Abstract
The stimulatory natural killer group 2 member D (NKG2D) lymphocyte receptor, initially discovered and expressed mostly on natural killer (NK) cells, T cells and natural killer T cells, can promote tumor immune surveillance. However, with increasing tumor grade, tumors themselves express NKG2D to self-stimulate oncogenic pathways. To confirm that cancer cells themselves express NKG2D, we have now investigated the role of the tumoral NKG2D in NK cell-mediated immune surveillance. Both anti-NKG2D and shRNA to that down-regulated tumoral NKG2D increased the number of cells in G1 phase and S phase, increased the expression of cyclin E–CDK2 and decreased P21. In addition, CD107a, IFN-γ and TNF-α increased when the cells were treated with anti-NKG2D which suggests that blocking tumoral NKG2D could augment tumor surveillance of NK cells. Altogether, tumoral NKG2D stimulates cell propagation and immune escape in acute myeloid leukemia cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural killer (NK) cells are key players in the immune surveillance of acute myeloid leukemia (AML). However, the majority of AML patients have decreased NK-mediated killing resulting in immune suppression. There are three potential mechanisms by which AML can be protected from NK-mediated immune surveillance: NK cell abnormalities, alterations in the AML cells which enable them to escape the NK surveillance [1]. Specially, the natural killer group 2 member D (NKG2D) and its ligands which are important immunotherapeutic proteins known to mediate tumor immunity have also been implicated in tumor proliferation, evasion and immune suppression [2–6].
NKG2D is a type II transmembrane-anchored lectin-like receptor expressed mostly on NK cell, T cells and natural killer T (NKT) cells. It interacts with the signaling adapter molecule DNAX-activating protein 10 (DAP10) in humans and with DAP10 and DAP12, in mice [7]. When NKG2D is ligated, DAP10 provides signals that lead to the recruitment of the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and a complex of GRB2. This results in the activation of protein kinase B (PKB/AKT) and mitogen-activated protein kinase (MAPK) signaling cascades [4, 8, 9]. NKG2D ligands in humans include MICA and MICB (MHC class I chain-related proteins A and B), which are encoded by genes in the MHC, and six structurally diverse proteins known as UL16-binding proteins (ULBP1–ULBP6) [1, 10]. NKG2D ligands are prominently expressed in malignancies as a result of induction of their transcription through cellular stress responses. Tumor cells of all types express MICA/MICB and at least one ligand from the ULBP family [11]. The tumor cells are therefore recognized by the immune system through the NKG2D ligands. Thus, the NKG2D receptor–ligand system provides a means for immune cells to recognize tumor cells and the tumor microenvironment resulting in cell-mediated cytotoxicity of the tumor cells. However, in a conceptual twist, it has been observed that a functional NKG2D–DAP10 complex is expressed on surface of certain tumor cells [5]. Unlike the NKG2D–DAP10 complex on effector cells, the NKG2D–DAP10 complex on tumor cells has been implicated in the growth of the tumor cell. Thus, primary breast and epithelial ovarian cancer specimens express the NKG2D receptor to competitively engage its ligands to self-stimulate oncogenic signaling pathway that can promote tumor angiogenesis and metastasis. Using RNAi-mediated targeting of NKG2D in orthotopic MCF-7 xenotransplant breast cancer models in mice, it was shown that tumoral NKG2D promoted tumor initiation [5]. Tumoral NKG2D was also found to promote metastatic disease [12] and activate oncogenic signaling cascades [4]. However, whether the expression of tumoral NKG2D affects cell cycle activity and tumor immunosurveillance in hematopoietic malignancies, such as AML, [13] remains unknown.
This study now addresses these questions through investigating whether expression of NKG2D–DAP10 in AML cells has tumor promoting ability in addition to its role in immune surveillance or immune suppression. The answer could provide in-depth understanding of immune escape in AML and a new therapeutic approach to targeting NKG2D-expressing tumor cells.
