Abstract
When a firearm projectile hits a biological target a spray of biological material (e.g., blood and tissue fragments) can be propelled from the entrance wound back towards the firearm. This phenomenon has become known as “backspatter” and if caused by contact shots or shots from short distances traces of backspatter may reach, consolidate on, and be recovered from, the inside surfaces of the firearm. Thus, a comprehensive investigation of firearm-related crimes must not only comprise of wound ballistic assessment but also backspatter analysis, and may even take into account potential correlations between these emergences. The aim of the present study was to evaluate and expand the applicability of the “triple contrast” method by probing its compatibility with forensic analysis of nuclear and mitochondrial DNA and the simultaneous investigation of co-extracted mRNA and miRNA from backspatter collected from internal components of different types of firearms after experimental shootings. We demonstrate that “triple contrast” stained biological samples collected from the inside surfaces of firearms are amenable to forensic co-analysis of DNA and RNA and permit sequence analysis of the entire mtDNA displacement-loop, even for “low template” DNA amounts that preclude standard short tandem repeat DNA analysis. Our findings underscore the “triple contrast” method’s usefulness as a research tool in experimental forensic ballistics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The analysis of bloodstain patterns, first mentioned in the nineteenth century [1] is an important aspect of forensic crime scene reconstruction. Caused by shots against biological targets a spray of biological material (e.g., blood and tissue) may be ejected a wound in the direction of the projectile’s trajectory, but some material is propelled from the entrance wound back towards the firearm. This phenomenon has become known as the “backspatter effect” and when contact shots or shots from short distances are applied backspattered biological material may be found on the inside surfaces of the firearm (e.g., the barrel inside), on the shooter (e.g., on his/her hands), and the shooter’s surroundings, where it can persist and be recovered for forensic analysis. As early as 1931, Weimann [2] demonstrated the forensic importance of traces and stains resulting from backspatter. Three years later, Brüning and Wiethold [3] first investigated and systematically evaluated traces of biological material on both outside and inside surfaces of firearms used in suicidal shootings, which was subsequently verified by other groups [4–6]. The persistence of DNA and RNA amenable for forensic analysis on internal parts of firearms has only recently been demonstrated [7–9].
However, criminal acts involving shooting firearms at biological targets cannot be planned or controlled; therefore experimental shootings using standardized ballistic models are indispensable for the systematic analysis of backspatter and the investigation of wound ballistic phenomena.
To facilitate efficient analysis with maximum utilization of evidence generated during expensive and cumbersome experimental shootings, Schyma et al. [10] developed the “triple contrast” method for the simultaneous analysis of backspatter encompassing short tandem repeat (STR) profiling of nuclear DNA (nDNA) and wound ballistic effects.
However, samples in forensic casework are frequently comprised of aged and/or degraded trace material of a reduced quantity and quality that may preclude standard STR profiling of nDNA. In these cases, the analysis of hyper-variable regions in the displacement loop (D-loop) of mitochondrial DNA (mtDNA) as a valid, reliable and sensitive alternative to nDNA typing is advisable, and robust methods for the analysis of the complete D-loop, even from highly degraded material, have already been described [11, 12].
Knowing the composition of biological material from a crime scene, e.g., whether a stain contains blood or semen, is complementary to DNA based identification and can be essential in reconstructing the course of events in criminal investigations. Today, numerous studies document the practicability of mRNA and microRNA (miRNA) analyses for body fluid identification (BFI) based on the detection of differentially expressed mRNA and miRNA [13]. Also, RNA can be co-extracted with DNA from small amounts of forensic samples [14] and even from backspatter recovered from the inner surfaces of firearms that had been fired at human targets [9].
The aim of the present study was to expand the applicability of the “triple contrast” method by evaluating its compatibility with forensic analysis of mtDNA and the simultaneous investigation of co-extracted mRNA and miRNA from backspatter collected from inside parts of firearms after experimental shootings.
Materials and methods
Blood collection and ethics
To generate ballistic samples we used EDTA-free blood drawn by venipuncture from three different healthy Caucasian individuals (one male, two females) with their informed consent. These persons had no physical contact whatsoever with any firearm and model construction used in this study. For comparison, DNA profiles had been generated from pristine volunteers’ blood samples in advance. The study protocol was reviewed and approved by the ethics committee of the Hospital of the University of Bonn.
Ballistic models and sample collection
For this study ten different firearms were used: six revolvers (two .38 Special, three .357 Magnum and one 9 × 19 mm) and four semi-automatic pistols (all 9 × 19 mm). Caliber .38 Special (Magtech Ammunition Company Inc., Lino Lakes, USA) lead round-nose ammunition (projectile weight: 10.2 g) and 9 × 19 mm (Fiocchi Munizioni S.p.A, Lecco, Italy) full metal jacketed bullets (projectile weight: 10.2 g) were fired.
