Abstract
Macrophages are cells of the innate immune system involved in critical activities such as maintaining tissue homeostasis and immune surveillance. Pro-inflammatory macrophages M1 are responsible for the inflammatory response, while M2 macrophages are associated with the immunosuppressive repair phase of tissue remodeling. Most cancers are associated with chronic inflammation, and a high number of macrophages in tumors have been associated with tumor progression. Much effort has been made in elucidating the mechanisms through which macrophages contribute to tumor development, yet much less is known about the initial mechanisms by which tumors modify macrophages. Our work has focused on identifying the mechanisms by which macrophages from tumor hosts are modified by tumors. We have shown that peritoneal macrophages are significantly altered in mice bearing advanced mammary tumors and are not M1 or M2 polarized, but express a mixture of both transcriptional programs. These macrophages are less differentiated and more prone to apoptosis, resulting in increased myelopoiesis as a compensation to regenerate macrophage progenitors in the marrow. Macrophages in the tumor microenvironment are also neither M1 nor M2 cells and through a display of different mechanisms are even more impaired than their peripheral counterparts. Finally, systemic blood monocytes, precursors of tissue macrophages, are also altered in tumor bearers and show a mixed program of pro- and anti-inflammatory functions. We conclude that there is evidence for local and systemic immune impairment in tumor hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macrophages are the main phagocytic and inflammatory cells in our bodies and are fundamental in the response of our innate immune system to infection and damage. Cancer is a disease strongly associated with chronic inflammation, and macrophages are significantly involved in cancer development. In chronically inflamed tissues, macrophages contribute to tumor initiation, whereas within developing tumors, macrophages help in the progression of the disease. A strong immunosuppressive microenvironment is established in advanced tumors, in great part contributed by the presence of immunosuppressive macrophages within tumors. However, it is still controversial whether in addition to a local immune deficiency there is also a systemic or generalized state of immunosuppression in cancer patients [1].
Evidence indicates that the healthy immune system is necessary for the control of malignant disease and that immunosuppression associated with cancer contributes to its progression. Tumors have developed strategies to successfully evade the host immune system, and various molecular and cellular mechanisms responsible for tumor evasion have been identified. Some of these mechanisms target macrophages, and the changes induced in these macrophages contribute to tumor development.
Our work seeks to analyze the mechanisms by which tumors can modify different populations of myelomonocytic cells, resulting in both local and systemic immunosuppression that further contributes to tumor progression. To do this, we have analyzed macrophages and their precursor blood monocytes as indicative of immune impairment. The results of our work show that macrophages from within the tumor, those in peripheral locations of tumor-bearing mice and also their precursor blood monocytes are all targets of immunosuppressive strategies in tumor hosts. Understanding the ways by which tumors modify these critical immune cells may contribute in part to the reversion of immunosuppression and to the control of cancer progression.
Macrophages: key players in tissue homeostasis and immune surveillance
Macrophages are cells of the innate immune system involved in critical regulatory activities in health and disease [2, 3]. Due to their phagocytic capacity, macrophages perform necessary housekeeping functions in healthy tissues, clearing away apoptotic cells and debris to maintain tissue homeostasis [4–6]. Macrophages are also responsible for the phagocytosis of apoptotic cells in embryogenesis [7, 8] and during tissue remodeling in organ development [9, 10]. Importantly, they participate as central sentinels in immune surveillance in the detection of infection and tissue damage. As important as they are in homeostasis, their roles in inflammation and disease are also essential [11]. In conjunction with the short-lived neutrophils, macrophages are the key executors of the acute inflammatory immune response, enabling multicellular organisms to rapidly react to infections and tissue damage [12, 13]. Together with dendritic cells (DC), macrophages are considered a bridge between innate and adaptive immunities in their roles as professional antigen-presenting cells (APC) and presenters of second signals to T lymphocytes.
Living and performing diverse functions in tissues, macrophages are phenotypically and functionally heterogeneous and remarkably plastic cells capable of adapting to different tissue microenvironments [3, 14, 15]. Macrophages are recognized as “resident” when they perform housekeeping functions in healthy tissues and are known as “inflammatory” when they are newly recruited into tissues in which infection or damage has been recently detected by resident macrophages. Resident macrophages exist in practically every tissue of multicellular organisms and receive different names according to their specific locations: In vertebrates there are two types of resident macrophages in the lungs, called alveolar and interstitial macrophages; they are referred to as osteoclasts when in the bone; microglia in the central nervous system; Kupfer cells in the liver; Langerhans cells in the skin; and marginal zone, red pulp and white pulp macrophages in the spleen [9]. Resident macrophages fulfill tissue-specific and niche-specific functions that range from homeostatic activities, such as clearance of cellular debris and iron processing, to central roles in tissue immune surveillance to detect damage or infection. On the other hand, inflammatory macrophages are cytotoxic effector cells that contribute to unleashing the physiologically required but destructive acute inflammatory response. Once tissue damage or infection is resolved through acute inflammation and the initiation of the adaptive immune response, a different regulatory macrophage subpopulation is involved in the feedback wave of immunosuppressive functions that restores homeostasis to the inflamed tissue [16–18].
Origin of tissue macrophages
Recent studies reveal differences in the origins of tissue macrophages, which may arise from hematopoietic (bone marrow) versus self-renewing embryo-derived populations. It is well known that tissue macrophages differentiate from bone marrow-derived blood monocytes, which are recruited into the tissues in situations of homeostasis or inflammation and become macrophages. However, at least in mice, other tissue macrophages have prenatal origins, some of which involve cells arising from a distinct yolk sac-derived embryonic macrophage lineage maintained by self-renewal [19, 20]. Until recently, macrophages were considered highly differentiated cells incapable of cell proliferation, so the only way macrophages could increase in numbers within tissues was considered to be through renewed blood monocyte tissue recruitment and macrophage differentiation. However, the latest evidence indicates that macrophages can proliferate in some tissue niches. Actually, low-level self-renewal during adulthood appears sufficient to maintain many tissue-resident macrophages [21], and tissues infected by parasites have shown a Th2 cell environment where IL-4 has been demonstrated to stimulate tissue macrophage proliferation [22]. It is possible that tissues contain diverse populations of both local self-renewing and peripherally derived macrophages both in mice and in humans [9].