Materials and methods
Cell lines and cell culture
Tumor lines U937, THP-1, K562, MD-MB-231, HCT116 preserved in our laboratory were cultured in RPMI 1640 medium or DMEM medium, containing 10 % (v/v) fetal bovine serum (FBS), penicillin (100 units/mL) and streptomycin (100 units/mL); human embryonic kidney cell line HEK293 and HEK293T preserved in our laboratory were grown in DMEM medium (high glucose), supplemented with 10 % (v/v) FBS. Cell culture media, supplements and trypsin powder were purchased from Life technologies (Basel, Switzerland).
RNA silencing and plasmid construction
Specific NKG2D (GenBank accession number AJ001687.1) shRNA (5′GGATCCCGGATGGGACTAGTACACATTCTTCAAGAGAGAATGTGTACTAGTCCCATCCTTTTTTCCAAGAATTC3′) sequences were designed, produced and cloned into pLVX-shRNA (AxyBio, China) vector. The plasmids were transfected into HEK293T by Lipofectamine 2000 (Invitrogen, USA) according to manufacturer’s instructions. Lentiviral plasmid supernatants were then collected after 48-h incubation at 37 °C. Subsequently, U937 and THP-1 were transfected with pLVX-shRNA-NKG2D by Lipofectamine 2000 and incubated at 37 °C for 72 h.
Flow cytometry and cell cycle analysis
Tumor cell lines were incubated with anti-MICA (MBL, Japan) or anti-NKG2D antibody (SAB, USA) for 1 h and then incubated with goat anti-rabbit IgG, FITC-conjugated (SAB, USA) or goat anti-mouse IgG, FITC (SAB, USA).Cell cycle analysis was conducted by determinating DNA content as described [6]. Briefly, U937 and THP-1 (1 × 106) were treated with 0, 5 or 40 nM of anti-NKG2D. After a 24-h incubation at 37 °C, cells were further treated with pLVX-shRNA-NKG2D for 48 h and subsequently cell cycle and apoptosis analysis (Beyotime Biotechnology, China). All of the assays were performed using a BD FACS flow cytometer, and the number of cells in cell cycle was calculated using the ModFit software.
Real-time quantitative PCR
U937 or THP-1 cells pretreated with 0, 2.5,10 or 40 nM of anti-NKG2D at 37 °C for 24 h were transfected with pLVX-shRNA-NKG2D and further incubated at 37 °C for 48 h. Prior to real-time quantitative PCR (RT-qPCR), total RNAs were extracted and reverse-transcribed using Trizol reagent and RT kit (Sangon, China). Primers are designed as follows: Cyclin E (GenBank accession number M73812.1) forward: 5′CACTTTCTTGAGCAACACCCT3′ and reverse: 5′TATGTCGCACCACTGATACCCT3′, CDK2 (GenBank accession number: X62071.1) forward: 5′AACAAGTTGACGGGAGAGGT3′ and reverse: 5′GAAGAGGAATGCCAGTGAGA3′, P21 (GenBank accession number S67388.1): forward: 5′CCCGTGAGCGATGGAACTT3′ and reverse: 5′CTTCCTGTGGGCGGATTAG3′. β-Actin (GenBank accession number HQ154074.1, primers are: forward: 5′CCTGTTCCTCCCTGGAGAAGAGCTATGAGCTG3′ and reverse: 5′GATCCACACAGAGTACTTGCGCTCAGGAGGAG3′) was quantified as reference. Relative mRNA expression was quantitated by SYBR Green using Step One Plus™ real-time PCR system and calculated using \({\varDelta \varDelta }C_{\text{t}}\) method.
