Abstract
Background
Iodine is an essential element for the biosynthesis of thyroid-stimulating hormone (TSH). Both excessive and deficient iodine are major risk factors for thyroid diseases, including thyroid dysfunction, thyroid nodules, and thyroid autoimmunity (TAI). This study aimed to elucidate the relationship between iodine status and the prevalence of thyroid diseases through a national cross-sectional epidemiological survey in Jiangxi province (China).
Methods
This population-based, cross-sectional study enrolled 2636 Chinese local inhabitants who aged over 18 years old from April to August in 2015. Physical examination was performed and biochemical indices, urinary iodine concentration (UIC), and TSH level were measured. The Chi-square test, nonparametric test, and 4 multivariate logistic regression models adjusted for risk factors were applied to analysis. Spearman correlation coefficients were calculated to investigate the relationship between iodine intake level and the prevalence of thyroid diseases.
Results
The median UIC was 176.4 μg/L, and a significant difference was found in median UIC between men (182.45 μg/L) and women (169.25 μg/L) (P = 0.03). Among these study subjects, 14.4%, 44.5%, 26.1%, and 15.0% had deficient, adequate, more than adequate, and excessive iodine concentrations, respectively. The prevalence rates of hyperthyroidism, subclinical hyperthyroidism, hypothyroidism, subclinical hypothyroidism, thyroid nodules, and TAI were 0.91%, 0.57%, 0.34% and 7.89%, 9.45%, and 12.7%, respectively. Significant differences were found in iodine status, waist circumstance, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), TSH, thyroid nodules, and TAI between men and women (P < 0.05). Compared with those with adequate UIC, subjects with excessive UIC had higher prevalence rates of thyroid dysfunction (odds ratio (OR) = 1.74, 95% confidence interval (CI): 1.40–2.54) and thyroid nodules (OR = 3.33, 95%CI 1.32–8.42). In addition, subjects with deficient and excessive UIC were at the higher risk of TAI compared with those with adequate UIC (OR = 1.68, 95%CI: 1.19–2.60; OR = 1.52, 95%CI: 1.04–2.96, respectively). UIC was positively correlated with the prevalence rates of thyroid nodules (r = −0.44, P < 0.01) and TAI (r = −0.055, P < 0.01). On the contrary, UIC was negatively correlated with the risk of thyroid dysfunction (r = −0.24, P > 0.05).
Conclusion
Adult inhabitants from Jiangxi province in the TIDE study were in the adequate iodine status. Excessive iodine status was noted as a risk factor for thyroid dysfunction and thyroid nodules. In addition, both iodine deficiency and excessive iodine were risk factors for TAI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iodine is a pivotal factor for maintaining normal growth, brain development, and thyroid function [1]. Iodine plays a key role in maintaining thyroid homeostasis, which is influenced by hormones through almost all nucleated cells and is essential for growth and metabolism. Iodine is actively concentrated by the thyroid and converted to organic iodine within follicular cells. The main hormones produced by the thyroid gland are thyroxine (T4) and triiodothyronine (T3). Excessive or inadequate iodine intake is associated with thyroid dysfunction. Thyroid dysfunction is common, identifiable, and easily treatable, including hypothyroidism and hyperthyroidism, while if it is not timely diagnosed and treated, which may lead to serious adverse effects, such as cardiovascular diseases, severe myxedema, and embolization.
According to the guideline of the World Health Organization (WHO), iodine status was classified into deficient, adequate, more than adequate, and excessive classes. In iodine-replete populations, thyroid autoimmunity (TAI) was prevalent among women of reproductive age and is the most frequent cause of thyroid dysfunction. The thyroid peroxidase (TPO) antibodies were positive in autoimmune thyroid diseases, such as Graves’ disease, Hashimoto thyroiditis, and post-partum thyroiditis. Terribly, the lack of iodine intake was ubiquitous in the world. Iodine deficiency disorders (IDDs) had still remained a crucial public health challenge,which had negatively affected within 1.9 billion people worldwide, particularly in Southeast Asia, South America, and Central Africa [2,3,4]. IDDs were found as the main cause of defects in the development of the central nervous system and impairment of cognitive function in children and lactating women, as well as thyroid nodules and thyroid dysfunction in different age groups. Since the 1990s, the WHO globally expanded the implementation of the universal salt iodization (USI) policy, and IDDs were eradicated at the national level in China in 2011. However, after several years of implementation of the USI policy, the increased prevalence of thyroid diseases reported in countries suffering from mild or severe iodine deficiency [5]. China followed the USI policy in 1996, as a vast area country, there were diverse climates, customs, products, and habits in China. The iodine status and prevalence of thyroid diseases remained elusive after the implementation of the USI policy. Therefore, the health authorities and scientists could not timely adopt appropriate strategies according to the above-mentioned situation. To date, no epidemiological study on the iodine status and prevalence of thyroid diseases was conducted in Jiangxi province (China) before and after the implementation of the USI policy. In the present study, the iodine status and prevalence of thyroid diseases in Jiangxi province were analyzed.
