Abstract
The purpose of this study was to analyze the presence of interleukins 6, 8, and 18 in post-mortem lung tissue of subjects deceased due to polytrauma. In addition to this, we have described different micromorphological features of lung tissue in ARDS cases associated with fatal traffic trauma. A total of 18 autopsy cases with ARDS after polytrauma and 15 control autopsy cases were analyzed in this study. From every subject, we collected one sample for each lung lobe. All of the histological sections were analyzed by using light microscopy, and for the purpose of ultrastructural analysis, we used transmission electron microscopy. Representative sections were further processed by way of immunohistochemistry analysis. Quantification of IL-6, IL-8, and IL-18-positive cells was conducted by applying the IHC score. We noticed that all samples of ARDS cases exhibited elements of the proliferative phase. Immunohistochemical analysis of lung tissue in patients with ARDS showed strong positive staining for IL-6 (2.8 ± 0.7), IL-8 (2.2 ± 1.3), and IL-18 (2.7 ± 1.2), while staining of the control samples resulted in no positivity to low/moderate positivity (for IL-6 1.4 ± 0.5; for IL-8 0.1 ± 0.4; for IL-18 0.6 ± 0.9). Only IL-6 correlated negatively with the patients’ age (r = −0.6805, p < 0.01). In this study, we described microstructural changes in lung sections of ARDS cases and control cases, as well as interleukins’ expression, demonstrating that autopsy material is as informing as tissue samples collected by performing open lung biopsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute respiratory distress syndrome (ARDS) is a syndrome of acute respiratory failure caused by noncardiogenic pulmonary edema [1]. It is a life-threating respiratory condition characterized by hypoxemia and stiff lungs that lead to the death of most patients unless mechanical ventilation is applied [2]. Incidence and mortality of ARDS are important so that patients could obtain adequate care and treatment. Criteria for the diagnosis for ARDS have changed over time. In 1994, American-European Consensus Conferences (AECC) published a statement on definitions, mechanisms, relevant outcomes, and clinical trial coordination that attempted to delineate and guide treatment [3] and defined tree criteria based on which the diagnosis is made: heavy hypoxia with PaO2/FiO2 ratio < 200 mmHg, bilateral opacities that are not fully explained by effusions and lobar/lung collapse or nodules [2,3,4,5,6,7,8]. Later, in 2012, Berlin Clinical Classification of ARDS was established to classify patients according to the etiology of ARDS. The current working definition proposes three mutually exclusive categories: mild (PaO2/FiO2 200–300 mmHg), moderate (PaO2/FiO2 100–200 mmHg), and severe (PaO2/FiO2 < 100 mmHg) [2,3,4,5,6], with widely varied reported mortality rates.
Potential biomarkers that indicate activation or damage of certain cell types may be useful in understanding the pathogenetic mechanisms and improving the diagnosis of ARDS [6]. Thrombin and proinflammatory cytokines activate endothelial cells, lead to the activation of plasminogen inhibitors, and suppress fibrinolysis [9, 10]. IL-6 is a proinflammatory cytokine that stimulates adhesive molecules expression in endothelial cells, suppresses fibrinolysis, and leads to endothelial capillary destruction [11]. IL-8 is significant neutrophil chemotactic factor [9]. Neutrophil migration is correlated with severity and volume of pulmonary parenchyma destruction in ARDS [11]. Activated neutrophils in ARDS produce numerous cytotoxic substances, including granular enzymes, bioactive lipid, numerous proinflammatory cytokines, and neutrophil extracellular traps (NETs). IL-18 is a proinflammatory cytokine that belongs to the IL-1 superfamily [16] and operates in synergy with IL-12 [9, 11,12,13].