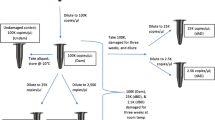
Ballistic models were constructed as described elsewhere [10]. Briefly, a triple mixture of 1 ml blood, 2 ml acrylic paint (CPM, Erkrath, Germany) and 2 ml Micropaque® (Guerbert, Brussels, Belgium), a barium sulphate-based radiocontrast agent, was sealed into thin 5 × 5 cm foil bags. Each bag was attached to a 0.5 l polyethylene bottle filled with 10 % gelatine “Ballistic III” (Gelita, Ebersbach, Germany) which was then covered with a 2–3 mm thick silicon layer and stored at 4 °C for 48 h until use.
Shots were fired with the muzzle touching the ballistic model. After each firing and before cleaning of the weapon, samples were collected using a modified double swab technique [15] by thoroughly wiping the inner surface of the proximal and distal part of the barrel with DNA-free forensic swabs (Sarstedt AG & Co, Nümbrecht, Germany) half moistened with sterile, desalted water, half dry.
Subsequently, the external and internal surfaces of the weapon were cleaned meticulously to prevent contamination.
DNA/RNA co-extraction, DNA quantification and STR profiling
DNA was extracted from collected samples using the PrepFiler® Forensic DNA Extraction Kit (Life Technologies, Darmstadt, Germany) according to the manufacturer’s instructions. 40–35 µl of the eluate was diverted for RNA analysis (see below). All DNA extracts were stored at −80 °C until further processing.
DNA concentration and potential PCR inhibition were quantified by quantitative PCR (qPCR) using the Plexor® HY System (Promega, Mannheim, Germany) according to the manufacturer’s protocol on an ABI Prism 7500 Sequence Detection System (Life Technologies).
STR-Multiplex-PCR was performed using the Powerplex® ESX 17 Kit (Promega) according to the provider’s protocol for selected samples with input amounts between 4 and 500 pg of DNA. PCR products were detected on ABI 310 Genetic Analyzer (Life Technologies). Analysis of raw data and DNA profile generation was done with the GeneMapper v3.2.1 software (Life Technologies). The analytical threshold for a positive result was set at 50 RFU.
Analysis of mitochondrial DNA
Due to the enhanced risk of contamination in mtDNA analysis, all procedures were performed in a working area dedicated to “low template” DNA. Also, prior to each work phase, all surfaces, machines, and devices utilized in the process were thoroughly cleansed using Roti®-Nukleinsäurefrei (Carl Roth, Karlsruhe, Germany) and Softa-Man® (B. Braun, Melsungen, Germany) to remove all traces of ambient nucleic acid contaminations.
The complete control region of the mtDNA was amplified according to the “Mito-Mini” method described by Eichmann and Parson [11]. Briefly, ten overlapping mini-amplicons are generated in two multiplex PCR assays which can then be sequenced.
Amplification was performed on a PTC-220 DNA Engine DYAD™ (MJ Research™, Waltham, USA) with the cycling conditions described in Eichmann and Parson: an initial denaturation step at 95 °C for 2 min followed by 39 cycles at 95 °C for 15 s, 57 °C for 10 s, and 72 °C for 10 s. Prior to sequencing, PCR products from all samples were examined via microfluid-gelelectrophoresis using the DNA 7500 Kit on an Agilent 2100 Bioanalyzer (both Agilent, Böblingen, Germany).
PCR products were then purified using ExoSAP-IT® (Affymetrix, Santa Clara, USA). For sequencing, the BigDye® Terminator v1.1 Cycle Sequencing Kit (Life Technologies) on a PTC-220 DNA Engine DYAD™ (MJ Research) was used as per manufacturer’s prescription and with a primer concentration of 0.5 µM for all sequencing reactions. The sequencing reaction products were cleared of residual dye terminators using Centri-Sep™ spin columns (Life Technologies) according to the manufacturer’s prescription. The sequence fragments were then separated via capillary electrophoresis on an ABI 310 Genetic Analyzer using POP-6 Polymer (both Life Technologies) and analysis of raw data was performed using Sequencing Analysis software v3.7 and SeqScape® software v2.1 (both Life Technologies).
Selection of blood specific genes of interest and reference genes for the normalization of miRNA- and mRNA-expression data
For the detection of blood and based on previous work [16–19] the following blood specific mRNA and miRNA were chosen: β-hemoglobin (HBB) and miR-16 (Table 1). These assays do not amplify genomich DNA (gDNA), as they are specific for miRNA and complementary DNA (cDNA) (exon junction spanning), respectively.
Also, a panel of nine candidate reference genes for normalization of miRNA expression from blood comprising miRNAs and small nucleolar RNAs (snoRNA) was compiled based on the work of Sauer et al. [17]. The resulting panel of normalization candidates consisted of miR-93, miR-191, RNU6-2, SNORA66, SNORA74A, SNORD24, SNORD38B, SNORD43, and SNORD49A.