Macrophage polarization
Paralleling the Th1/Th2 division of CD4+ T helper cells, macrophages have been classified into M1 (also known as “classically” activated) and M2 (or “alternatively” activated) [23–25]. Th1-related cytokines such as IFNγ, as well as microbial stimuli such as lipopolysaccharide (LPS), polarizes macrophages to M1. M1 macrophages are characterized by IL-12hi, IL-23hi, TNFαhi, IL-10low, CXCL9hi, CXCL10hi, ROIhi, RNIhi, COX1low, COX2hi and iron uptake phenotype, among other features, and are involved in inflammatory, microbicidal and at least in vitro tumoricidal activities. In contrast, Th2 cytokines such as IL-4 and IL-13 polarize macrophages to M2 [17, 26]. These macrophages exhibit IL-12low, TNFαlow, IL-10hi, IL-1decoyRhi, IL-1RAhi, Arginase1hi, CCL17hi, CCL18hi, CCL22hi, CCL24hi, COX1hi, COX2low, iron release, increased phagocytic activity and high expression of scavenging, mannose and galactose receptors [14]. M2 macrophages reduce inflammation, promote tissue remodeling and repair, help in parasite clearance and in tumor progression and possess immunoregulatory functions. Several other stimuli such as IL-10, glucocorticoid hormones, apoptotic cells and immune complexes have also been reported to induce macrophages to an M2-like immunosuppressive phenotype [11]. M1 macrophages are involved in the inflammatory response, for which they are also known as pro-inflammatory macrophages, and are thus associated with tissue destruction and mutation induction through the free radicals these macrophages produce (RNI and ROI). In contrast, M2 macrophages are associated with the immunosuppressive repair phase of tissue remodeling; these macrophages operate in the feedback response that terminates inflammation and results in restoration of tissue homeostasis.
Macrophage heterogeneity in tissues
Although the notion of M1/M2 macrophage polarization was initially described in vitro and was defined as an “operational” concept unfolding “extremes of a continuum” in the phenotype and function of macrophages [26], such polarization states have been observed also in vivo in mice and humans, under physiological and pathological conditions. Macrophages that are present in physiological settings such as developing embryonic tissues or normal lean adipose tissues, or that participate in pathological situations such as parasitic infections, allergy and tumors, all resemble M2 or M2-like phenotypes [9, 27]. Whether M1–M2 macrophage switches in phenotype and function occur normally in vivo or whether new waves of circulating blood monocytes are recruited into tissues to become differently polarized macrophages is a controversial issue. Immunosuppressive, regulatory macrophages are required for the resolution of inflammation and restoration of tissue integrity by removing debris and promoting the proliferation and differentiation of parenchymal cells. Thus, the M1 to M2 switch during the progression of the inflammatory response requires the twofold role of macrophages in coordinating the onset of inflammation and subsequently promoting healing and repair. There is currently an active debate about the mechanisms underlying this switch between the pro-inflammatory and the suppressive status in macrophages. This shift could reflect a transcriptional reprogramming between their alternative polarization states so that plastic macrophages may switch between different transcriptional programs [28]. Alternatively, the shift between macrophage polarization profiles could reflect a new wave of blood monocyte recruitment, resulting in new macrophages exhibiting features of a different activation status. These two interpretations are not necessarily mutually exclusive, and both things could at the same time occur in tissues: Early-recruited M1 inflammatory macrophages might undergo a reprogramming to M2-type cells, and newly bone marrow-generated suppressive blood monocytes may also replenish tissues with M2 macrophages. In vivo settings are characterized by a great variety of microenvironmental conditions reflecting the diversity of cells and molecules that are present and continuously expressed; therefore, it should not be surprising to find mixtures of M1 and M2 macrophages coexisting in a particular tissue or organ.
Homeostatic macrophages resident in healthy tissues were understood until recently as housekeeping “inert” non-polarized (Mo) cells involved in the silent phagocytosis of apoptotic cells, in contrast to the M1- or the M2-polarized “activated” macrophages. However, this vision has been challenged by new findings on immunosuppressive M2-like macrophages occurring in the vast majority of homeostatic tissues, as mentioned above, probably as an expression of the physiologic requirement to protecting homeostatic tissues against overwhelming uncontrolled inflammation.
Macrophages may be actively and dynamically transcribing different genetic programs to adapt to the changing conditions of the diverse microenvironments in which they live. A note of caution should be taken into consideration against the concept of macrophage polarization. This concept should not be understood as an irreversible cellular differentiation in one of two antagonist subsets, but as the dynamic, adaptive and reversible changes constantly occurring as response of macrophages to varying microenvironments [29, 30]. Hence, as an alternative to the concept of stable macrophage subsets, other authors have proposed the model of “functional adaptivity” or “functional plasticity” of macrophages as a basis for understanding macrophage function in physiologically, dynamic settings [31]. Thus, the concept of tissue macrophage phenotype should not be understood as an immutable end-stage set of characteristics and features but rather as a dynamic and plastic state maintained by reversible homeostatic mechanisms exerted through adaptation to tissue-specific yet changing microenvironments. Addressing the mechanistic bases of these complex population dynamics in vivo is of outmost priority, although it will be extremely challenging [28].
Role of macrophages in tumorigenesis
Most cancers are associated with chronic inflammation, a process that sets in when the resolution of acute inflammation fails [32, 33]. Chronic inflammation results in the installment of a permanent state of damage in a tissue with the persistent action of inflammatory cells such as macrophages. Inflammation and immunosuppression are two opposing responses of the immune system linked in different ways to cancer progression: Earlier stages of tumor development are associated with chronic inflammation, while established advanced cancers induce a stage of immunosuppression [34].
Given that macrophages are key players of the inflammatory response, it is anticipated that they will exhibit significant roles in the different stages of tumorigenesis. In fact, the release of mutation-inducing free radicals, such as reactive oxygen intermediates and reactive nitrogen intermediates (ROI/RNI) as part of the macrophage cytotoxic/inflammatory response, does contribute to tumor initiation [35, 36]. Macrophages also play important roles in tumor progression by releasing factors that promote angiogenesis, invasion, extracellular matrix remodeling/repair and metastasis and by recruiting additional immune suppressor cells to the tumor [26, 37].