Cell proliferation assay
The assay was performed by seeding 1 × 104 cell/well U937, THP-1 or K562 (NKG2D negative cell) onto 96 plates. Cells were treated with different concentrations of anti-NKG2D (0, 0.25, 0.5, 1, 2, 5, 10, 20 and 40 nM) at 37 °C for 24, 48 or 72 h. Following incubation, cell viability was measured with MTT assay. The OD value of each well was read at 570 and 630 nm by a micro-plate reader (thermo), and the inhibitory rate calculated using the formula:
Immunoblotting
1 × 106 cells /well of U937 or THP-1 were treated with series of concentrations of anti-NKG2D (0, 5 and 20 nM) for 24 h and transfected with lentiviral plasmids pLVX-shRNA-NKG2D for 48 h. Whole cell extracts were harvested using RAPI buffer (Beyotime, China). Proteins were subsequently resolved by electrophoresis and then transferred onto PVDF membranes. The membranes were blocked and incubated with primary antibodies anti-β-actin (SAB, USA), anti-AKT (Cell Signaling, USA), anti-AKT (Ser473) (EPITOMICS, CA), anti-p-P38 MAPK (Anbo, USA) and anti-P38 MAPK (SAB, USA) at 37 °C for 2 h. Subsequently, the membrane was washed with TBS and TBST in turn and incubated with anti-mouse/anti-rabbit antibodies conjugated with horseradish peroxidase (HRP). The membranes were developed with enhanced ECL chemiluminescence reagent (Millipore, Billerica, USA) and exposed on a Bio-Rad detection system.
NK degranulation assay and release of cytokines
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Lympholyte-H (Cedarlane, Netherland). Target cells were treated with anti-NKG2D (0, 5 or 20 nM) or pLVX-shRNA-NKG2D in six-well plates for 4 h at 37 °C and subsequently washed with PBS followed by co-incubation with PBMCs (1 × 106) at a ratio of 1:100 (target:effector) for another 4 h. Degranulation of NK cells was analyzed by flow cytometric analysis of LAMP-1 (CD107a) expression as described [14]. To detect the release of cytokines, cells were washed with 2 % FBS–PBS, fixed and permeabilized by Inside Stain Kit (Miltenyi Biotec, Germany) for 20 min. After being washed with 2 % FBS–PBS, the samples were incubated with antihuman-IFN-γ-PE or antihuman-TNF-α-PE for 10 min in the dark room and the stained cells were observed by flow cytometer (BD).
ELISA
To confirm the release of cytokines IFN-γ and TNF-α, supernatant of the above culture was harvested, in which the content of IFN-γ and TNF-α was detected using commercial ELISA kits (keyGEN BioTECH, China) according to the manufacturer’s instructions. The detectability of IFN-γ was 8 pg/mL and TNF-α 5 pg/mL. All concentrations were expressed as mean of triplicates.
ADCC assay
The release of lactate dehydragenase (LDH) from target cells was detected using CytoTox 96 Nonradioactive Cytotoxicity assay (Promega, Madison, USA). As previously described, the target cells were treated with anti-NKG2D (20 nM) and then cocultured with PBMCs at 1:5, 1:10 and 1:30 (target:effector) ratio. LDH in the supernatant was analyzed following manufacturer’s protocol. Controls for spontaneous LDH release in effector and target cells, as well as target maximum release, were prepared. The calculation of cytotoxicity percentage is as follows:
Results and discussion
Expression of MICA and NKG2D on cancer cells
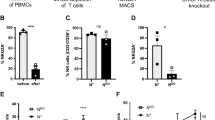
The expression of MICA and MICB by tumor cells has been previously reported [11, 13]. Additionally, the ability of these ligands to induce cytotoxic activity in effector cells expressing NKG2D has been described [15]. Also, the ability of certain tumor cells to express NKG2D has also been reported elsewhere [4, 13]. Hence, tumor cells U937, THP-1, K562, MD-MB-231 and HCT116 were screened for the over-expression of MICA and NKG2D using flow cytometry. HEK293 cell was used as a control group. Whereas K562 and MD-MB-231 over-expressed MICA, THP-1 expressed low levels (Fig. 1a). In contrast, U937 and HCT116 did not express MICA. It has been reported elsewhere that leukemic myelomonocytic U937 and THP-1 cell lines express MICA and MICB, but they are immediately shed from the tumor cell [11, 13, 16], possibly explaining why MICA was not detected on U937 and THP-1 cells in the current study. Effector cells that over-express cognate NKG2D are able to recognize tumor cells via the NKG2D ligand interaction, which leads to cell-mediated cytotoxicity of the tumor cells [15, 17, 18]. Therefore, shedding of MICA from tumor cells could result in the escape of immune surveillance by tumor cells and has been established as one of the mechanisms employed by tumor cells to escape immune surveillance. Another important mechanism adopted by tumor cells to escape immune surveillance is the expression of NKG2D, which has been implicated in tumor proliferation [5]. U937, THP-1, K562, MD-MB-231 and HCT116) were therefore examined for the expression of NKG2D. U937 and THP-1 were found to over-express NKG2D, while K562, MD-MB-231 and HCT116 cell lines did not express NKG2D (Fig. 1b). Therefore, the expression of NKG2D on tumor cells is limited to particular tumor cells. Lentiviral plasmids were used to interfere with the expression of NKG2D on U937 and THP-1 (Fig. 1c, d) which could therefore serve as control in subsequent studies.