To the best of our knowledge, various authors detected different proinflammatory cytokines in bronchoalveolar fluid and lung tissue sampled during open lung biopsy, but only few with detection of interleukin in autopsy-sampled lung tissue. Therefore, the aim of this study is to analyze the presence of interleukins 6, 8, and 18 in post-mortem lung tissue of subjects deceased due to multiple traumas. In addition to this, we have demonstrated that different micromorphological features of lung tissue in ARDS are associated with fatal traffic trauma.
Material and methods
A total of 18 autopsy cases with ARDS after polytrauma and 15 control autopsy cases (cases of natural death, without ARDS, pulmonary pathology, and trauma) were analyzed. All cases in the experimental group were pedestrians with various injuries, but without lung injuries. From every subject, we collected five lung tissue samples: one sample for each lung lobe. The including criteria were adult age (≥ 18 years) and diagnosis of ARDS according to the Berlin criteria (2012). The excluding criteria were the following: age under 18 years, deceased who died within 3 days after being admitted to the hospital, presence of obstructive lung disease, pulmonary embolism, pneumothorax, or bronchopleural fistula appearing within less than 2 weeks after lobectomy, cardiac edema, increased intracranial pressure, myasthenia gravis, muscle dystrophy, as well as other causes of ARDS (sepsis, operations, burns). All autopsies (in both groups) were performed within 24 h post-mortem.
Light microscopy analysis
After sampling, the tissue was fixed in an automatic sample processor Leica ASP6025, embedded in paraffin blocks and stained with hematoxylin and eosin (HE). Light microscopy was performed by using Olympus BX45 microscope. A total of 165 histological sections were analyzed, and representative sections were further processed by way of immunohistochemistry analysis.
Immunohistochemical analysis
Immunohistochemical analysis of lung tissue in patients with ARDS was conducted on representative paraffin sections by using Ultravision LP-HRP polymer detection kit (Thermo Scientific, TL-125-HL) with various antibodies: anti-IL6 (Sigma-Aldrich, SAB4301665, dilution 1:20), anti-IL8 (Sigma-Aldrich, HPA057179, dilution 1:100), and anti-IL18 (Sigma-Aldrich, HPA003980, dilution 1:500). After incubation with antibodies, visualization was performed with 3,3′-diaminobenzidine (DAB).
Digital images of HE sections were made with a Leica DM4000B LED (Leica Microsystems, Wetzlar, Germany) microscope with a Digital Camera (Leica DFC295) and LAS 4.4 Software.
Quantification of IL-6, IL-8, and IL-18-positive cells was conducted by applying the IHC score calculated by way of a 10-high-power-fields method (× 400 magnification), where the total proportion of cells staining positively at any intensity was scored as 0 (no cell staining), 1 (less than 25% cells stained; weak, sparse staining), 2 (26 to 50% cells stained, focal positivity), 3 (50 to 75% cells stained, diffuse positivity), and 4 (more than 75% cells stained, strong positivity).
Transmission electron microscopy analysis
For the purpose of ultrastructural analysis of lung tissue changes in ARDS, tissue samples were fixed in 3% glutaraldehyde and postfixed in 1% OsO4. After dehydration in a series of graded alcohols and propylene oxide, samples were embedded in epoxy resin (Sigma-Aldrich, 45,345) and cut by using an ultramicrotome (Leica UltraCut UCT, Leica Microsystems, Wetzlar, Germany). Ultrathin sections were contrasted with uranyl acetate and lead citrate and then analyzed on a transmission electron microscope (Morgagni 268D, FEI, Hillsboro, OR).
Statistical analysis was conducted by implementing the SPSS software (Chicago, IL), using Student’s t-test and Pearson correlation coefficient. A p value below 0.05 was considered as statistically significant and below 0.01 as highly statistically significant.
Results
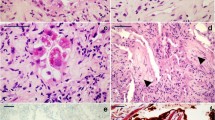
The general characteristics observed in ARDS cases and control cases are presented in Table 1. By using light microscopy, we noticed that all samples of ARDS cases exhibited elements of the proliferative phase, which is consistent with clinical data, given that all were lethal outcomes within 10 days of admission. The analyzed histology sections showed the presence of hyaline membranes, interstitial edema, and a certain degree of intra-alveolar fibrinous exudate. The walls of a great number of alveoli were mostly congested, with disruption and focal alveolar hemorrhage (Fig. 1).
