17 and their corresponding cytokines were higher in RA PB compared to HC and OA PB; it was also higher in RA SF compared to OA SF. The frequencies of Tregs were diminished in RA PB compared to HC PB but similar in RA SF compared to OA SF. Nevertheless, all the cell frequencies were higher in RA SF compared to RA PB. The percentage of Tregs expressing CXCR3 and CCR6 was significantly high in both the PB and SF of RA compared to HC PB and OA SF respectively. Also, the ability of Tregs from RA PB to suppress autologous Teff cell proliferation was reduced as compared to HC PB Tregs.
T cells and fibroblast-like synoviocytes (FLS), two important cell types in the RA joint, interact either directly or indirectly to cause irreversible joint damage. In addition, these cells also interact with other immune as well as non-immune cells present in the joint to promote damage [27]. Although the frequency of these cells determines the overall inflammation and joint damage, the molecular mechanisms underlying their interactions are not known. Data on Treg cell frequencies in RA is inconclusive with some studies reporting increased [28], decreased [20], or similar Treg cell frequencies [29] in RA. Such dysregulation or a tilt in balance of Tregs might be either by the loss or hampered suppressive function of Tregs or by the autoantibody-mediated overwhelming inflammatory processes [30].
We observed higher frequencies of Th17 cells and lower Tregs compared to HC in the PB. Similarly compared to OA, Th17 cells were higher, and Tregs were similar in RA emphasizing the role of Th17 in RA. Our findings appear similar to van Hamburg et al., who also had reported increased Th17 cells in PB of treatment-naïve early RA, and these cells when co-cultured with RA synovial fibroblasts produced matrix metalloproteinases that are capable of joint erosion [31]. However, those who have studied treatment-experienced RA patients have found either lower frequencies of Th17 [32] or similar frequencies when compared to inflammatory RA [29]. Penatti et al. reported lower frequencies of circulating Th17 cells in RA PB when compared to inflammatory OA patients [25]. More recently, Paradowska-Gorycka et al. observed a similar percentages of Th17 cells in peripheral blood of RA and OA [33]. These conflicting results on the frequencies of Th17 cells in RA might be due to different patient population and different level of disease activity and treatments used.
With regard to Tregs, Paradowska-Gorycka et al. had reported lower percentage of Tregs in RA compared to OA, whereas we observed that Treg (CD4+CD25hiCD127lo/−) frequencies between RA and OA were comparable [25, 33]. Similar to our report, Moradi et al. reported comparable frequencies of Tregs (CD4+CD25+/highCD127low/−) between RA and OA in PB [34], and Kawashiri et al. in PB reported similar frequency of Tregs (CD4+CD25+CD127low/−) in DMARD-naïve RA but a lower frequency in active RA on treatment compared to controls. But when Kawashiri et al. gated the Tregs for CD25 high, a lower frequency of Tregs (CD4+CD25highCD127low/−) was observed in DMARD-naïve RA as well as in active RA on treatment compared to controls [35]. One of the reasons for this variation might have been related to markers used to identify Tregs, as some groups use CD127− while others use CD127low as a Treg marker highlighting the importance of using homogenous cell surface markers to identify a specific Treg cell population. In our study, we also have taken CD127lo and CD127− as one population as has been done by other groups as it appears to be more specific Tregs [25, 26, 34, 35].
Considering the contradictory results of Th17 and Treg cells, we evaluated Th17/Treg ratio. In line with the report by Wang et al., we also found an increased ratio of Th17/Treg (when compared to HC), thus suggesting that in treatment-naïve active RA, there could be excess of Th17 and reduced Tregs cells in circulation indicating a broken balance in Th17/Treg [36]. These altered Th17/Tregs in active RA have been demonstrated previously, and Tregs in an inflammatory environment have been shown to exhibit Th17-like characteristics and plasticity. In fact, treatment with TNF inhibitors has been shown to restore Treg function in RA [21, 29].
Likewise, in the RA-SF, we observed elevated frequencies of all the effector T cell subsets (Th1, Th2, and Th17) but a similar frequency of Tregs on comparison with OA-SF. In line with our findings, Moradi et al. also reported that the Tregs were significantly enriched in SF compared to PB in RA, and Tregs were found with almost similar frequency in the synovial compartments of both RA and OA. They also found that Tregs were higher especially in the synovial membrane rather than SF, thus postulating that RA pathophysiology may not relate to only a Treg deficiency per se [34]. Supporting this hypothesis, Penatti et al. found that Tregs were significantly increased in RA in both the synovial compartment and the peripheral blood as compared to inflammatory OA [25].