Materials and methods
Subjects and study design
Epidemiological data were collected from the Thyroid disease, Iodine nutrition and Diabetes Epidemiology (TIDE) survey. As a part of the TIDE survey, this population-based, cross-sectional, epidemiological survey was performed in Jiangxi province from April to August 2015. Totally, 2665 adults were enrolled, of whom 2636 effectively attended, indicating a response rate of 98.9%. The inclusion criteria were as follows: (1) Patients’ age over 18 years old; (2) Han ethnic group; (3) Patients who lived at the study sites for at least 5 years; (4) No history of iodine utilization over the past 3 months; (5) Availability of medications for patients. Pregnant women were excluded. Informed consent was obtained from all study subjects prior to enrollment, and the study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of China Medical University.
The questionnaire, anthropometric measurement, and blood sampling
Firstly, patients’ demographic and clinical data were collected. Demographic and clinical data included age, gender, status of residence, marital status, salt origin, history of intake of kelp and laver over the past three days, the use of medications containing iodine, and history of thyroid diseases.
Anthropometric data, including blood pressure (systolic blood pressure (SBP) and diastolic blood pressure (DBP)), heart rate (HR), waist circumstance (WC), height, and body weight were measured by recommended standard procedures. In brief, blood pressure and HR were average values of two separate measurements taken at 5-min intervals. Body weight and height were measured without shoes or heavy garments. Body mass index (BMI, kg/m2) was calculated as the ratio of an individual’s weight (kg) divided by the height in meters squared (m2). WC was measured in the erect position at the middle of the lowest rib and at the superior border of the iliac crest.
Fasting blood and urine samples were obtained between 8:00 am and 10:00 am to determine blood chemical parameters using an automatic biochemical analyzer (Mindray Medical Co., Ltd., Shanghai, China). Centralized serum thyroid-stimulating hormone (TSH), thyroid peroxidase antibodies (TPoAb), and thyroid globulin antibodies (TgAb) were tested by chemiluminescent immunoassay. Free thyroxine (FT4) and free triiodothyronine (FT3) were tested unless TSH was abnormal. Total cholesterol (TC) and triglyceride (TG) levels were determined by enzymatic methods. Serum low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels were measured by the direct method. Serum uric acid (UA) was measured by the uric acid enzymatic-peroxide enzyme coupling method. Centralized urinary iodine concentration (UIC) was measured by inductively coupled plasma mass spectrometry (ICP-MS; PerkinElmer, Waltham, MA, USA) using an iodine standard (Inorganic Ventures, Christiansburg, VA, USA). All the experiments were carried out by experienced laboratory technicians. Furthermore, thyroid ultrasonography was performed by trained ultrasound physicians. All study subjects underwent thyroid ultrasound by experienced radiologists using a portable instrument (LOGIQ 100 PRO, GE, Milwaukee, WI, USA, with 7.5 MHz linear transducers).
Assessment of iodine status
According to the classification system of the WHO, iodine status was divided into four classes: deficient (<100 μg/L), adequate (100–199 μg/L), more than adequate (200–299 μg/L), and excessive (>300 μg/L) [6].
Diagnosis of thyroid dysfunction, thyroid nodules, and TAI
The definition of hyperthyroidism was TSH < 0.27 mIU/L, together with FT4 > 22.00 pmol/L and FT3 > 6.80 pmol/L. Subclinical hyperthyroidism was defined as TSH < 0.27 mIU/L and the normal levels of FT4 and FT3. Hypothyroidism was defined as TSH > 4.2 mIU/L and FT4 < 12.00 pmol/L. Subclinical hypothyroidism was defined as TSH > 4.2 mIU/L and a normal FT4 level. Thyroid nodule was defined as a normal thyroid size, which was >5 mm determined by thyroid ultrasound. TPoAb >34 IU/ml and/or TgAb >115 IU/ml were defined as positive autoimmune antibodies. Patients with positive TPO antibodies were mainly considered to suffer from TAI [7].
Data collection and statistical analysis
In the present study, data collection was performed by trained medical professionals at the Second Affiliated Hospital of Nanchang University (Nanchang, China). The EpiData (EpiData Association, Odese, Denmark) database was established, and all data were analyzed by SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Continuous variables were presented as mean with standard deviation (SD) or median with interquartile range (IQR) based on the data distribution. Mann–Whitney U test or t test was used to compare differences between the two groups. Categorical variables were presented as the frequency with percentage, and the Chi-square test was used to compare differences between the two groups. Statistical signiffcance was deffned by a two-sided P value < 0.05. To explore possible sources of bias, differences in clinical characteristics among four UIC-based groups were also analyzed. The independent risk factors for thyroid diseases were determined by the multivariate logistic regression analysis, and odds ratios (ORs) with 95% confidence intervals (95%CIs) were calculated. For the analysis, thyroid dysfunction, thyroid nodules, and TAI were considered dependent variables. Age, gender, status of residence, smoking status, height, body weight, HR, blood pressure, WC, UIC, TPoAb, TgAb, and TSH were regarded as independent variables.