Tumors are structures comprised of tumor and non-tumor cells, which include endothelial cells, pericytes, fibroblasts, stromal, mesenchymal cells, innate and adaptive immune cells. These tumor and non-tumor cells and the many different factors they express form the tumor microenvironment. Macrophages not only constitute the most abundant immune cell type comprising tumor microenvironments but also significantly modulate tumor development. In fact, high numbers of macrophages in tumors (tumor-associated macrophages, TAMs) have been associated with tumor progression in animals and humans and with poor tumor prognosis in the clinics [38–40].
Before TAMs can modulate tumor development, tumor cells and other cells and factors from the tumor microenvironment modify or “educate” these macrophages [41, 42]. By shaping macrophage functions, tumors induce them to express pro-inflammatory and immunosuppressive traits, all of which end up facilitating the different stages of tumor progression [33, 37]. Much effort has been made in elucidating the mechanisms through which macrophages contribute to tumorigenesis, yet much less is known about the initial mechanisms by which tumors modify macrophages.
Our work in the past several years has focused on identifying the mechanisms by which macrophages from tumor hosts are modified by the presence of a tumor. To investigate this, we have used syngeneic mouse mammary tumor models. We have been interested in understanding whether only local tumor-associated macrophages are affected by the tumor, or whether also peripheral macrophages at non-tumor niches and their precursor circulating blood monocytes are impacted by the disease as well.
In the following sections, we will present an overview of our studies comparing local (tumor microenvironment TAMs) and peripheral (peritoneal macrophages and circulating blood monocytes) myelomonocytic cells from tumor hosts exposed to the transplantable mouse mammary tumor model D1-DMBA3, an estrogen-receptor-negative malignant murine mammary tumor [43], which we have compared with a different mammary tumor model, the 4T1 (an estrogen-receptor-positive malignant murine breast tumor), both syngeneic to BALB/c mice.
Peripheral, non-tumor-associated peritoneal macrophages are significantly altered in mammary tumor-bearing hosts
We were interested in examining whether advanced tumors may induce peripheral immune deficiency in the host. To this end, we investigated whether macrophages recruited to the peritoneal cavity of mammary tumor-bearing mice differed in their pro-inflammatory capacities from their normal counterparts, i.e., a similar macrophage subpopulation isolated from normal, non-tumor-bearing age- and sex-matched control mice. The peritoneal cavity is considered a non-tumor, peripheral, distal location when analyzing breast tumors. Peritoneal-elicited macrophages from mice bearing advanced D1-DMBA-3 mammary tumors were found to be immunologically dysfunctional, either constitutively or in response to LPS activation. Among other deficiencies, they exhibit depressed mRNA and protein levels of pro-inflammatory molecules IL-12 and inducible nitric oxide synthase (iNOS). We found that these defects were associated with impaired binding activities of pro-inflammatory transcription factors NFκB and C/EBP to their respective sites on the IL-12 and iNOS gene promoters. Interestingly, lower NFκB nuclear translocation but no difference in the amounts of IκBA, pIκBA or IKKα was found in these macrophages [44].
Elevated constitutive NFκB activity has been previously demonstrated in tumor cells and myeloid cells from tumor hosts [45]. However, we were among the first to report the opposite observation: a decreased constitutive expression and activity of NFκB in macrophages from mice bearing advanced (4-week) tumors; interestingly, mice bearing earlier tumors (1–2 weeks) showed an upregulation of NFκB in these peripheral macrophages. Thus, our work provided initial evidence to support the notion that tumor initiation is associated with inflammation (increased NFκB activity and the downstream pro-inflammatory cytokines NFκB regulates), whereas tumor progression is associated with immunosuppression (decreased NFκB).
Moreover, the finding of these deficiencies in peritoneal macrophages, which in contrast to TAMs are not in direct contact with the tumor, further underscored the potency of tumor-derived factors that are capable of acting far from the tumor milieu on peripheral peritoneal macrophages. We confirmed a similar downregulation of NFκB and C/EBP transcription factors in peritoneal macrophages from mice bearing other advanced tumors such as 4T1 mammary tumors and RENCA renal tumors.
Peripheral macrophages from mice bearing advanced tumors are not M1 or M2 polarized; instead, they express a mixture of both transcriptional programs
Peritoneal macrophages from mice bearing advanced tumors do not exhibit a definite polarization program. In addition to being deficient in IL-12 and nitric oxide, they are also impaired in the production of other main pro-inflammatory cytokines and chemokines, such as IL-1β, IL-6, TNF-α, CCL2 and M-CSF. Among the crucial intermediates that operate upstream of NFκB in the TLR/NFκB signaling pathway, these peripheral macrophages from tumor hosts exhibit a dramatic reduction in kinase IRAK-1 and a significant upregulation of the inhibitor kinase IRAK-M; these cells also show a general decrease in the mRNA expression of most of the MAPKs examined, including a substantial reduction in NFκB-inducing kinase (NIK), JNK, p38 and ERK1/2.
Moreover, STAT1 transcription factor, which is involved in upregulating genes upon stimulation by IFNs and is associated with the activation of a Th1-type of antitumor immune response, is profoundly decreased in these peritoneal macrophages, as is the case of a critical STAT1-regulated chemokine, CXCL10, distinctive of M1 macrophages, which is also diminished. Interestingly, these macrophages upregulate TGFβ production [46] but do not exhibit increased expression of the anti-inflammatory cytokine IL-10, as expected from M2 macrophages. No enhanced expression of other suppressor markers shared by M2 macrophages is detected in these cells, such as VEGF, matrix metalloproteinase-9 or arginase.
Overall, these peripheral macrophages exhibit major dysfunctional expression and signaling of several M1 pro-inflammatory proteins, and pathways yet show the absence of a clear-cut M2 transcriptional program. Consequently, we conclude that peritoneal macrophages from tumor bearers exhibit a mixed M1/M2 phenotype. Amazingly, these macrophages can nevertheless become high producers of IL-12 or of IL-10, when experimentally they switch to express high amounts of IL-12p70 by culturing them in the presence of LPS supplemented with IFN-γ. Moreover, they can be induced to produce elevated amounts of IL-10 by culturing them with peptidoglycan, a TLR-2 ligand [47].