Tregs are conventionally non-inflammatory (rather they have suppressive properties), but in an inflammatory milieu, they may expand into Teff cells and become pro-inflammatory. They can be identified by the expression of CXCR3 (Th1-like Treg) and CCR6 (Th17-like Treg) chemokine receptors [17, 37, 38]. CXCR3+ and CCR6+ Tregs can actively migrate to synovial joint or can be produced locally [39,40,41] to aggravate tissue damage by promoting inflammatory cytokine/chemokine production [5, 42]. Data concerning the frequency and the plasticity of Tregs in RA are controversial partly because of different cell surface markers used by different groups to characterize Tregs. Besides this, the patient population studied has also been heterogeneous in terms of their disease activity and treatments [21, 43]. So, in our study, we attempted to study the frequency of these CXCR3 (Th1-like) and CCR6 (Th17-like) expressing Tregs in this homogenous group of treatment-naïve, early RA patients, and we also used widely accepted phenotypic markers to characterize Treg subsets. What we found was an increased frequency of these CXCR3 expressing Th1-like and CCR6-expressing Th17-like cells in both the RA-PB and RA-SF compared to HC-PB and OA-SF respectively. In RA PB, Zhang et al. observed increased number and proportion of both Th1-like and Th17-like Tregs compared to HC PB, whereas in RA SF, only Th1-like cells were higher compared to both RA and HC PB. Additionally, they explored the functional capacity of Th1-like Tregs and found lower suppression capacity of the same. There was also negative correlation between proportion of Th1-like Tregs (in SF) and disease activity in RA. Importantly, not all their RA patients were treatment naïve [17].
While quantifying the T cell subset–specific cytokines in the circulation, we observed elevated levels of only Th1 and Th17 subset cytokines in RA compared to controls, but all the measured cytokines were elevated in RA synovial fluid compared to the RA periphery. Our current results in periphery are different from our earlier findings where we reported an elevation in all the T-cell subset–specific cytokines in RA. This could be due to inclusion of RA patients with established disease on different treatments in the previous study [44]. Additionally, this might point that Th1 and Th17 are the predominant cellular subsets in the periphery in treatment-naïve early RA, but as the disease progresses, all the cellular subsets might be involved in RA pathogenesis. Although the involvement of all cellular subsets is reflected in this study by their elevated cytokines in SF, it is not mirrored immediately in the periphery. Raza et al. reported increased pro-inflammatory cytokines in synovial fluid of early RA vs OA suggesting that early synovitis bound to develop into RA has a distinct cytokine profile [45]. We also noted that cytokines corresponding to all the T cell subsets were elevated in the synovial compartment of RA compared to OA suggesting a distinct inflammatory process in early RA.
A positive correlation observed between Th-like Treg (CXCR3+ Tregs and CCR6+ Tregs) proportion and Th1 and Th17 T cell subset–specific cytokines in both PB and SF suggests an enhanced Treg cell plasticity in the presence of pro-inflammatory cytokines. The likely implication of our findings could be an initial selective expansion of pro-inflammatory T cell subsets and their cytokines in the circulation which in turn might have facilitated their active migration and accumulation in the joint contributing to cellular as well as cytokine imbalance in the joint.
In RA presence of RF and ACPA (seropositive RA) is associated with joint damage and extra-articular manifestations [46, 47]. The relationship of Teff subsets with seropositive status and disease activity status has been reported variably. For example, Alvandpur et al. found that that ACPA + patients have an increased frequency of IFN-γ-producing Th1 and IL-17A-producing Th17 cells compared to ACPA negative [44]. With respect to disease activity, Kawashiri et al. found that in the circulation, the lowest number of Tregs was in those with active RA, while the number of Tregs of RA who were in remission was equal to that in controls [35]. Our analysis based on ACPA status and disease activity showed increased Th1 and Th17 cells in ACPA positive and highly active disease groups while the Treg frequencies remained unaffected by ACPA status and disease activity in the periphery. Our observation on Tregs contradicts that of Kawashiri et al. and may be attributable to the treatment or the heterogeneous patient population they included. However, in the synovial fluid (our study), only the frequency of Th17 cells was increased in ACPA-positive and high-disease subgroups. These findings indicate that Th1 and Th17 phenotypes might be selectively amplified in the periphery by ACPA antibodies and high disease but only Th17 cells dominate at the site of inflammation reiterating that periphery does not mirror synovial milieu in early RA. Likewise, the frequency of Th-like Tregs (CXCR3+ Tregs and CCR6+ Tregs) was higher in ACPA-positive and high-disease groups in the peripheral blood but was similar in SF suggesting that antibody and disease activity–dependent Treg plasticity could be initiated mainly in the periphery, and these cells may later be home to SF to exacerbate the disease.