Assessment of the nine selected candidate reference genes was performed using the GenEx software v6 (multiD Analyses, Göteborg, Sweden), which integrates the geNorm [22] and NormFinder [23] algorithms to evaluate relative gene expression stability. In addition the MS Excel-based “BestKeeper” [24] algorithm was applied, which compares expression levels by pair-wise correlation analysis. Based on this analysis we chose SNORA66 as best suited reference gene (Table 1).
For the selection of candidate reference genes for mRNA expression data normalization we applied the online accessible “Genevestigator” v4-35-1 (Nebion AG, Zurich, Switzerland), a multi-organ microarray data browsing algorithm [25]. To facilitate reference gene selection, “Genevestigator” offers the “RefGenes” application [26]. Using this tool, we chose ribosomal protein L37a (RPL37A) as the reference gene exhibiting minimal expression variance and similar expression intensity across a large set of relevant gene expression arrays for our pair of genes (gene of interest:reference gene) (Online Resource 1).
RNA quantification and integrity assessment
Prior to all RNA processing, all surfaces, machines, and devices utilized in the processes were thoroughly cleansed using RNase-Zap® (Ambion, Austin, TX, USA) and Roti®-Nukleinsäurefrei (Carl Roth) to remove all traces of ambient RNases and nucleic acid contaminations. Only RNase-free reagents and plastic consumables were used and RNA was kept on ice during processing or was stored at −80 °C until further proceedings.
Total quantity and quality of RNA in the eluate diverted from DNA extraction as described above were measured using QuantiFluor® RNA Dye on a Quantus™ Fluorometer (both Promega) and the RNA 6000 Pico Kit on an Agilent 2100 Bioanalyzer (both Agilent, Böblingen, Germany), respectively. For both methods, 1 µl of DNA extraction eluate was used and all measurements were performed according to the manufacturers’ recommendations. RNA quality is represented by the “RNA integrity number” (RIN) as described elsewhere [27]. Note, that RNA was not extracted separately using any dedicated extraction kit but was taken directly from the eluate from the DNA extraction procedure.
Based on quantification results, all individual samples were diluted to a final concentration of 2 ng/µl with RNAse free water. If RNA yield could not be detected fluorometrically, the maximum input volumes for the reverse transcription reactions were used instead.
Reverse transcription (RT) and qPCR
cDNA was synthesized from mRNA and miRNA using the High Capacity Reverse Transcription Kit with random hexamer primers and the TaqMan® MicroRNA Reverse Transcription Kit with target-specific stem-loop primers (both Life Technologies) according to manufacturer’s protocols and in total volumes of 20 µl and 15 µl, respectively. Each mRNA RT reaction consisted of 20 ng of total RNA, 1× RT Buffer, 100 mM dNTPs, 1× Random Primers, and 50U MultiScribe™ Reverse Transcriptase. Each miRNA RT reaction comprised 10 ng of total RNA, 1× RT Primers, 50U MultiScribe™ Reverse Transcriptase, 1 mM dNTPs, 3.8U RNase Inhibitor, and 1× RT Buffer.
All RT reactions were performed on a T3 Thermocycler (Biometra, Göttingen, Germany) with the following cycling conditions for mRNA: 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 min. For RT of miRNA: 16 °C for 30 min, 42 °C for 30 min, and 85 °C for 5 min.
To detect potential cross-reactivity with genomic DNA, H2O controls (i.e., RT reactions with water instead of RNA template) and RT(−) controls (i.e., RT reaction without reverse transcriptase) were set up.
RNA was kept on ice during all steps and RT reaction products were stored at −20 °C until further proceedings. For mRNA, qPCR was performed using target-specific TaqMan® Assays for the gene of interest and the reference gene (Table 1) and the TaqMan® Universal PCR Master Mix, No AmpErase® UNG (all Life Technologies), according to manufacturer’s prescription. 4 µl of the corresponding RT reaction product were used in each qPCR with 1× TaqMan® Universal PCR Master Mix and 1× specific TaqMan®Assay adding up to a total reaction volume of 20 µl.
For miRNA, qPCR reactions were performed using two target-specific TaqMan® Assays for the gene of interest and the reference gene (Table 1) and the TaqMan® Universal PCR Master Mix II, No AmpErase® UNG (both Life Technologies) as per manufacturer’s protocol: Each 20 µl reaction contained 1.3 µl of the respective RT reaction product, 1× TaqMan® Universal PCR Master Mix and 1× specific TaqMan®Assay.
All reaction components were kept on ice during processing.
All sample/assay combinations were run in technical duplicates conducted in MicroAmp® Optical 96-Well Reaction Plates (Life Technologies), both for mRNA and miRNA, with the following qPCR cycling conditions: 95 °C for 10 min, followed by 40 cycles with 95 °C for 15 s and 60 °C for 1 min. All qPCR were performed on an ABI Prism 7500 Sequence Detection System (Life Technologies).