Transmission electron microscopy analysis of ARDS lungs showed that alveolar space was filled with edema and hyaline membranes. Hyaline membranes appeared homogeneous and extended along and partially covered the denuded epithelial basement membranes. Nuclei of some of the alveolar epithelial cells showed chromatin condensation and fragmentation, i.e., features of apoptotic cells. Most alveolar epithelial cells were necrotic and were missing in most parts of the ARDS lungs. Endothelial cells in capillaries were necrotic, and the endothelial basement membrane was thickened. No obvious proliferation of fibroblasts was observed in ARDS lung samples (Fig. 2). In the control samples, the ultrastructure of alveolar epithelial cells types I and II and of endothelial cells did not show the abovementioned pathological features and hallmarks of ARDS.
a Most alveolar epithelial cells were necrotic and were missing in most parts of ARDS lungs. b, c, d TEM images of ARDS showed only small isolated areas with collagen fibers and with no obvious proliferation of fibroblasts. e, f Nuclei of some alveolar epithelial cells showed chromatin condensation and fragmentation, features of apoptotic cells. g Endothelial cells in capillaries were necrotic and with thickened endothelial basement membrane. h In alveoli, the hyaline membranes appear homogeneous and extend along and partially cover the denuded epithelial basement membranes. i The alveolar space of ARDS lungs was filled with edema
Immunohistochemical analysis of lung tissue in patients with ARDS showed strong positive staining for IL-6 (2.8 ± 0.7), IL-8 (2.2 ± 1.3), and IL-18 (2.7 ± 1.2), while staining of the control samples resulted in no positivity to low/moderate positivity (for IL-6 1.4 ± 0.5; for IL-8 0.1 ± 0.4; for IL-18 0.6 ± 0.9) (Fig. 3). Differences in staining of the abovementioned ILs between lungs of patients with ARDS and lungs of the control samples were highly significant (p < 0.01).
In our analysis of IL-6, IL-8, and IL-18, we correlate interleukin staining positivity level with the patients’ age, duration of hospital stays, and the leukocyte and granulocyte number. Pearson correlation coefficient showed that only IL-6 correlated negatively with the patients’ age (r = −0.6805, p < 0.01) (Fig. 4). No other significant correlations have been observed.
Discussion
Etiology of ARDS is diverse. Most common causes are classified as direct (pneumonia, aspiration of gastric content, lung contusion, inhalation lung injury, reperfusion pulmonary edema) and indirect causes (sepsis, multiple trauma, cardiopulmonary bypass, acute pancreatitis, drug abuse) [6]. Despite the ongoing efforts to improve therapy, traumatized patients experience mortality rate of approximately 20%, often due to developing a “post-injury syndrome,” with ARDS being the most common complication during hospitalization [14]. Among the potential mechanisms involved in the “post-injury syndrome,” inflammasomes might become promising targets of future research [15]. “Post-injury syndrome” is referred to as a disorder that leads to local and systemic release of endogenous mediators acting as threat signals (damage-associated molecular patterns — DAMPs) [15]. Recognition of DAMPs by the innate immune system triggers both an intense proinflammatory immune response and a concomitant anti-inflammatory immune response [15].
By using light microscopy, we found certain characteristic features of lung tissue: hyaline membranes, interstitial edema, and increased number of neutrophils. Those histological hallmarks are consistent with the exudative phase of ARDS [17, 18]. In the exudative phase, the earliest changes detectable by light microscopy are capillary congestion, interstitial and alveolar edema, and intra-alveolar hemorrhage, which was demonstrated in our study sample. A far more distinctive histological feature is reflected in the fact that hyaline membranes were most prominent along the alveolar septa [19]. In an attempt to better understand the pathophysiology and immune reactions in ARDS, finding these histological hallmarks in autopsy material is very significant and as informative as open lung biopsy, considering that open lung biopsy is an invasive procedure for a patient on mechanical ventilation [20,21,22,23].