Peripheral macrophages from hosts bearing advanced tumors are more prone to apoptosis, which is associated with increased myelopoiesis as a compensatory mechanism to regenerate macrophage progenitors in the bone marrow
As expected from a decreased antiapoptotic NFκB expression and function, these peripheral macrophages from tumor-bearing mice are more apoptotic than their normal counterparts, with dramatically decreased BCL-x, notably increased caspase-3 activation and a TUNEL assay indicative of significant cellular apoptosis. Therefore, peripheral macrophages are not only dysfunctional but are also less in numbers.
Because the tumor induces decreased NFκB expression and enhanced apoptosis in peripheral macrophages, we hypothesized that a compensatory increase in the generation of myeloid progenitors could be occurring in the bone marrow of tumor bearers to compensate for this macrophage loss. It has been previously described that enhanced myelopoiesis is associated with the presence of tumor-derived factors in tumor hosts [48, 49]. However, we were the first to describe increased myelopoiesis in tumor hosts as a result of a compensatory mechanism to equilibrate for the loss of macrophages due to apoptosis; we compellingly demonstrated that macrophage depletion is associated with a specific increase in bone marrow progenitors committed to macrophage but not other myeloid lineages [47].
Peripheral macrophages from tumor hosts are less differentiated than their normal counterparts but are not typical myeloid-derived suppressor cells
Elevated expression of a group of myeloid markers is considered indicative of macrophage differentiation. Peritoneal macrophages from mice bearing advanced tumors appear as a homogeneous cell population of macrophages of larger size and increased vacuolation, with significantly lower levels of F4/80, CD68, CD115 and CD11b than similar macrophages from their normal counterparts, consistent with the phenotype of newly recruited blood monocytes (F4/80low, CD68low, CD115low, CD11blow). These results suggest that peritoneal macrophages from tumor hosts are less differentiated than their normal counterparts. Interestingly, they also exhibit upregulation of the granulocytic myeloid marker Gr-1.
Myeloid-derived suppressor cells (MDSC) are a group of poorly differentiated myeloid cells that appear in the bone marrow, spleen, blood and the tumor microenvironment of tumor hosts, inducing immunosuppression and promoting tumor progression [50, 51]. In mice, they are characterized by a CD11bhi and Gr 1hi phenotype.
Peripheral macrophages from tumor-bearing mice with a CD11blow and Gr 1hi expression pattern also lack expression of other characteristic markers of MDSC, such as elevated arginase and nitric oxide. In contrast to these peritoneal macrophages, splenic MDSC isolated from these tumor-bearing mice do not express F4/80 [52]. Therefore, peritoneal macrophages from tumor hosts do not correspond to the definition of MDSC.
Our data suggest that peritoneal macrophages are a less differentiated cell population and represent monocytes recently recruited from the blood that may become apoptotic during differentiation to macrophages [47].
Factors derived from tumor cells and not from non-tumor cells, such as TGFβ, PGE2 and IL-11, downregulate NFκB and C/EBP expression: possible involvement of TGFβ-mediated increase in macrophage proteasomal activity
We in vitro cultured peritoneal macrophages from normal healthy mice with supernatants from different murine mammary, lung and kidney tumor cell lines and also with supernatants from the murine non-tumor fibroblast cell line 3T3. Our results confirmed that only supernatants from tumor cell lines and not from non-tumor cells were associated with diminished NFκB and C/EBP protein expression in these normal macrophages [47].
Among the many factors known to be secreted by most of the tumor cell lines, and particularly by the DA3 cell line isolated from the D1-DMBA3 mammary tumor used in our studies, we showed that treatment of normal peritoneal macrophages with TGFβ and PGE2, individually and additively, resulted in significant downregulation of some of the NFκB and C/EBP family member proteins. We had previously shown that IL-11, produced by the DA3 tumor cell line, reduced C/EBP expression in normal macrophages [53].
Given that upregulation of ubiquitin/proteasomal pathways has been described under cancer-induced cachexia [54], we examined the possible role of this proteolytic machinery in the decrease in NFκB and C/EBP proteins in macrophages from tumor hosts. Using the proteasome inhibitor MG-132 to block the proteasome machinery in macrophages from normal and tumor-bearing animals, we concluded that peritoneal macrophages from tumor hosts display higher ubiquitination and proteolysis compared with those from normal mice. We also observed that NFκB and C/EBP protein downregulation is reversed when these macrophages are treated with this proteasome inhibitor [55].
Studies on the Smad family proteins, which are the key signal transducers of the TGF-ß family ligands, have revealed the ability of Smads to interact with various components of the 26S proteasome system [56]. In breast cancer cell lines, TGF-ß increases the activity of the proteasome [57]. We speculate that TGF-ß-induced modulation of proteasomal function may in part mediate the immunosuppressive effects of TGF-ß in macrophages by accelerating degradation of inflammatory mediators.
Further studies to elucidate the role of TGF-ß in proteasome function in macrophages are required to assess the possible involvement of the proteasome in immune regulation by TGF-ß, particularly as it regards to increased NFκB and C/EBP proteolysis. Thus, proteasome degradation may contribute, at least in part, to NFκB and C/EBP impairment in macrophages from tumor bearers [55].
Macrophages in the tumor microenvironment reveal greater impairment and are more prone to apoptosis and less differentiated than peripheral peritoneal macrophages from mice bearing advanced tumors
Once we determined that macrophages from peripheral sites in tumor-bearing mice were significantly impacted by the presence of the tumor, we sought to compare their degrees of alteration with the ones shown by TAMs from animals bearing the same tumor model. Because of their location within the tumor, TAMs are exposed to higher concentration gradients of tumor factors and to the combined effect of additional cells and molecules at the tumor microenvironment.
The first thing that surprised us when comparing the two macrophage subpopulations from the same tumor model was their contrasting expression patterns of main pro-inflammatory (IL-12p70, IL-12p40, IL-23, IL-6 and TNFα) and anti-inflammatory (IL-10) cytokines. TAMs do not express IL-12p70 at all but constitutively upregulate IL-12p40, IL-23, IL-6 and IL-10. Significantly, the two related pro-inflammatory cytokines, IL-12p70 (with antitumor properties) and IL-23 (with pro-tumor properties), show strikingly different patterns of gene and protein expression in peritoneal macrophages and TAMs.