For all quantification reactions the internal PCR control from the Quantifiler® Human DNA Quantification Kit (Life Technologies) was used as an inter plate calibrator. Data was collected during the 60 °C step by the SDS software v2.0.6 (Life Technologies). Raw fluorescence was recorded by the SDS software and then exported for further analysis.
Data analysis, normalization, statistics, and MIQE compliance
To calculate quantification cycle (Cq)-values and amplification efficiencies from SDS spread sheet exported Rn-values from mRNA and miRNA qPCR reactions, the LinRegPCR analysis program v2015.1 [28] was applied. For calculation of Cq-values a common threshold was set to −0.7 log (fluorescence). Cq-values deviating more than one cycle from the duplicate median were excluded from further processing. Normalization of computed Cq-values of genes of interest was performed using the GenEx software v6 (multiD Analyses). A Cq-value of < 35 was considered to indicate successful PCR implying a specific signal and thus RNA quality suitable for expression analysis.
Data analysis, interpretation, and statistical calculations (t tests in this study) were performed using MS EXCEL and SPSS software v.22 (SPSS Inc., Chicago, IL, USA). p values < 0.05 were interpreted as significant.
To facilitate reliable and unequivocal interpretation of the qPCR results reported herein, all information that is rated “essential” according to the MIQE guidelines [29] is reported, where applicable.
Results
DNA quantification and STR profiling of triplex mixtures
After experimental shots, biological material was collected from the anterior and posterior half of the barrel and analyzed separately. Positive DNA quantification results were obtained for all collected samples, however a large variance of DNA yield was observed between samples from different barrel parts as well as weapon types and blood donors. The highest DNA concentration (4.10 ng/µl) was obtained for a sample recovered from the anterior barrel half of a revolver 0.357 Magnum whereas the lowest DNA amount (0.0002 ng/µl) resulted from a sample collected from the posterior barrel half of a revolver 0.38 Special.
In general and as was to be expected, samples recovered from the front half of a barrel contained significantly more DNA (median 0.25 ng/μl, ±1.29 SD) than samples obtained from the rear half (median 0.01 ng/μl, ±0.19 SD; p = 0.001). Notably though, DNA yields varied between the weapon types (pistol vs. revolver) with samples collected from pistols exhibiting lower median DNA concentrations (0.14 ng/µl, ±1.06 SD) than those collected from revolvers (0.24 ng/µl, ±0.96 SD).
To assess the success rate of STR typing from “triplex mixture” samples as limited by DNA amount, DNA profiles were generated for selected samples with varying DNA yields and rated according to the following result categories:
-
“++”, full STR profiles (17 of 17 possible STR systems), “+”, identifiable profiles (at least 8–16 of 17 possible STR systems), and “−”, not identifiable profiles (<8 of 17 possible STR systems).
-
“++” was achieved for samples with an STR-PCR input amount of >100 pg of DNA, “+” was achieved with an STR–PCR input amount of >40 pg up to ≤100 pg (except #16, Table 2, which produced a “−” despite of an input DNA amount of 80 pg), and “−” was obtained if the STR–PCR input amount of DNA was <40 pg.
Table 2 STR typing and mtDNA sequencing results of selected samples across a range of DNA amounts
The majority of DNA profiles consisted of eight or more successfully amplified and correctly typed STR loci (82 %). In one case (#13, Table 2) a mixed STR profile was obtained, indicating residual contamination from previous shootings. PCR inhibition was not observed in any case.
Analysis of mtDNA
Analysis of mitochondrial DNA was performed for selected samples with a DNA yield ≤0.007 ng/µl and these results are summarized in Table 2. For all tested samples, at least 8 of 10 fragments were successfully amplified as demonstrated via microfluid–gelelectrophoresis (an example is shown in Online Resource 2). To analyze mtDNA haplotypes all amplified fragments, representing the entire D-loop region of mtDNA, were sequenced for selected samples that exhibited a DNA amount of less than 100 pg and for which only partial or no STR profiles at all had been obtained (Table 2). In 73 % of samples and down to a DNA amount of 4 pg a complete alignment of all (10/10) amplicon sequences to the CRS reference sequence [30] was obtained. For the remaining samples at least 8 of 10 sequenced amplicons could be aligned. In general, sequence quality differed between multiplexes 1 and 2. Multiplex 2 amplicons on average exhibited lower sequence quality than multiplex 1 amplicons though overall a high sequence quality of completely aligned sequences was observed. Only in one case (#19, Table 2) was a complete alignment of HVI and HVII not possible. All haplotypes obtained correctly matched those of the pristine blood positive controls. In the case indicating contamination (#13, Table 2) in its STR profile, described above, a corresponding contamination was noticed in the mtDNA sequence (Fig. 1).