In this research, we attempted to establish a connection between IL-6, 8, and 18 and lethal outcome of ARDS in young and otherwise healthy individuals who suffered trauma of various degree, but which led to them developing life-threatening respiratory failure. Our staining showed the presence of interleukins 6, 8, and 18 in all lung tissue samples of ARDS cases. Cells from samples of lungs prior diagnosed as one with ARDS showed an increase in expression of IL-6, 8, and 18. In our sample, all sections sampled from lungs with ARDS showed a presence of interleukin antibody for IL-6, IL-8, and IL-18. None of those interleukins was present in the control group. In many previous studies, authors reported presence or increased levels of those interleukins as they tried to connect them with such malicious outcomes [3, 24,25,26,27,28,29,30,31,32,33,34]. In this study, not only did we obtain results consistent with research by other authors, namely the presence of interleukins 6, 8, and 18 in lung tissue, but we also identified them in autopsy material. The importance of our findings is multiple, and with these results, we could open the door to easier, cheaper, and less invasive sampling of lung tissue, but with the same quality of molecular information.
Different authors attempted to explain the role of interleukin 6 in pathophysiology of ARDS in trauma patients. Our results showed that IL-6 correlated with a younger age of the deceased and with a shorter survival interval. This result is in concordance with the works of other authors, who suggested that after suffering a blunt trauma, ARDS would more likely develop in younger, male Caucasians [35]. Considering that IL-6 is a proinflammatory cytokine responsible for activation of the innate immune response to trauma, which could lead ether to healing, but could also be a major driver of complications and fatal outcome after injury [36, 37], Niesler et al. suggested that alveolar macrophages activated by blunt trauma release chemokines, inflammatory, and proinflammatory cytokines [37].
Even with the ongoing development of safety systems in traffic and lower numbers of trauma patients worldwide, the outcome of ARDS still poses a challenge to the emergency care units. In order to decrease the mortality rate, it is necessary to make an effort in understanding the role of mediators of inflammation in the development of ARDS. In this paper, we presented our results showing that autopsy material is as informing as tissue samples collected by performing open lung biopsy, but with a clear advantage. By sampling post-mortem specimens, one can provide a representative sample while at the same time avoiding ethical issues of performing an invasive and expensive procedure.
Key points
-
1.
This study analyzed the presence of interleukins 6, 8, and 18 in post-mortem lung tissue of subjects with ARDS deceased due to multiple traumas, as well as in tissue of control cases, and described micromorphological features of lung tissue.
-
2.
Immunohistochemical analysis of autopsy lung tissue in patients with ARDS showed strong positive staining for IL-6, IL-8, and IL-18.
-
3.
IL-6 is in negative correlation with the patients’ age.
-
4.
Autopsy material is as informing as tissue samples collected by performing open lung biopsy.
References
Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40(1):31–9. https://doi.org/10.1055/s-0039-1683996.
Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis. 2013;5(3):326–34. https://doi.org/10.3978/j.issn.2072-1439.2013.04.05.
Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–50. https://doi.org/10.5858/arpa.2015-0519-RA.
Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev. 2017;26:160116. https://doi.org/10.1183/16000617.0116-2016.
Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad med J. 2011;87:612. https://doi.org/10.1136/pgmj.2011.118398.
Spadaro S, Park M, Turrini C, Tunstall T, Thwaites R, Mauri T, et al. Biomarkers for acute respiratory distress syndrome and prospects for personalized medicine. J Inflamm (Lond). 2019;15(16):1. https://doi.org/10.1186/s12950-018-0202-y.