Cancer cell proliferation assay by tumoral NKG2D and cell cycle analyses
After establishing that certain tumor cells express the NKG2D–DAP10 complex, we sought to investigate the function of the NKG2D on tumor cells by blocking the tumoral NKG2D with anti-NKG2D and observing whether there is an effect on the growth of U937 and THP-1 which have been shown to express NKG2D (Fig. 1b). As shown in Fig. 2a, anti-NKG2D dose dependently inhibited proliferation in both U937 and THP-1. In contrast, anti-NKG2D had no effect on the proliferation of NKG2D negative K562. Taken together, these studies suggest that NKG2D on tumor cells aids tumor growth and proliferation. Further investigations were however required to establish the specific aspect of the NKG2D–NKG2D ligand pathway responsible. Cell cycle analysis of U937 and THP-1 following treatment with varying concentrations of the anti-NKG2D showed that the number of cells in G1 phase and S phase increased and remained relatively high compared with the number of cells in G2 phase (Fig. 2b, c), consistent with the data obtained when the expression of tumoral NKG2D on the cells was disrupted using lentiviral plasmids. These data indicate that blocking NKG2D on tumor cell could sufficiently inhibit the proliferation of NKG2D-expressing tumor cells suggesting that NKG2D on tumor cells is involved in tumor proliferation.
a Anti-NKG2D dose dependently inhibited the proliferation of U937 and THP-1, however, exhibited no significant inhibition on K562. b, c Cell cycle analyses of U937 and THP-1 in the presence or absence of anti-NKG2D antibody, and U937shRNA and THP-1shRNA, by PI staining and quantitative evaluation by flow cytometry data based on ModFit software. The number of cells in G1 phase and S phase increased and remained high with increasing concentrations of anti-NKG2D in U937, THP-1, U937shRNA and THP-1shRNA (values represented mean ± SD, n = 3, *p < 0.05, **p < 0.01, ***p < 0.001 versus untreated control)
Levels of cyclin E–CDK2 are altered by expression of tumoral NKG2D
Cyclin E and CDK2 play a crucial role in the transition from G1 to S phase of the cell cycle. Cyclin E binds to G1 phase CDK2, which is a requirement for the transition from G1 to S phase of cell cycle that precedes cell division [19]. In contrast, P21 protein inhibits the activity of cyclin-CDK2, CDK1 and CDK4/6 complexes and thus functions as a regulator of cell cycle progression at G1 phase and S phase [20, 21]. Therefore, to further confirm that NKG2D on tumor cells is involved in tumor proliferation, the expression levels of these proteins in NKG2D-expressing U937 and THP-1 treated with varying concentrations of anti-NKG2D were evaluated using real-time quantitative PCR (Fig. 3). Anti-NKG2D significantly influenced the expression levels of cyclin E, CDK2 and P21 in both U937 and THP-1 with the expression levels of cyclin E and CDK2 increasing with increasing concentration of anti-NKG2D, whereas expression of P21 decreased with increasing concentration of anti-NKG2D. Taken together, these data indicate that down-regulating or blocking tumoral NKG2D results in an increase in the number of cells at the G1 phase and S phase.