Representative electropherograms of successful STR profiling and mtDNA sequencing. A Exemplary STR profile of a sample obtained from the posterior barrel half of a revolver cal. 357 Magnum with a template DNA amount of 40 pg (donor A). a Exemplary sequencing electropherogram of the same sample described using primer F160947. B Exemplary STR profile of a sample obtained from the posterior barrel half of a revolver cal. 357 Magnum with a template DNA amount of 100 pg (donor B), showing evidence of contamination b exemplary sequencing electropherogram of the same sample described using primer F160947, showing evidence of contamination. STR loci: (1) amelogenin; (2) D3S1358; (3) TH01; (4) D21S11; (5) D18S51
Quantity and integrity of co-extracted RNA
Total RNA (processed from eluates diverted from DNA extraction) exhibited considerable variation in quantity and quality (RIN) among all samples and quantification results and RIN values are summarized comprehensively in Online Resource 3.
Overall, the highest RNA yield (6.30 ng/µl) was obtained for a sample recovered from the anterior barrel half of a revolver 0.357 Magnum but an RNA amount above the limit of detection could only be determined in 49 % of samples. In 75 % of samples collected from pistol type weapons no RNA could be detected. In contrast, RNA was detectable in 67 % of samples recovered from revolvers and also exhibited a slightly higher average RNA concentration (0.56 ng/µl, ±1.27 SD) than those taken from pistols (0.55 ng/µl, ±1.37 SD). RNA integrity in terms of RIN was low on average, as expected, and did not vary significantly between weapon types (revolvers: average RIN: 1.7, ±1.0 SD; pistols: average RIN: 1.4, ±1.2 SD).
Comparing the different barrel halves, samples collected from the anterior half exhibited higher average RNA quantity but lower RNA quality (1.05 ng/µl, ±1.71 SD; RIN: 1.3, ±1.2 SD) than those that were taken from the posterior half (0.07 ng/µl, ±0.12 SD; RIN: 1.8, ±1.1 SD).
Expression analysis of mRNA and miRNA
To assess the general suitability for forensically relevant downstream analyses of RNA isolated from “triple mixture” samples, expression levels of blood specific mRNA (HBB) as well as miRNA (miR-16), were determined by qPCR in selected samples (Table 3). The C q -values calculated and normalized employing the LinReg and GeneEx softwares, respectively, varied between the tested RNAs.
Overall, normalized expression of HBB was blood specific (C q,n < 35) in all selected samples. For five samples (#3, #4, #5, #7, and #12) the C q -values for SNORA66 were >35, these samples were excluded from further analysis. Lower C q -values (representing high start amounts of RNA) were exhibited by miRNA miR-16 (25.59, ±1.55 SD) than mRNA HBB (28.41, ±2.78 SD). The reference gene RPLA37A exhibited lower C q -values (30.43, ±1.31 SD) than SNORA66 (32.31, ±0.75 SD).
All negative controls showed no or unspecific results (C q > 35) for all tested samples.
Discussion
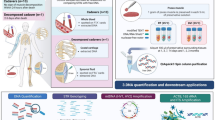
In the present study we are first to describe the simultaneous extraction and forensic analysis of nuclear and mitochondrial DNA, mRNA and miRNA from traces of backspatter collected from the inside surfaces of firearms used in experimental shootings. Additionally, we demonstrate the compatibility of these analyses with multiple stained samples according to the “triple contrast” method [10].
Since its first description in 1925 by Brüning [31], backspatter pattern analysis has become an important tool for both forensic research and routine crime scene reconstruction, and extensive literature on the topic is available [3, 32].
To make use of the information encoded in backspatter patterns encountered in cases of firearm-related crimes the conditions and circumstances for the generation and distribution of backspatter has to be understood. Therefore, systematic investigation of backspatter in controlled experimental settings employing standardized ballistic models [9, 33, 34] is a valuable research approach which is less challenging to realize and to replicate and thus more reproducible than shots at living animals [35–37]. Recently Kunz et al. [38] used spongy material in their models to address the distribution of bloodstains spattered back onto the weapon and the shooter’s hand. The ballistic model used in this study was devised and tested by Lux et al. [9].
In forensic casework, STR genotyping is considered the gold standard for DNA-based identification because of its robustness and high power of discrimination. Courts et al. [34] previously demonstrated the persistence of biological traces in gun barrels after experimental shootings. Their results indicated that DNA isolated from backspatter on internal surfaces of firearms could yield full STR profiles eligible for forensic identification. The nDNA concentrations reported herein varied between 0.0002 ng/µl and 4.10 ng/µl which is consistent with the results of Courts et al. and a study by Schyma et al. [7] who described the persistence of biological traces in gun barrels after fatal contact shots. Both studies indicated that an STR profile eligible for forensic identification required DNA amounts >0.001 ng/µl. Additionally, Lux et al. quantified DNA from backspatter on the inside parts of seven firearms used for suicidal headshots, resulting in concentrations between 0.0015 ng/µl up to 0.0733 ng/µl [9].