Our results demonstrate the existence of dissimilar transcriptional patterns of IL-12p40, IL-12p35 and IL-23p19 gene expression in these two macrophage subpopulations, resulting in TAMs showing a shift toward features that supported tumor progression, such as constitutive IL-23 (which is not expressed by peritoneal macrophages) and total block of IL-12p70 production (which was produced at lower levels in peritoneal macrophages). Surprisingly, we also observed that in contrast to TAMs isolated from D1-DMBA3 (ER−) mammary tumors, TAMs isolated from 4T1 ER+ mammary tumors do not produce IL-23 at all, indicating that the patterns of TAM polarization even within a similar tumor type may be different, reflecting variations in the molecular, biochemical and microenvironmental characteristics of different tumors [58].
Despite the fact that many authors have shown prototypical M2 features in TAMs isolated from a variety of tumors, our data demonstrate that the peritoneal and tumor-associated macrophages studied in these tumors do not correspond to clear-cut M1 or M2 activation profiles, but instead display mixtures of both polarization programs.
Our results also reveal that peritoneal macrophages and TAMs exhibit different NFκB and C/EBP expression patterns: Both transcription factors are downregulated to higher degrees in TAMs, and the mechanisms used to modulate NFκB and C/EBP expression are different in these two macrophage subpopulations. Whereas NFκB p65 and c-rel are more profoundly diminished in TAMs, p50 is in contrast dramatically upregulated, with inhibiting p50 homodimers formed in TAMs but not in peritoneal macrophages. Interestingly, other authors working with different experimental and clinical tumors have also demonstrated the presence of p50 homodimers in TAMs [59, 60].
In contrast, STAT1, which is downregulated in peritoneal macrophages from tumor bearers, shows a dramatic increase in TAMs (in its constitutive and activated forms) as has also been reported in other tumor models [61, 62]. STAT3, involved in the signaling of the pro-tumor cytokine IL-23 [63], exhibits an increased expression in TAMs, yet no alteration in peritoneal macrophages of tumor bearers when compared with peritoneal macrophages of normal mice.
Altogether, our data demonstrate that there is a gradual alteration, either down- or upregulation, in the expression of critical transcription factors in peripheral macrophages and TAMs, with the more significant changes observed in TAMs [58].
Our work also reveals that macrophages at the tumor microenvironment are even more susceptible to apoptosis than macrophages in the periphery. Resting TAMs express significantly higher levels of activated caspase-3 with augmented expression of pro-apoptotic p53 and lower levels of Bcl-x compared with peritoneal macrophages. Therefore, we demonstrate that there is definitely an important contribution of macrophage cell death in tumor hosts to the compensatory myelopoiesis that occurs in the bone marrows of mice bearing advanced tumors.
Regarding their cellular differentiation, TAMs express even lower levels of the myeloid differentiation markers F4/80, CD11b, CD115 and CD68 than peripheral macrophages from tumor hosts, so they can be considered less differentiated cells than peripheral macrophages. Interestingly, Gr1 is upregulated in TAMs to higher levels than those seen in peritoneal macrophages of tumor hosts.
Inducible nitric oxide synthase (iNOS), arginase and MMP-9 are three enzymes critically involved in the pro-tumor activities of myeloid cells such as TAMs and MDSC. Our studies reveal that peritoneal macrophages and TAMs display opposite expression patterns and enzymatic activities of these markers, but neither peritoneal nor tumor-associated macrophage subpopulations correspond to the classical phenotypical and functional definition of suppressor MDSC, which simultaneously overexpress CD11b and Gr1 and upregulate iNOS and arginase [58].
Macrophage functions are differently altered in TAMs and in peripheral macrophages
Phagocytosis is one of the most important functions of macrophages as cells of the innate immune system, both in homeostasis and in inflammation. We investigated whether the presence of the tumor alters this function in macrophages from tumor hosts. Our results indicate that peritoneal and tumor-associated macrophages from tumor bearers have a similar degree of impaired ability to phagocytose zymosan particles compared with peritoneal macrophages from normal mice but that in the presence of an extrinsic inhibitor of phagocytosis, TAMs show significantly higher phagocytic capacity than peritoneal macrophages [58].
Macrophages are also professional antigen-presenting cells that contribute to T lymphocyte activation and proliferation. We assessed peritoneal macrophages from tumor hosts and TAMs in their ability to modulate cytokine-induced T cell proliferation. Our studies demonstrate that the presence of the tumor induces macrophages to inhibit cytokine-induced T cell proliferation in general, with TAMs displaying a much stronger T cell proliferation inhibitory capability than macrophages from the periphery.
Overall, our results confirm that the tumor microenvironment is the location that mostly alters macrophage activities, due to the intimate contact of macrophages with tumor cells and other cells and factors. We found that these local modifications in the tumor niche are more profound than the changes acquired by macrophages residing in non-tumor, peripheral locations, to which lower tumor factor gradients reach. The complex mixture of cellular components, tumor-derived factors and cytokines/chemokines within the tumor microenvironment may induce TAMs to express a different subset of molecules that are not expressed by macrophages in the periphery of tumor bearers. This could lead to altered macrophage functions that may in turn promote metastatic traits in tumor cells and facilitate tumor progression.
Remarkably, neither peripheral nor tumor local macrophage subpopulations express a definite polarization program; instead, both types of macrophages display mixed features of classical and alternative polarization programs. One possible explanation to these facts in particular case of the tumor microenvironment could be that populations of pure M1 and M2 macrophages could coexist in different niches of the tumor microenvironment and that isolation procedures may result in the disruption of the original tissue architectures, giving the impression of mixtures of M1 and M2 transcriptional programs in TAMs. An alternative explanation may be that individual macrophages could be actively switching programs and simultaneously expressing features of M1 and M2 activation programs, so that in a given niche, macrophages expressing both M1 and M2 programs could exist. In any case, our studies clearly demonstrate that mixtures of pro-inflammatory and immunosuppressive traits are identified in peripheral and also tumor-associated macrophages from tumor hosts, although it is difficult to conclude at this point of our studies whether these are mixed populations of classically and alternatively activated macrophages or whether these are macrophages that simultaneously express both activation programs.