Tumoral NKG2D induces PI3K–AKT signal pathway
It has been suggested elsewhere that the NKG2D–DAP10 complex on tumor cells could engage NKG2D ligands on adjacent tumor cells and lead to the activation of oncogenic PI3K–AKT–mammalian target of rapamycin (mTOR) signaling axis and downstream effectors, and trigger phosphorylation of ERK and JNK in MAPK cascades downstream Grb2 and PI3K, respectively [4]. This implies that the tumor cells co-opt expression of NKG2D to complement its ligands for self-stimulation that can trigger oncogenic signaling cascades that promote tumor growth. To confirm that the expression of NKG2D on tumor cell promotes tumor growth and malignant dissemination, U937 and THP-1 cells treated with varying concentrations of anti-NKG2D were compared with the knockdown cell lines U937shRNA and THP-1shRNA. As anticipated, the anti-NKG2D dose dependently inhibited the phosphorylation of P38 MAPK and AKT in U937 and THP-1 consistent with the data obtained when the knockdown cell lines U937shRNA and THP-1shRNA (Fig. 4a, b) were cultured without anti-NKG2D, consistent with the fact that NKG2D on tumor cell promotes tumor growth and malignant dissemination. Thus, blocking NKG2D expressed on tumors could inhibit their growth suggesting that tumoral NKG2D can selectively be targeted for therapeutic purposes.
a, b Western blotting analysis for signaling pathway of p-AKT, AKT, p-P38 MAPK and P38 MAPK on whole cell extracts from U937 (a) and THP-1 (b), treated with different concentrations of anti-NKG2D and U937shRNA and THP-1shRNA. c, d Percentages of p-AKT/AKT and p-P38/P38 indicating NKG2D-mediated signaling cascade
Induction of NK degranulation and release of cytokines
PBMCs express NKG2D and therefore are activated by NKG2D ligands on target cells such as tumor cells. Activating PBMCs results in the production of CD107a, IFN-γ and TNF-α. To investigate whether down-regulating or blocking tumoral NKG2D with anti-NKG2D would enhance production of CD107a, IFN-γ and TNF-α by PBMCs, U937 or THP-1 was pre-treated with various concentrations of anti-NKG2D and subsequently cocultured with PBMCs at an effector: to target ratio of 100:1. Production of CD107a, IFN-γ and TNF-α by the PBMCs increased with increasing concentration of anti-NKG2D, suggesting that the NKG2D on U937 and THP-1 was blocked by the anti-NKG2D (Figs. 5, 6). Therefore, the PBMCs were able to interact with sufficient NKG2D ligands on the tumor cells to result in the activation of the PBMCs with subsequent production of CD107a, IFN-γ and TNF-α. CD107a, IFN-γ and TNF-α synergistically enhance PBMCs cytotoxic activity through NF-κB-dependent up-regulation of ICAM-1 expression in target cells [22]. Staining showed relatively high expression of CD107a, IFN-γ and TNF-α in U937shRNA and THP-1shRNA compared with untreated U937 and THP-1. However, the expression levels of these markers were lower in U937shRNA and THP-1shRNA compared to U937 and THP-1 treated with 40 nM anti-NKG2D. Thus, these studies indicate that down-regulating or blocking of tumoral NKG2D could induce cell-mediated cytotoxicity of the tumor cell. ELISA analysis showed that the production of IFN-γ and TNF by NK cells cocultured with U937, THP-1, U937shRNA or THP-1shRNA increased with increasing concentration of anti-NKG2D (Fig. 7a, b) consistent with the fact that blocking tumoral NKG2D could reduce the possible interactions between adjacent tumor cells via the NKG2D ligand pathway therefore enhancing binding between the NKG2D ligand expressing tumor cells and NKG2D-expressing effector cells and subsequently leading to the activation of the effector cell and cell-mediated cytotoxicity in the tumor cell.