These findings are confirmed by our own results in that comparable ranges of DNA yields were obtained and all samples with DNA amounts >0.001 ng/µl exhibited identifiable STR profiles.
In general and as was to be expected, samples recovered from the front half of firearm barrels contained about one order of magnitude more DNA than samples obtained from the rear half. This significant difference between DNA amounts implies a decline of backspatter with increasing distance from the muzzle. The intra-barrel difference was less pronounced in revolvers than in pistols.
STR analysis can be compromised if only highly degraded and/or minuscule amounts of nuclear DNA are available. While only one large molecule of linear nDNA is present per cell, mtDNA in contrast, is comparably short and circular and thus not only exhibits enhanced resistance against degradation but is also present in hundreds, and up to a thousand, copies per cell. Consequently, mtDNA though being less discriminative has long since been employed in forensic casework in cases where STR profiling was unsuccessful due to limited or degraded nDNA [39], although it is mostly used only for the exclusion of non-perpetrators given its lack of specificity as compared to STR profiling. However, and to the best of our knowledge, there is as yet no account in the current forensic literature of the assessment of mtDNA isolated from backspatter on the inside surfaces of firearms. We therefore applied the “Mito-Mini” method specifically designed by Eichmann and Parson [11] for “highly degraded samples” to analyze the complete D-loop of the mtDNA in selected samples with nDNA yields insufficient for STR profiling, and found that down to an nDNA amount of 4 pg, representing less than the nDNA content of a single cell, a complete alignment of all ten amplicon sequences was possible. These results demonstrate that robust protocols for mtDNA analysis, such as “Mito-Mini” or “Mini-Midi-Mito” [40], can serve as alternative approaches for samples with degraded or very low amounts of nDNA, typically encountered in backspatter, that preclude standard short tandem repeat DNA analysis. Disadvantages of the “Mito-Mini” method and forensic mtDNA analysis in general are their high susceptibility to contamination, cumbersome and labor intensive procedures, relatively high costs, and reduced power of discrimination as compared to STR profiling. Therefore, mtDNA analysis is often regarded as a means of last resort to tackle otherwise unaccessible genetic evidence. As such however, mtDNA analysis clearly has its worth and can, as shown here, suitably be employed in the investigation of traces of micro backspatter.
In 1994, Phang et al. first introduced reverse transcription PCR based mRNA-based profiling as a potential tool to forensic genetics [41] which was later taken on by other groups [42–45]. International blind trial exercises of RNA based body fluid identification (BFI) [16, 46], dedicated workshops at forensic genetic conferences, and the implementation of RNA analysis in forensic routine casework in some countries [47], have helped to raise consciousness to the vast potential of forensic RNA analysis. Since 2009, to sidestep the inherent instability of mRNA, the first reports of forensic micro-RNA analysis were presented [18, 48, 49], proving their potential as forensically relevant markers [50]. Due to their short size (18–24 bp) and superior stability miRNA are substantially less prone to degradation than mRNA, which is a crucial asset to forensic investigation and forensic miRNA analysis and their usefulness is now constantly being explored further.
In this study, the presence of blood in the “triple contrast” stained sample material was demonstrated via the detection of expression of blood specific mRNA and miRNA, proving the compatibility of “triple contrast” doping of ballistic models with RNA analysis from backspatter. Whereas normalized blood specific expression of HBB mRNA was detectable in all tested samples, normalization of miRNA expression data failed in some samples that exhibited too low expression of the endogenous control (SNORA66) probably due to insufficient RNA yield.
Conclusion
We have demonstrated that “triple contrast” stained biological material from backspatter collected from the inside surfaces of gun barrels can successfully be subjected to the full bandwidth of forensic nucleic acid analyses, encompassing nuclear and mitochondrial DNA, mRNA, and miRNA.
We show that mtDNA analysis qualifies as an alternative approach to standard STR profiling in molecular ballistics if only small amounts of, and/or highly degraded nDNA, can be retrieved from traces of backspatter. Additionally, we demonstrate the compatibility of “triple contrast” staining of sample material in experimental shootings with forensic body fluid identification based on parsimonious co-extraction and quantitative analysis of blood specific mRNA and miRNA.
Thus, our findings considerably expand the versatility of the “triple contrast” method by broadening its applicability in experimental shootings to the simultaneous analysis of wound ballistic phenomena and all forensically relevant nucleic acid species. This allows for a comprehensive and “economical” realization of experimental shootings, underscoring the method’s usefulness as a research tool in experimental forensic ballistics.
Key points
-
1.
The “triple contrast” method of assessing backspatter can be expanded to encompass forensic co-analysis of DNA and RNA and permit sequence analysis of the entire mtDNA displacement-loop, even for “low template” DNA amounts that preclude standard short tandem repeat DNA analysis, demonstrating its versatility and economy.