A logistic regression model was used to estimate the effects of UIC on the risk of thyroid diseases. In order to reduce the effects of confounders, these variables were included in the multivariate logistic regression analysis according to the results of the univariate analysis. The basic statistical mode (model 1) was unadjusted. Model 2 was adjusted for gender and age based on the model 1. In model 3, SBP, DBP, WC, HR, and BMI were adjusted based on model 1. In model 4, some biochemical variables, such as fasting blood glucose (FBG), 2 h oral glucose tolerance test (2h-OGTT), TC, LDL, UA, TPoAb, and TgAb were included as covariates, in addition to confounding factors in the model 3. The results were expressed as OR with 95% CI.
Results
Characteristics of the study population
A total of 2636 study subjects (1354 men and 1282 women) were included in the present study (men: 41.8 ± 16.4 years old; women: 41.97 ± 16.9 years old). The clinical characteristics of study subjects are summarized in Table 1. There were statistically significant differences in WC, SBP, DBP, TC, TSH, TAI, thyroid nodules, UIC, and UA between the two genders. However, no significant differences were found in age, FPG, 2h-OGTT, HR, TG, LDL, HDL, and BMI between the two genders.
Iodine status
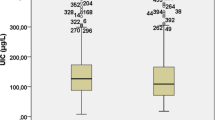
The median UIC and the distribution of subjects with different UICs were presented in Fig. 1. The median UIC was 176.4 μg/L in adults. A significant difference was found in median UIC between men (182.5 μg/L) and women (169.2 μg/L) (P = 0.03, Fig. 1A). Among all subjects, 380 (14.4%), 1173 (44.5%), 688 (26.1%), and 395 (15.0%) subjects were in groups of deficient iodine, adequate iodine, more than adequate iodine, and excessive iodine, respectively (Fig. 1B).
Prevalence of thyroid dysfunction, thyroid nodules, and TAI
As shown in Table 2, the prevalence of hyperthyroidism, subclinical hyperthyroidism, hypothyroidism, and subclinical hypothyroidism were 0.91%, 0.57%, 0.34%, and 7.89%, respectively. The prevalence of thyroid nodules and TAI were 9.45% and 12.7%, respectively.
Association between UIC and thyroid dysfunction, thyroid nodules, and TAI using the multivariate logistic regression analysis
As presented in Table 3, compared with the adequate iodine group as reference, patients in the excessive iodine group were at a higher risk of thyroid dysfunction in models 1, 2, 3, and 4, and ORs were 1.74 (95%CI: 1.20–2.54, P = 0.003), 1.79 (95%CI: 1.24–2.74, P = 0.002), 1.85 (95%CI 1.20–2.66, P = 0.002), and 1.79 (95% CI: 1.20–2.66, P = 0.004), respectively.
As shown in Table 4, compared with the adequate iodine group, patients in the excessive iodine group were at a higher risk of thyroid nodules in models 1, 2, 3, and 4, and ORs were 3.33 (95% CI: 1.32–8.42, P = 0.01), 3.16 (95%CI: 1.24–8.02, P = 0.02), 2.64 (95%CI: 1.03–6.75, P = 0.04), and 2.58 (95% CI: 1.0–6.59, P = 0.05), respectively.
As presented in Table 5, patients with TAI in the iodine group were analyzed. Compared with the adequate iodine group, patients in the deficient iodine, more than adequate iodine, and excessive iodine groups were at a higher risk of TAI, and adjusted ORs were 1.68 (95%CI: 1.19–2.60, P = 0.01), 1.26 (95%CI: 0.93–1.70, P = 0.14), and 1.52 (95%CI: 1.04–2.96, P = 0.02), respectively. The U-shaped relationship between iodine status and TAI in adults was confirmed.
As illustrated in Fig. 2, patients in the excessive iodine group were at a high risk of thyroid dysfunction and thyroid nodules. Importantly, there was a U-shaped relationship between iodine intake and TAI, indicating both iodine deficiency and excessive iodine were risk factors for TAI in adults.
The spearman correlation coefficient between iodine status and prevalence of thyroid diseases
Thyroid dysfunction was negatively correlated with iodine status (r =−0.24, P > 0.05). However, a positive correlation was found between thyroid nodules and UIC (r =−0.044, P < 0.01), as well as between TAI and UIC (r = −0.055, P < 0.01).