Previous reports state that during the autoinflammatory processes, Tregs lose their FOXP3 expression and become effector T cells affecting their stability and ability to suppress effector T cells [43, 48]. Our in vitro experiments performed to check this revealed that RA Tregs had significantly reduced FOXP3 gene expression and inhibitory function compared to Tregs from HC. The plausible explanation could be that Treg plasticity initiated in periphery might promote homing of Th-like Tregs to the synovium to encounter the already overwhelming inflammatory process there and consequently either tilt the Treg balance by promoting further expansion of Th-like Tregs or by reducing their inhibitory function.
Further research on contribution of synovial inflammatory milieu on Treg cell conversion to effector T cell types, loss of function, and associated transcriptional regulation might shed light on the plasticity of Treg cells in RA.
Limitation of our study is that T-helper subsets and Th-like Treg cells were defined solely by the expression of surface markers, while intracellular cytokine production was not examined. We have also not classified Tregs as resting Treg (rTreg) cells, activated Treg (aTreg) cells, and cytokine-secreting unsupported T (non-Treg) cells based on CD45RA positivity and FOXP3 expression (MFI); as a result, the Treg fraction could have cytokine-secreting unsupported T (non-Treg) cells. The function of sorted conventional Treg was examined in the in vitro analysis, but the functional potential of Th1-like Treg and that of Th17-like Treg was not evaluated, which would have provided evidence for contribution of these cells to the functional loss of Tregs. Furthermore, the disease control group was OA patients who were all older than 50 years. Knowing that T cell subsets can vary with age, the disease control we used was less than ideal [49].
Conclusion
To conclude, we observed that in women with treatment-naïve early RA, effector and regulatory T cell frequencies and their proportions were altered. Both the Tregs and “Th1-like and Th17-like Tregs” were higher in RA SF irrespective of their ACPA status and disease activity. From these data and previous literature, we think that Th1 and Th17 cells plus the cytokines have created an inflammatory environment for polarization of Tregs to CXCR3 + and CCR6 + Tregs (Th1- and Th17-like phenotypes) leading to propagation of inflammation in the joint cavity. Though we have not studied the suppressive function of Th-like Tregs, we assume that their function is defective as reported by others [17]. Our study is of a cross-sectional nature in treatment-naïve population; we feel that a longitudinal study would be important to see for T cell dynamics with treatment. Moreover, studies to understand the mechanisms of functional compromise of Tregs in RA and the possibility of restoring its function are needed.
Data Availability
Data and materials are available upon request.
References
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. https://doi.org/10.1038/nature01661.
Morita Y, Yamamura M, Kawashima M, et al. Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1669–76. https://doi.org/10.1002/1529-0131(199809)41:9%3c1669:AID-ART19%3e3.0.CO;2-G.
Kusaba M, Honda J, Fukuda T, Oizumi K. Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1998;25:1466–71.
James EA, Rieck M, Pieper J, et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 2014;66:1712–22. https://doi.org/10.1002/art.38637.
Aldridge J, Ekwall A-KH, Mark L, et al. T helper cells in synovial fluid of patients with rheumatoid arthritis primarily have a Th1 and a CXCR3+Th2 phenotype. Arthritis Res Ther. 2020;22:245. https://doi.org/10.1186/s13075-020-02349-y.
Henriques A, Gomes V, Duarte C, et al. Distribution and functional plasticity of peripheral blood Th(c)17 and Th(c)1 in rheumatoid arthritis. Rheumatol Int. 2013;33:2093–9. https://doi.org/10.1007/s00296-013-2703-6.
van Amelsfort JMR, Jacobs KMG, Bijlsma JWJ, et al. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. https://doi.org/10.1002/art.20499.