-
2.
Samples of backspatter collected from the inside surfaces of firearms are amenable to the full bandwidth of forensic nucleic acid analyses.
-
3.
Analysis of mitochondrial DNA can be used as an alternative approach if standard STR profiling fails because of low template amounts and/or degradation of the sample.
References
Gross H. Handbuch für Untersuchungsrichter, Polizeibeamte, Gendarmen u.s.w. 2nd ed. Graz: Leuschner & Lubinsky; 1894.
Weimann W. Über das Verspritzen von Gewebsteilen aus Einschussöffnungen und seine kriminalistische Bedeutung. Dtsch Z Gerichtl Med. 1931;17:92–105.
Brüning A, Wiethold F. Die Untersuchung und Beurteilung von Selbstmörderschusswaffen. Dtsch Z Gerichtl Med. 1934;23:71–82.
Stone IC. Observations and statistics relating to suicide weapons. J Forensic Sci. 1987;32:711–6.
Stone IC. Characteristics of firearms and gunshot wounds as markers of suicide. Am J Forensic Med Pathol. 1992;13:275–80.
Visser JM. Detection and significance of blood in firearms used in contact gunshot wounds. Dissertation: University of Pretoria; 2003.
Schyma C, Madea B, Courts C. Persistence of biological traces in gun barrels after fatal contact shots. Forensic Sci Int Genet. 2013;7:22–7.
Courts C, Gahr B, Madea B, Schyma C. Persistence of biological traces at inside parts of a firearm from a case of multiple familial homicide. J Forensic Sci. 2014;59:1129–32.
Lux C, Schyma C, Madea B, Courts C. Identification of gunshots to the head by detection of RNA in backspatter primarily expressed in brain tissue. Forensic Sci Int. 2014;237:62–9.
Schyma C, Lux C, Madea B, Courts C. The ‘triple contrast’ method in experimental wound ballistics and backspatter analysis. Int J Legal Med. 2015;. doi:10.1007/s00414-015-1151-0.
Eichmann C, Parson W. ‘Mitominis’: multiplex PCR analysis of reduced size amplicons for compound sequence analysis of the entire mtDNA control region in highly degraded samples. Int J Legal Med. 2008;122:385–8.
Alonso A, Albarran C, Martin P, Garcia P, Garcia O, de la Rua C, et al. Multiplex–PCR of short amplicons for mtDNA sequencing from ancient DNA. Prog Forensic Genet. 2003;9(1239):585–8.
Sijen T. Molecular approaches for forensic cell type identification: on mRNA, miRNA, DNA methylation and microbial markers. Forensic Sci Int Genet. 2014;. doi:10.1016/j.fsigen.2014.11.015.
Grabmüller M, Madea B, Courts C. Comparative evaluation of different extraction and quantification methods for forensic RNA analysis. Forensic Sci Int Gen. 2015;16:195–202.
Pang BC, Cheung BK. Double swab technique for collecting touched evidence. Leg Med (Tokyo). 2007;9:181–4.
Haas C, Hanson E, Bar W, Banemann R, Bento AM, Berti A, et al. mRNA profiling for the identification of blood-Results of a collaborative EDNAP exercise. Forensic Sci Int Genet. 2010;5:21–6.
Sauer E, Madea B, Courts C. An evidence based strategy for normalization of quantitative PCR data from miRNA expression analysis in forensically relevant body fluids. Forensic Sci Int Genet. 2014;11:174–81.
Courts C, Madea B. Specific micro-RNA signatures for the detection of saliva and blood in forensic body-fluid identification. J Forensic Sci. 2011;56:1464–70.
Lindenbergh A, de Pagter M, Ramdayal G, Visser M, Zubakov D, Kayser M, et al. A multiplex (m)RNA-profiling system for the forensic identification of body fluids and contact traces. Forensic Sci Int Genet. 2012;6:565–77.
Wong L, Lee K, Russell I, Chen C. Endogenous controls for real-time quantitation of miRNA using TaqMan microRNA assays. Application Note (Applied Biosystems). 2010:1–8.
Griffiths-Jones S. The microRNA registry. Nucleic Acids Res. 2004;32:D109–11.
Vandesompele J, De Preter K, Pattyn F, Poppe B, van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034.
Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–50.
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–15.
Zimmermann P, Bleuler S, Laule O, Martin F, Ivanov NV, Campanoni P, et al. Expression data—a public resource of high quality curated datasets representing gene expression across anatomy, development and experimental conditions. BioData Min. 2014;7:18.
Hruz T, Wyss M, Docquier M, Pfaffl MW, Masanetz S, Borghi L, et al. RefGenes: identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC Genom. 2011;12:156.
Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3.
Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45.
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22.
Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147.
Brüning A. Beiträge zur Untersuchung und Beurteilung von Geschossen usw. Arch Kriminol. 1925;77:85–6.