TAMs are found in the proximity of suppressor T lymphocytes in the tumor microenvironment
Another mechanism by which TAMs mediate immunosuppressive effects is by promoting the local accumulation of suppressive regulatory CD4+ T cells (Tregs) in the tumor microenvironment [64]. Tumor-associated regulatory T cells efficiently inhibit CD4+ T cell-dependent immune responses and suppress the proliferation and IFNγ production of antigen-experienced CD8+ T cells.
Our results demonstrate that increased numbers of TAMs and CD3+ cells in the tumor microenvironment correlate with tumor progression. Both TAMs and CD3+ cells co-localized in the tumor stroma of 3-week and increasingly in 4-week tumors. Additionally, peritumoral presence of macrophages and CD3+ cells was noticed in 4-week tumors. These results clearly indicate that TAMs and CD3+ cells accumulate and co-localize in higher numbers preferentially in advanced tumors.
The high levels of TGFβ, IL-6 and IL-23 existing in D1-DMBA-3 tumors (mostly contributed by TAMs) have been related to induction of Tregs and Th17 cells, two cell types associated with tumor progression [65–67]. Interestingly, IL-17 also produced by “altered” tumor-infiltrating Tregs in colorectal tumors is associated with a more aggressive tumor behavior [68]. Detection of Tregs, IL-17-producing CD3+ cells and TAMs co-localizing within the mammary tumor microenvironment suggests the possibility of a cross talk between all these immunosuppressive cell types in advanced tumors.
Blood monocytes, precursors of tissue macrophages, are also altered in tumor-bearing mice, yet they do not exhibit a unique polarization pattern as the macrophages they later become
The results of our work showed that tumor-bearing mice exhibit different degrees of phenotypic alteration and functional impairment in two distinctive macrophage subpopulations located at different distances from the tumor, within the tumor or peripheral to it. Our results strongly indicate that the proximity to the tumor microenvironment results in the highest degree of macrophage alteration but that tissue macrophages residing far from the tumor are also affected to a significant degree that still impairs their phenotype and function. However, little was known about whether similar defects exist in macrophage precursor stages as blood monocytes.
We addressed the experimental challenge of comparing blood monocytes from tumor hosts and normal animals to investigate whether they were significantly different cell types. We thus examined blood monocytes isolated from the same mammary tumor-bearing mice from which we studied macrophage populations and investigated whether these monocytes are already altered in their polarization profiles before becoming tissue macrophages. We also sought to analyze whether they correspond to inflammatory or resident monocyte subtypes similar to the M1/M2 macrophage polarized states.
Phenotypic characterization of monocytes found in mice bearing infections has allowed the identification of two discrete monocyte subsets. These two populations have been described as being CD115+Ly6ChiCX3CR1loCCR2+CD62L+ inflammatory monocytes and CD115+Ly6CloCX3CR1hiCCR2−CD62L− resident monocytes. Inflammatory monocytes are selectively recruited to inflamed tissues and lymph nodes and are the main producers of TNF-α and IL-1. However, the resident monocytes have a longer half-life and exhibit a constitutive long-range crawling on the luminal side of the endothelium within most blood vessels [69–74]. Although extensive data have been collected describing the functions of monocytes during inflammation as a result of infection, sufficient evidence is lacking regarding the characteristics of monocytes in the context of tumor burden.
As expected from an increased myelopoiesis in tumor bearers, we found augmented numbers of circulating blood monocytes in tumor-bearing mice, when we compared them to normal age- and sex-matched controls. Tumor-derived factors condition not only tumor microenvironment and peripheral immune niches, but also the bone marrow, leading to abnormal myelopoiesis and subsequent modification of circulating myeloid cells. Surprisingly, we found that monocytes from tumor-bearing mice express reduced levels of the same myeloid differentiation markers that were downregulated in the two studied subpopulations of macrophages from tumor bearers (CD11b, F4/80, CD68 and CD115). Moreover, downregulation of MHC II, CD62L and the pro-angiogenic marker Tie-2 [75] was observed in these cells, whereas Gr-1 and Ly6C were upregulated. Gene microarray analysis performed by us for the first time in blood monocytes from tumor hosts indicated that these cells express the transcript for C3 complement (and C5R protein), and a mixture of pro-inflammatory and anti-inflammatory cytokines and chemokines. Interestingly, CCR2 and CX3CR1, which are crucial in monocyte definition as inflammatory or resident, respectively, were both upregulated. Finally, monocytes from tumor bearers produce low levels of nitric oxide and lack arginase activity when compared to their normal counterparts [76]. Thus, these monocytes cannot be considered MDSC either, since typical MDSC upregulate nitric oxide and arginase.
Taken together, our studies suggest that blood monocytes from tumor-bearing mice are significantly altered but elude the rigid classification of pro-inflammatory or anti-inflammatory phenotypes and rather exhibit a mixture of these two opposite activation profiles, as the macrophages they later become.
Conclusions and future directions
Defining macrophage subsets by membrane protein and functional phenotype during an inflammatory episode might be akin to defining chameleons by the color pattern they display as they move across an artist’s paint palette [31].
Macrophages are extremely malleable cells that constantly modify their phenotype to adapt to the tissue microenvironments where they live. This plasticity seems to be associated with the macrophage capacity to turn on and off different gene transcriptional programs and thus express various sets of proteins and functions. The initial concept of macrophage polarization viewed these cells as entities that could express definite groups of genes and proteins that allow them to behave as either pro-inflammatory or immunosuppressive cells. It was believed that macrophages either could be inert inactive (Mo) cells that could participate in housekeeping phagocytic functions, or could become activated as pro-inflammatory (M1, classically activated) or suppressor (M2, alternatively activated) cells. Evidence is now accumulating, showing that in vivo macrophages do not necessarily exhibit these dichotomous behaviors or clear-cut gene expression patterns as they show in vitro, where the concept of macrophage polarization was first described.
We have been interested in studying the interplay that exists between a developing tumor and the innate immune system of a tumor host, particularly focusing on the role of macrophages, central inflammatory cells in our bodies that are also innate immune cells with significant roles in cancer development and in tumor prognosis. A body of information has been collected on the mechanisms by which macrophages promote tumor development, but much less is known about the initial steps by which tumors modify macrophages.