Della Torre V, Badenes R, Corradi F, Racca F, Lavinio A, Matta B, et al. Acute respiratory distress syndrome in traumatic brain injury: how do we manage it? J Thorac Dis. 2017;9(12):5368–81. https://doi.org/10.21037/jtd.2017.11.03.
Han S, Mallampalli RK. The acute respiratory distress syndrome from mechanism to translation. J Immunol. 2015;194:855–60. https://doi.org/10.4049/J.Immunol.14002513.
Hughes KT, Beasley MB. Pulmonary manifestations of acute lung injury-more than just diffuse alveolar damage. Arch Pathol Lab Med. 2017;141(7):916–22. https://doi.org/10.5858/arpa.2016-0342-RA.
Ming Wong J, Leong J, Lee J, Albani S, Yeo J. Insights into the immuno-pathogenesis of acute respiratory distress syndrome. Ann Transl Med. 2019;7(19):504. https://doi.org/10.21037/atm.2019.09.28.
Dinarello C. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103(1 Pt 1):11–24. https://doi.org/10.1016/S0091-6749(99)70518-X.
Hupert L, Matthay M, Ware L. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40(1):31–9. https://doi.org/10.1055/s-0039-1683996.
Máca J, Jor O, Holub M, Sklienka P, Burša F, Burda M. Past and present ARDS mortality rates: a systematic review. Respir Care. 2017;62(1):113–22. https://doi.org/10.4187/respcare.04716.
Haider T, Halat G, Heinz T, Hajdu S, Negrin L. Thoracic trauma and acute respiratory distress syndrome in polytraumatized patient: a retrospective analysis. Minerva Anestesiol. 2017;83(10):1026–33. https://doi.org/10.23736/S375-9393.17.11728-1.
Bortolotti P, Faure E, Kipnis E. Inflammasomes in tissue damages and immune disorders after trauma. Front Immunol. 2018;9:1900. https://doi.org/10.3389/fimmu.2018.01900.
McKee CA, Lukens JR. Emerging roles for the immune system in traumatic brain injury. Front Immunol. 2016;7:556. https://doi.org/10.3389/fimmu.201600556.
Thille AW, Esteban A, Fernandez-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Care Med. 2013;187(7):761–767. https://doi.org/10.1164/rccm.201211-198OC on 31 Jan 2013.
Morales MB, Pires-Neto R, Inforsato N, Lancas T, da Silva LF, Saldiva PH, Mauad T, Carvalho C, Amato M, Dolhnikoff M. Small airway remodeling in acute respiratory distress syndrome: a study in autopsy lung tissue. Crit Care. 2011.
Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21(3):435–66. https://doi.org/10.1016/s0272-5231(05)70158-1.
Papazian L, Thomas P, Bregeon F, Garbe L, Zandotti C, Saux P, Gaillat F, Drancourt M, Auffray JP, Gouin F. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology. 1998;88(4):935–44. https://doi.org/10.1097/00000542-199804000-00013.
Hill JD, Ratliff JL, Parrott JC, Lamy M, Fallat RJ, Koeniger E, Yaeger EM, Whitmer G. Pulmonary pathology in acute respiratory insufficiency: lung biopsy as a diagnostic tool. J Thorac Cardiovasc Surg. 1976;71(1):64–71.
Barbas CS, Capelozzi VL, Hoelz C, Magaldi RB, de Souza R, Sandeville ML, de Campos JR, Werebe E, Andrade Filho LO, Knobel E. Impact of open lung biopsy on refractory acute respiratory failure. J Bras Pneumol. 2006;32(5):418–23.
Fraire AE, Cooper SP, Greenberg SD, Rowland LP, Langston C. Transbronchial lung biopsy. Histopathologic and morphometric assessment of diagnostic utility. Chest. 1992;102(3):748–52. https://doi.org/10.1378/chest.102.3.748.