a Production of IFN-γ and TNF-α by PMBCs cocultured with U937shRNA or U937 after pre-treating with anti-NKG2D at E:T ratio (100:1) for 4 h evaluated by ELISA. b Secretion of IFN-γ and TNF-α by PBMCs coculturing with THP-1shRNA or THP-1 after pre-treating with increased concentrations of anti-NKG2D at E:T ratio (100:1) for 4 h are assessed by ELISA. c The cytotoxic activity of anti-NKG2D is assessed by pre-treating U937 or THP-1 with anti-NKG2D and measuring the released LDH. As expected, the release of the LDH increased with increasing E/T (effector/target) ratio
Cytotoxic activity of anti-NKG2D
ADCC assay was used to confirm the ability of anti-NKG2D to induce cell-mediated cytotoxicity NKG2D-expressing U937 or THP-1 pre-treated with anti-NKG2D and subsequently cocultured with PBMCs at E:T ratios of 5:1, 10:1 and 30:1 were monitored for lactate dehydrogenase (LDH) release (Fig. 7c). Cell-mediated cytotoxicity of the target cells increased when the E:T ratio was increased suggesting that anti-NKG2D has cytotoxic activity and could therefore mediate cytotoxicity of NKG2D-expressing tumor cells.
Conclusion
AML can escape from NK cell-mediated immune surveillance [1]. In the present study, we have investigated whether NKG2D expressed by the tumor is involved in NK cell-mediated immune surveillance in AML. Furthermore, the current study has confirmed that unlike the NKG2D–DAP10 complex on effector cells, the NKG2D–DAP10 complex on some breast, ovarian and prostate cancer cells plays a crucial role in the malignant dissemination of the tumors [4]. In the present study, we wanted to determine whether tumoral NKG2D expressed by AML tumor cells could lead to tumor growth. To investigate the role of the expression of NKG2D on cancer cells, we used shRNA targeting human NKG2D to establish NKG2D-deficient U937 and THP-1 cell lines.
In addition, we used anti-NKG2D to block the NKG2D signaling pathway in the AML cell lines U937 and THP-1. This treatment inhibited the phosphorylation of P38 MAPK and AKT signals known to drive tumor growth [23]. In addition, blocking of NKG2D expressed in the tumor with varying concentrations of anti-NKG2D resulted in more cells in G1 and S and fewer in G2 of the cell cycle. Consistent with this, quantitative PCR analyses demonstrated that down-regulation or blocking of NKG2D expressed in the tumors could increase the expression levels of cyclin E and CDK2 and decrease the expression level of marker P21, implying that more tumor cells were in G1 and S.
Moreover, NKG2D is an activating receptor expressed on NK cells and T cell lymphocytes [24]. Shedding of the NKG2D ligands MICA, MICB and ULBPs from cancer cell leads to their escape from immune recognition [1, 3]. Blocking of NKG2D on tumor cells augmented cell-mediated cytotoxicity in the NKG2D-expressing tumor cells when these cell lines were cocultured with PBMCs. Also, increased expression of CD107a, IFN-γ and TNF-α was observed when NKG2D-expressing tumor cells were pre-treated with anti-NKG2D and subsequently cocultured with PBMCs, suggesting that the PBMCs were activated by binding to NKG2D ligands on the tumor cell. Thus, down-regulation or blocking the NKG2D on the tumor cells facilitated the binding between PBMCs and tumor cells.
In conclusion, the current study has demonstrated that AML cells U937 and THP-1 can express NKG2D and this NKG2D expressed in the tumors could be involved in tumor growth and proliferation and may serve as a therapeutic target for anti-tumor therapy and immunotherapy.
References
Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26(9):2019–26. doi:10.1038/leu.2012.87.