Macrophages in the tumor microenvironment have been described by many authors as straightforward M2 immunosuppressive cells contributing to tumor invasion, angiogenesis, extracellular matrix remodeling and metastasis. However, there is also evidence that M2-like macrophages are found in physiologic, healthy and non-inflamed homeostatic tissues. We compared the effects that the presence of a syngeneic mammary tumor has on the phenotype and functions of three different myeloid cell types in tumor-bearing mice: macrophages in intimate contact with the tumor microenvironment (TAMs), macrophages far from the tumor, in the periphery (peritoneal macrophages) and macrophages’ precursors, the systemic circulating blood monocytes. We could not find evidences of definite M1 or M2 activation programs in any of these three cell populations isolated from mice bearing advanced tumors. Instead, macrophages from the tumor, the periphery and blood monocytes all clearly exhibited different and unique mixtures of pro-inflammatory and anti-inflammatory phenotypes and functions, probably as a result of the variety of stimuli they received in the different microenvironments from which they were all isolated.
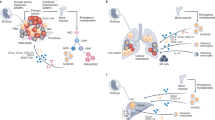
Our work represents the first comprehensive comparative analysis of the differences between three myeloid cell subpopulations exposed to different conditions and environments in tumor hosts. Definitely, it is the tumor microenvironment, with the proximity to tumor cells and the factors they produce, in addition to other immune and non-immune cells and factors present in the tumor stroma, the location that more robustly modifies macrophages and induces a strong immunosuppression in the host. We demonstrated that different tumor models exhibit diverse TAM phenotypes and activation patterns, due to their exposure to different microconditions within each tumor, as other authors have shown as well [77]. We have also confirmed that there is a measurable systemic immune impact in tumor hosts, since peritoneal macrophages and blood monocytes from these mice exhibit immune impairment, decreased degrees of cellular differentiation and altered functions as compared with their normal counterpart populations (Fig. 1).
Our results enable us to conclude that there is not only local immunosuppression in the tumor microenvironment of cancer patients, contributing to tumor development and disease progression, but that also a certain degree of systemic immune deficiency exists in tumor hosts, judging from the alterations that these peripheral myeloid cells also show. The dysfunction and apoptosis of these local and peripheral macrophages and monocytes in the tumor-bearing host create an immune imbalance that contributes to the progression of the disease as well.
Reversal of existing immune dysfunction and normalization of macrophage and monocyte homeostasis in patients with cancer needs to be a part of future cancer immunotherapy. Therapeutic strategies are being designed to repair the immune imbalance and recover the original functions of tumor macrophages. Much effort is currently devoted to reversing macrophage adverse traits in tumor hosts and to “re-educating” them back to non-tumor-promoting cells. Tumor microenvironment could be targeted and modified so that it is unfavorable for tumor cells to grow. Deleting TAMs, re-educating them in antitumor responses and blocking their recruitment into tumors are some of the strategies proposed to reverse the deleterious effects of these cells in cancer development. However, the permanent success of such strategies may be questionable given that the same factors and conditions that initially modified macrophages still remain, and new waves of TAMs can be negatively educated again. In addition, since TAMs reside within tissues, accessing them is limited. Blood monocytes could be better targeted and manipulated by less invasive means but again their “re-education” may be subject to the same limitations as for the TAMs.
Tumor-derived factors and their indirect targets, such as transcription factors, cytokines and chemokines, are ultimately the causes of macrophage changes both in the periphery and in the tumor microenvironment of tumor hosts and may be better targets to control in cancer. Targeting identifiable tumor factors (TGFβ, PGE2, etc.) or their downstream indirect targets, such as transcription factors (NFκB, STAT3), cytokines (IL-23, IL-10) and chemokines (CCL2), may well result in more permanent therapeutic responses than re-educating resilient TAMs or monocytes to help them promote tumor rejection.
Apparently, the rule is no rule at all: Different tumors seem to contain different populations of TAMs and other immune cells with mixed profiles of molecules and functions. Detailed characterization of the cellular and molecular composition of a tumor microenvironment should be carried out, as part of the initial therapeutic assessment of a tumor, in order to better decide the strategy to follow, i.e., deleting, reprogramming or targeting cells or molecules for therapeutic benefit.
References
Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998;64(3):275–90.
Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–17. doi:10.1002/eji.200737638.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi:10.1038/nri1733.
Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15(2):243–50. doi:10.1038/sj.cdd.4402184.
Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27(5):244–50. doi:10.1016/j.it.2006.03.005.
Uderhardt S, Herrmann M, Oskolkova OV, Aschermann S, Bicker W, Ipseiz N, et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834–46. doi:10.1016/j.immuni.2012.03.010.
Lichanska AM, Browne CM, Henkel GW, Murphy KM, Ostrowski MC, McKercher SR, et al. Differentiation of the mononuclear phagocyte system during mouse embryogenesis: the role of transcription factor PU.1. Blood. 1999;94(1):127–38.
Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol. 2007;308(1):232–46. doi:10.1016/j.ydbio.2007.05.027.
Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–95. doi:10.1038/ni.2705.
Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9(4):259–70. doi:10.1038/nri2528.
Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53(1–3):11–24. doi:10.1007/s12026-012-8291-9.
Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41(4):457–65.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. doi:10.1038/nri2448.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Investig. 2012;122(3):787–95. doi:10.1172/JCI59643.
Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175(1):342–9.
Duan M, Li WC, Vlahos R, Maxwell MJ, Anderson GP, Hibbs ML. Distinct macrophage subpopulations characterize acute infection and chronic inflammatory lung disease. J Immunol. 2012;189(2):946–55. doi:10.4049/jimmunol.1200660.
Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi:10.1016/j.immuni.2010.05.007.
Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2013;. doi:10.1007/s10456-013-9381-6.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. doi:10.1126/science.1194637.
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi:10.1126/science.1219179.
Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol. 2011;41(8):2155–64. doi:10.1002/eji.201141817.
Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–8. doi:10.1126/science.1204351.
Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi:10.1038/nri978.
Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–12.
Schreiber RD. Identification of gamma-interferon as a murine macrophage-activating factor for tumor cytotoxicity. Contemp Top Immunobiol. 1984;13:171–98.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Investig. 2007;117(1):175–84. doi:10.1172/JCI29881.
Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–61. doi:10.1038/nri3088.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96. doi:10.1038/ni.1937.