Cao D, van Vollenhoven R, Klareskog L, et al. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335-346. https://doi.org/10.1186/ar1192.
Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. https://doi.org/10.1093/intimm/dxm014.
Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. https://doi.org/10.1084/jem.20060772.
Koenen HJPM, Smeets RL, Vink PM, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. https://doi.org/10.1182/blood-2008-01-133967.
Voo KS, Wang Y-H, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–8. https://doi.org/10.1073/pnas.0900408106.
Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–65. https://doi.org/10.1053/j.gastro.2010.12.002.
Duhen T, Duhen R, Lanzavecchia A, et al. Functionally distinct subsets of human FOXP3+ treg cells that phenotypically mirror effector Th cells (Blood (2012) 119, 19 (4430–4440)). Blood. 2012;119:4430–40. https://doi.org/10.1182/blood-2011-11-392324.
Beriou G, Costantino CM, Ashley CW, et al. IL-17–producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. https://doi.org/10.1182/blood-2008-10-183251.
Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORγt+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010;107:19402–7. https://doi.org/10.1073/pnas.1008247107.
Zhang R, Miao J, Zhang K, et al. Th1-like Treg cells are increased but deficient in function in rheumatoid arthritis. Front Immunol. 2022;13:863753. https://doi.org/10.3389/fimmu.2022.863753.
Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–5. https://doi.org/10.1038/nm.2389.
McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918–26. https://doi.org/10.4049/jimmunol.1003099.
Lawson CA, Brown AK, Bejarano V, et al. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford). 2006;45:1210–7. https://doi.org/10.1093/rheumatology/kel089.
Lina C, Conghua W, Nan L, Ping Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:596–605. https://doi.org/10.1007/s10875-011-9542-6.
Aldridge J, Pandya JM, Meurs L, et al. Sex-based differences in association between circulating T cell subsets and disease activity in untreated early rheumatoid arthritis patients. Arthritis Res Ther. 2018;20:150. https://doi.org/10.1186/s13075-018-1648-2.
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. https://doi.org/10.1002/art.27584.
Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–9. https://doi.org/10.1136/ard.2009.113100.
Penatti A, Facciotti F, De Matteis R, et al. Differences in serum and synovial CD4+ T cells and cytokine profiles to stratify patients with inflammatory osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2017;19:103. https://doi.org/10.1186/s13075-017-1305-1.
Yu N, Li X, Song W, et al. CD4(+)CD25 (+)CD127 (low/-) T cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35:1773–80. https://doi.org/10.1007/s10753-012-9496-8.
Tu J, Huang W, Zhang W, et al. Two main cellular components in rheumatoid arthritis: communication between T cells and fibroblast-like synoviocytes in the Joint Synovium. Front Immunol. 2022;13:922111. https://doi.org/10.3389/fimmu.2022.922111.
Furuzawa-Carballeda J, Lima G, Jakez-Ocampo J, Llorente L. Indoleamine 2,3-dioxygenase-expressing peripheral cells in rheumatoid arthritis and systemic lupus erythematosus: a cross-sectional study. Eur J Clin Invest. 2011;41:1037–46. https://doi.org/10.1111/j.1365-2362.2011.02491.x.
Nie H, Zheng Y, Li R, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19:322–8. https://doi.org/10.1038/nm.3085.
Qiu R, Zhou L, Ma Y, et al. Regulatory T cell plasticity and stability and autoimmune diseases. Clin Rev Allergy Immunol. 2020;1:52–70.
van Hamburg JP, Asmawidjaja PS, Davelaar N, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. https://doi.org/10.1002/art.30093.
Wang T, Sun X, Zhao J, et al. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis. 2015;74:1293–301. https://doi.org/10.1136/annrheumdis-2013-204228.
Paradowska-Gorycka A, Wajda A, Romanowska-Próchnicka K, et al. Th17/Treg-related transcriptional factor expression and cytokine profile in patients with rheumatoid arthritis. Front Immunol. 2020;11:572858. https://doi.org/10.3389/fimmu.2020.572858.
Moradi B, Schnatzer P, Hagmann S, et al. CD4+CD25+/highCD127low/- regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints—analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther. 2014;16:R97. https://doi.org/10.1186/ar4545.