Betz P, Peschel O, Stiefel D, Eisenmenger W. Frequency of blood spatters on the shooting hand and of conjunctival petechiae following suicidal gunshots wounds to the head. Forensic Sci Int. 1995;76:47–53.
Schyma C. Wounding capacity of muzzle-gas pressure. Int J Legal Med. 2012;126:371–6.
Courts C, Madea B, Schyma C. Persistence of biological traces in gun barrels–an approach to an experimental model. Int J Legal Med. 2012;126:391–7.
Karger B, Nusse R, Schroeder G, Wustenbecker S, Brinkmann B. Backspatter from experimental close-range shots to the head I. Macrobackspatter. Int J Legal Med. 1996;109:66–74.
Karger B, Nusse R, Troger HD, Brinkmann B. Backspatter from experimental close-range shots to the head. II. Microbackspatter and the morphology of bloodstains. Int J Legal Med. 1997;110:27–30.
Karger B, Nusse R, Bajanowski T. Backspatter on the firearm and hand in experimental close-range gunshots to the head. Am J Forensic Med Pathol. 2002;23:211–3.
Kunz SN, Brandtner H, Meyer HJ. Characteristics of backspatter on the firearm and shooting hand—an experimental analysis of close-range gunshots. J Forensic Sci. 2015;60:166–70.
Budowle B. Mitochondrial DNA: a possible genetic material suitable for forensic analysis. In: Lee HC, Gaensslen RE, editors. Advances in forensic science. Chicago: Year Book Medical Publishers; 1990. p. 76–97.
Berger C, Parson W. Mini-midi-mito: adapting the amplification and sequencing strategy of mtDNA to the degradation state of crime scene samples. Forensic Sci Int Genet. 2009;3:149–53.
Phang TW, Shi CY, Chia JN, Ong CN. Amplification of cDNA via RT-PCR using RNA extracted from postmortem tissues. J Forensic Sci. 1994;39:1275–9.
Bauer M, Kraus A, Patzelt D. Detection of epithelial cells in dried blood stains by reverse transcriptase–polymerase chain reaction. J Forensic Sci. 1999;44:1232–6.
Juusola J, Ballantyne J. Messenger RNA profiling: a prototype method to supplant conventional methods for body fluid identification. Forensic Sci Int. 2003;135:85–96.
Bauer M, Gramlich I, Polzin S, Patzelt D. Quantification of mRNA degradation as possible indicator of postmortem interval—a pilot study. Leg Med (Tokyo). 2003;5:220–7.
Miller CL, Diglisic S, Leister F, Webster M, Yolken RH. Evaluating RNA status for RT-PCR in extracts of postmortem human brain tissue. Biotechniques. 2004;36:628–33.
van den Berge M, Carracedo A, Gomes I, Graham EA, Haas C, Hjort B, et al. A collaborative European exercise on mRNA-based body fluid/skin typing and interpretation of DNA and RNA results. Forensic Sci Int Genet. 2014;10:40–8.
Lindenbergh A, Maaskant P, Sijen T. Implementation of RNA profiling in forensic casework. Forensic Sci Int Genet. 2013;7:159–66.
Hanson EK, Lubenow H, Ballantyne J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal Biochem. 2009;387:303–14.
Zubakov D, Boersma AW, Choi Y, van Kuijk PF, Wiemer EA, Kayser M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int J Legal Med. 2010;124:217–26.
Courts C, Madea B. Micro-RNA—a potential for forensic science? Forensic Sci Int. 2010;203:106–11.
Wang Z, Zhang J, Luo H, Ye Y, Yan J, Hou Y. Screening and confirmation of microRNA markers for forensic body fluid identification. Forensic Sci Int Genet. 2013;7:116–23.
Acknowledgments
The authors would like to thank the DFG (Deutsche Forschungsgemeinschaft) and the SNF (Swiss National Science Foundation) for funding this project. The expert technical assistance of Julia Brünig and Marion Sauer is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12024_2015_9695_MOESM1_ESM.tif
Selection of a reference gene for mRNA expression data normalization using the “RefGenes” online resource (TIFF 1469 kb)
12024_2015_9695_MOESM2_ESM.tif
Imaging of PCR products resulting from two multiplex PCRs using “Mito-Mini” primer sets in “low template” samples. PCR products were separated via microfluid-gelelectrophoresis on an Agilent 2100 Bioanalyzer; Primer: primer name; bp: base pairs (TIFF 1320 kb)
Rights and permissions
About this article
Cite this article
Grabmüller, M., Schyma, C., Euteneuer, J. et al. Simultaneous analysis of nuclear and mitochondrial DNA, mRNA and miRNA from backspatter from inside parts of firearms generated by shots at “triple contrast” doped ballistic models. Forensic Sci Med Pathol 11, 365–375 (2015). https://doi.org/10.1007/s12024-015-9695-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-015-9695-3