Stout RD, Watkins SK, Suttles J. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol. 2009;86(5):1105–9. doi:10.1189/jlb.0209073.
Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76(3):509–13. doi:10.1189/jlb.0504272.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7. doi:10.1016/j.ccr.2005.02.013.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi:10.1038/nature07205.
Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18(1):11–8. doi:10.1016/j.gde.2007.12.007.
Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Investig. 2008;118(7):2516–25. doi:10.1172/JCI35073.
Nowarski R, Gagliani N, Huber S, Flavell R. Innate immune cells in inflammation and cancer. Cancer Immunol Res. 2013;1(2):77–84.
Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. doi:10.1038/nrc1256.
Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–65. doi:10.1002/path.1027.
Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi:10.1186/1471-2407-12-306.
Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15(4):1069–74. doi:10.1677/ERC-08-0036.
Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–12. doi:10.1158/0008-5472.CAN-05-4005.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi:10.1016/j.cell.2010.03.014.
Medina D, DeOme KB. Response of hyperplastic alveolar nodule outgrowth-line D1 to mammary tumor virus, nodule-inducing virus, and prolonged hormonal stimulation acting singly and in combination. J Natl Cancer Inst. 1969;42(2):303–10.
Torroella-Kouri M, Ma X, Perry G, Ivanova M, Cejas PJ, Owen JL, et al. Diminished expression of transcription factors nuclear factor kappaB and CCAAT/enhancer binding protein underlies a novel tumor evasion mechanism affecting macrophages of mammary tumor-bearing mice. Cancer Res. 2005;65(22):10578–84. doi:10.1158/0008-5472.CAN-05-0365.
Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–59. doi:10.1038/nri1703.
Torroella-Kouri M, Lopez DM. Mammary tumor-derived TGF-b1 impairs crucial innate immune responses in tumor hosts. J Immunol Immunopathol. 2003;5(1):31–8.
Torroella-Kouri M, Silvera R, Rodriguez D, Caso R, Shatry A, Opiela S, et al. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69(11):4800–9. doi:10.1158/0008-5472.CAN-08-3427.
Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–52. doi:10.1038/nri1498.
Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Investig. 2007;117(5):1155–66. doi:10.1172/JCI31422.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi:10.1038/nri2506.
Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–52. doi:10.1038/nrc3581.
Ilkovitch D, Lopez DM. Urokinase-mediated recruitment of myeloid-derived suppressor cells and their suppressive mechanisms are blocked by MUC1/sec. Blood. 2009;113(19):4729–39. doi:10.1182/blood-2008-08-176438.
Torroella-Kouri M, Keith JC, Ivanova M, Lopez DM. IL-11-induced reduction of C/EBP transcription factor binding may contribute to the IL-12 downregulation in tumor-bearing mice. Int J Oncol. 2003;22(2):439–48.
Muscaritoli M, Bossola M, Battista Doglietto G, Rossi Fanelli F. The ubiquitin/proteasome system in cancer cachexia. New Jersey: A Modern Approach Springer-Verlag; 2006.
Perry G, Iragavarapu-Charyulu V, Harhaj EW, Torroella-Kouri M. Role of the proteasome in the downregulation of transcription factors NFkappaB and C/EBP in macrophages from tumor hosts. Oncol Rep. 2010;23(3):875–81.
Wang T. The 26S proteasome system in the signaling pathways of TGF-beta superfamily. Front Biosci. 2003;8:d1109–27.
Petrel TA, Brueggemeier RW. Increased proteasome-dependent degradation of estrogen receptor-alpha by TGF-beta1 in breast cancer cell lines. J Cell Biochem. 2003;88(1):181–90. doi:10.1002/jcb.10353.
Rodriguez D, Silvera R, Carrio R, Nadji M, Caso R, Rodriguez G, et al. Tumor microenvironment profoundly modifies functional status of macrophages: peritoneal and tumor-associated macrophages are two very different subpopulations. Cell Immunol. 2013;283(1–2):51–60. doi:10.1016/j.cellimm.2013.06.008.
Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA. 2009;106(35):14978–83. doi:10.1073/pnas.0809784106.
Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, et al. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66(23):11432–40. doi:10.1158/0008-5472.CAN-06-1867.
Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107(5):2112–22. doi:10.1182/blood-2005-01-0428.
Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174(8):4880–91.
Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15(2):114–23. doi:10.1016/j.ccr.2008.12.018.
Terme M, Ullrich E, Aymeric L, Meinhardt K, Coudert JD, Desbois M, et al. Cancer-induced immunosuppression: IL-18-elicited immunoablative NK cells. Cancer Res. 2012;72(11):2757–67. doi:10.1158/0008-5472.CAN-11-3379.
Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi:10.1016/S0065-230X(10)07003-X.
Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–64. doi:10.1084/jem.20090207.
Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50(5):980–9. doi:10.1016/j.jhep.2008.12.033.
Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, et al. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA. 2010;107(14):6430–5. doi:10.1073/pnas.0913683107.
Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. doi:10.1146/annurev.immunol.021908.132557.
Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86(5):398–408. doi:10.1038/icb.2008.19.
Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82.
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61. doi:10.1126/science.1178331.
Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82(2):244–52. doi:10.1189/jlb.0307191.
Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol. 2010;17(1):53–9. doi:10.1097/MOH.0b013e3283324f80.
De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–26. doi:10.1016/j.ccr.2005.08.002.
Caso R, Silvera R, Carrio R, Iragavarapu-Charyulu V, Gonzalez-Perez RR, Torroella-Kouri M. Blood monocytes from mammary tumor-bearing mice: early targets of tumor-induced immune suppression? Int J Oncol. 2010;37(4):891–900.
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–39. doi:10.1158/0008-5472.CAN-09-4672.
Acknowledgments
I thank all members of my group, past and present, who have contributed to the work and ideas discussed in this manuscript. Special thanks to Risset Silvera and Giselle Perry who actively and significantly contributed to this research, as well as to Ozzie Perez, who prepared the included figure. Research has been supported by the National Institutes of Health Grants R21 CA153172 and KO1 CA101926 from MTK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torroella-Kouri, M., Rodríguez, D. & Caso, R. Alterations in macrophages and monocytes from tumor-bearing mice: evidence of local and systemic immune impairment. Immunol Res 57, 86–98 (2013). https://doi.org/10.1007/s12026-013-8438-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-013-8438-3