Kawashiri S-Y, Kawakami A, Okada A, et al. CD4+CD25(high)CD127(low/-) Treg cell frequency from peripheral blood correlates with disease activity in patients with rheumatoid arthritis. J Rheumatol. 2011;38:2517–21. https://doi.org/10.3899/jrheum.110283.
Wang W, Shao S, Jiao Z, et al. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int. 2012;32:887–93. https://doi.org/10.1007/s00296-010-1710-0.
Gao N, Cui W, Zhao LM, et al. Contribution of Th2-like Treg cells to the pathogenesis of Takayasu’s arteritis. Clin Exp Rheumatol. 2020;38(Suppl 124):48–54.
Li N, Wei W, Yin F, et al. The abnormal expression of CCR4 and CCR6 on Tregs in rheumatoid arthritis. Int J Clin Exp Med. 2015;8:15043–53.
Kim CH. Migration and function of Th17 cells. Inflamm Allergy Drug Targets. 2009;3:221–8.
Nevius E, Gomes AC, Pereira JP. A comprehensive review of inflammatory cell migration in rheumatoid arthritis. Clin Rev Allergy Immunol. 2016;51:59–78. https://doi.org/10.1007/s12016-015-8520-9.
Mellado M, Martínez-Muñoz L, Cascio G, et al. T cell migration in rheumatoid arthritis. Front Immunol. 2015;6:384. https://doi.org/10.3389/fimmu.2015.00384.
Paulissen SMJ, van Hamburg JP, Dankers W, Lubberts E. The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine. 2015;74:43–53. https://doi.org/10.1016/j.cyto.2015.02.002.
Jiang Q, Yang G, Liu Q, et al. Function and role of regulatory T cells in rheumatoid arthritis. Front Immunol. 2021;12:1021. https://doi.org/10.3389/fimmu.2021.626193.
Alvandpur N, Tabatabaei R, Tahamoli-Roudsari A, et al. Circulating IFN-γ producing CD4+ T cells and IL-17A producing CD4+ T cells, HLA-shared epitope and ACPA may characterize the clinical response to therapy in rheumatoid arthritis patients. Hum Immunol. 2020;81:228–36. https://doi.org/10.1016/j.humimm.2020.02.008.
Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–95. https://doi.org/10.1186/ar1733.
Lindqvist E, Eberhardt K, Bendtzen K, et al. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2005;64:196–201. https://doi.org/10.1136/ard.2003.019992.
Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Investig. 2006;116:961–73. https://doi.org/10.1172/JCI25422
Liu Z, Gu J, Qin Z, et al. Decreased Foxp3 and function of Tregs caused immune imbalance and liver injury in patients with autoimmune liver diseases post-liver transplantation. Ann Transl Med. 2020;8:534–534. https://doi.org/10.21037/atm.2020.03.203.
Jj G, F F, Mm C, et al (2015) Naive T cell maintenance and function in human aging. Journal of immunology (Baltimore, Md : 1950) 194 https://doi.org/10.4049/jimmunol.1500046
Funding
The work was supported by Department of Science and Technology-Science and Engineering Research Board (DST-SERB), India (SERB/F/0253/2016–17), and JIPMER Intramural Research Fund (JIP/Res/Intra-PhD/01/2014–15 and JIP/Res/Intra-PhD/O2/2015–16).
Author information
Authors and Affiliations
Contributions
VSN, KV, and CMM contributed to the conception and the design of the study. VSN and CKG recruited patients and collected clinical data. KV performed experiments, analyzed the data, and drafted the manuscript. SNB was involved in experiments and statistical analyses. MTT, CMM, CKG, and PBN contributed to interpretation of the data, carefully read the article, and suggested edits. VSN, CMM, and MTT critically revised the article for important intellectual content. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the JIPMER institute ethics committee and conducted the following Principles of the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. Protocol No. JIP/IEC/2014/10/483 dated 30.01.2015.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kommoju, V., Mariaselvam, C.M., Bulusu, S.N. et al. Conventional Tregs in treatment-naïve rheumatoid arthritis are deficient in suppressive function with an increase in percentage of CXCR3 and CCR6 expressing Tregs. Immunol Res 72, 396–408 (2024). https://doi.org/10.1007/s12026-023-09444-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-023-09444-7