Allen TC, Fudala R, Nash SE, Kurdowska A. Anti-interleukin 8 autoantibody: interleukin 8 immune complexes visualized by laser confocal microscopy in injured lung. Arch Pathol Lab Med. 2007;131(3):452–6. https://doi.org/10.5858/2007-131-452-AAICVB.
Allen TC, Kurdowska A. Interleukin 8 and acute lung injury. Arch Pathol Lab Med. 2014;138(2):266–9. https://doi.org/10.5858/arpa.2013-0182-RA.
Capelozzi VL, Allen TC, Beasley MB, Cagle PT, Guinee D, Hariri LP, et al. Molecular and immune biomarkers in acute respiratory distress syndrome: a perspective from members of the Pulmonary Pathology Society. Arch Pathol Lab Med. 2017;141(12):1719–27. https://doi.org/10.5858/arpa.2017-0115-SA.
Ware LB, Koyama T, Zhao Z, Janz DR, Wickersham N, Bernard GR, May AK, Calfee CS, Matthay MA. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17(5):R253. https://doi.org/10.1186/cc13080.
Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185(11):1225–34. https://doi.org/10.1164/rccm.201201-0003OC. Epub 2012 Mar 29.
Fujishima S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J Intensive Care. 2014;2(1):32. https://doi.org/10.1186/2052-0492-2-32.
Bime C, Casanova N, Oita RC, Ndukum J, Lynn H, Camp SM, et al. Development of a biomarker mortality risk model in acute respiratory distress syndrome. Crit Care. 2019;23(1):410. https://doi.org/10.1186/s13054-019-2697-x.
Chen S, Yang C, Zhu L, Jiang J. Plasma protein biomarkers of ALI and ARDS. Intern Clin Exp Med. 2017;10(8):12308–14.
Unver N, McAllister F. IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018;41:10–7. https://doi.org/10.1016/j.cytogfr.2018.04.004. Epub 2018 Apr 18.
Swaroopa D, Bhaskar K, Mahathi T, Katkam S, Raju YS, Chandra N, Kutala VK. Association of serum interleukin-6, interleukin-8, and Acute Physiology and Chronic Health Evaluation II score with clinical outcome in patients with acute respiratory distress syndrome. Indian J Crit Care Med. 2016;20(9):518–25. https://doi.org/10.4103/0972-5229.190369.
Pires-Neto RC, Morales MM, Lancas T, Inforsato N, Duarte MI, Amato MB, de Carvalho CR, da Silva LF, Mauad T, Dolhnikoff M. Expression of acute-phase cytokines, surfactant proteins, and epithelial apoptosis in small airways of human acute respiratory distress syndrome. J Crit Care. 2013;28(1):111.e9-111.e15. https://doi.org/10.1016/j.jcrc.2012.05.013.
Kasotakis G, Stanfield B, Haines K, Vatsaas C, Alger A, Vaslef SN, Brooks K, Agarwal S. Acute respiratory distress syndrome (ARDS) after trauma: improving incidence, but increasing mortality. J Crit Care. 2021;64:213–8. https://doi.org/10.1016/j.jcrc.2021.05.003.
Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. 2018;19(4):327–41. https://doi.org/10.1038/s41590-018-0064-8.
Niesler U, Palmer A, Radermacher P, Huber-Lang MS. Role of alveolar macrophages in the inflammatory response after trauma. Shock. 2014;42(1):3–10. https://doi.org/10.1097/SHK.0000000000000167.
Funding
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 145007) and SASA (Grant No. F-35).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The authors received approval for this research from the Ethical Committee of the Faculty of Medicine, University in Belgrade (permission no. 1322/X-31).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuzmanović, J., Savić, S., Bogdanović, M. et al. Micromorphological features and interleukin 6, 8, and 18 expressions in post-mortem lung tissue in cases with acute respiratory distress syndrome. Forensic Sci Med Pathol 20, 1–7 (2024). https://doi.org/10.1007/s12024-022-00572-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-022-00572-4