Park SW, Bae JH, Kim SD, Son YO, Kim JY, Park HJ, et al. Comparison of level of NKG2D Ligands between normal and tumor tissue using multiplex RT-PCR. Cancer Investig. 2007;25(5):299–307. doi:10.1080/07357900701208824.
Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–85.
Benitez AC, Dai ZP, Mann HH, Reeves RS, Margineantu DH, Gooley TA, et al. Expression, signaling proficiency, and stimulatory function of the NKG2D lymphocyte receptor in human cancer cells. P Natl Acad Sci USA. 2011;108(10):4081–6. doi:10.1073/pnas.1018603108.
El-Gazzar A, Cai X, Reeves RS, Dai Z, Caballero-Benitez A, McDonald DL, et al. Effects on tumor development and metastatic dissemination by the NKG2D lymphocyte receptor expressed on cancer cells. Oncogene. 2014;33(41):4932–40. doi:10.1038/onc.2013.435.
Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991;173(4):1017–20.
Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730–2.
Upshaw JL, Leibson PJ. NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes. Semin Immunol. 2006;18(3):167–75. doi:10.1016/j.smim.2006.03.001.
Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi:10.1038/ni1581.
Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7(9):737–44. doi:10.1038/nri2144.
Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96(12):6879–84.
Cai X, Dai ZP, Reeves RS, Caballero-Benitez A, Duran KL, Delrow JJ, et al. Autonomous stimulation of cancer cell plasticity by the human NKG2D lymphocyte receptor coexpressed with its ligands on cancer cells. PLoS One. 2014. doi:10.1371/journal.pone.0108942.
Weiss-Steider B, Soto-Cruz I, Martinez-Campos CA, Mendoza-Rincon JF. Expression of MICA, MICB and NKG2D in human leukemic myelomonocytic and cervical cancer cells. J Exp Clin Cancer Res CR. 2011;30:37. doi:10.1186/1756-9966-30-37.
Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22. doi:10.1016/j.jim.2004.08.008.
Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17(1):19–29. doi:10.1016/S1074-7613(02)00333-3.
Mistry AR, O’Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology. 2007;121(4):439–47. doi:10.1111/j.1365-2567.2007.02652.x.
Zhang T, Sentman CL. Cancer Immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells. Cancer Res. 2011;71(6):2066–76. doi:10.1158/0008-5472.CAN-10-3200.
Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781–90. doi:10.1038/nri1199.
Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K, et al. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell. 1998;1(4):553–63.
Rodriguez R, Meuth M. Chk1 and p21 cooperate to prevent apoptosis during DNA replication fork stress. Mol Biol Cell. 2006;17(1):402–12. doi:10.1091/mbc.E05-07-0594.
Dolezalova D, Mraz M, Barta T, Plevova K, Vinarsky V, Holubcova Z, et al. MicroRNAs regulate p21(Waf1/Cip1) protein expression and the DNA damage response in human embryonic stem cells. Stem Cells. 2012;30(7):1362–72. doi:10.1002/stem.1108.
Wang RP, Jaw JJ, Stutzman NC, Zou ZC, Sun PD. Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol. 2012;91(2):299–309. doi:10.1189/jlb.0611308.
Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi:10.1016/S0065-230X(09)02002-8.
Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol. 2006;16(5):333–43. doi:10.1016/j.semcancer.2006.07.008.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (NSFC81473125), Specialized Research Fund for the Doctoral Program of Higher Education (20130096110007), Jiangsu Province Qinglan Project (2014) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. We are grateful to Professor Sherie L. Morrison of University of California Los Angeles for her revision on this article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Mingying Tang and Desmond Omane Acheampong have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tang, M., Acheampong, D.O., Wang, Y. et al. Tumoral NKG2D alters cell cycle of acute myeloid leukemic cells and reduces NK cell-mediated immune surveillance. Immunol Res 64, 754–764 (2016). https://doi.org/10.1007/s12026-015-8769-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8769-3