Discussion
In this cross-sectional, epidemiological study, the iodine nutritional status and the prevalence of thyroid diseases were assessed in Jiangxi province, China. Among 2636 study subjects, a significant association was found between iodine status and the prevalence of thyroid diseases. It was revealed that adults who had low or high UIC were at the risk of thyroid diseases, suggesting that iodine status played an important role in the progression of thyroid diseases.
Iodine was an important raw material for the synthesis of thyroxine, which was closely associated with human health, especially for the brain and central nervous system. Both iodine deficiency and excessive iodine increased the prevalence of thyroid diseases [8]. Median UIC, a validated biomarker for the assessment of iodine status, facilitated the monitoring of thyroid function [9]. In the present study, it was found that the median UIC was 176.4 μg/L, which reflected normal iodine consumption in Jiangxi province. Which was in general accord with the TIDE study in mainland China (the median UIC was 177.89 μg/L) [10]. Moreover, 14.4%, 44.5%, 26.1%, and 15.0% of study subjects had deficient, adequate, more than adequate, and excessive iodine concentrations, respectively. The results of the present study were consistent with those previously reported in mainland China [10,11,12], which could be related to the implementation of the USI policy in China.
Moreover, in the present survey, it was indicated that the prevalence rates of hyperthyroidism, subclinical hyperthyroidism, hypothyroidism, and subclinical hypothyroidism were 0.91%, 0.57%, 0.34%, and 7.89%, respectively. Compared with the TIDE study in mainland China [10], the prevalence of subclinical hypothyroidism in this study was lower (12.93% vs 7.89%), which may due to the use of different TSH reference values by the manufacturer [13]. Regrettably, mechanisms that iodine induced subclinical hypothyroidism have still remained elusive. Two assumptions were presented in the following. Firstly, chronic iodine stimulation could be a factor for iodine-induced hypothyroidism because of the Wolff−Chaikoff effect [14]. Secondly, thyroid inflammatory response was aggravated by the high iodine intake, which could be related to the role of IP10 and CD4+ T lymphocytes [15]. Other thyroid dysfunction in this study was similar to the TIDE study in mainland China [10]. In addition, subjects in the excessive iodine group were at a higher risk of thyroid dysfunction compared with those in the adequate iodine group, which was consistent with Shan et al.’s findings [16].
The prevalence of thyroid nodules was 9.45% in the present study, which was slightly lower than that in mainland China from the TIDE study (20.43%) [10] and Germany (23.4%) [17]. Several epidemiological studies have demonstrated that the prevalence of thyroid nodules increased with age because of degenerative changes in the thyroid [18, 19]. In the present study, it was found that the prevalence of thyroid nodules was elevated with increasing iodine levels. Over 3 times the risk in the excessive iodine group would lead to the development of thyroid nodules compared with the adequate iodine group. However, the results were not perfectly consistent with those of a previous study, in which the U-shaped relationship was explored between iodine level and the prevalence of thyroid nodules [20]. The explanation of epidemiological difference was that IDDs were almost eradicated after the implementation of the USI policy for more than 25 years. Moreover, numerous studies reported that the risk of thyroid nodules increased when UIC was >400 μg/L [21, 22]. There is no exact mechanism underlying association of excessive iodine with the risk of thyroid nodules, and additional studies are therefore needed to provide direct evidence.
TAI is frequently diagnosed in cases with positive TPO antibodies. In the present study, the prevalence of 12.7% in TAI cases was lower than the TIDE study in mainland China (19.89%), the difference was attributed to the population size, iodine taking, diets and climate [10]. Compared with adequate iodine status, individuals with deficient, more than adequate, and excessive iodine were found at a higher risk of TAI. The U-shaped relationship between iodine level and the risk of TAI in adults was shown in previous studies [22, 23], which was consistent with the results of the present study. Furthermore, a strong correlation was noted between the risk of TAI and iodine status. Although the mechanisms that iodine-induced abnormal TPO antibodies have remained elusive, some hypotheses have been raised. Firstly, excessive iodine intake increased thyroglobulin immunogenicity and the risk of TAI by unmasking a cryptic epitope on thyroglobulin, which initiated the autoimmune process during development of the TAI [24]. Secondly, excessive iodine intake resulted in oxidative stress, lipid oxidation, and thyroid gland injury [25]. Finally, the direct stimulatory effect of iodine intake on immune cells could be involved in the development of TAI [15].
In the four models, subjects with excessive iodine intake were at the risk of thyroid dysfunction. In model 2, after adjusting for age and gender, the risk of thyroid dysfunction had no significant association with excessive iodine intake, which could be different from Deng et al.’s findings [26], and it could be attributed to the involvement of older subjects in Deng et al.’s study. Moreover, the relationship between the prevalence of thyroid nodules and UIC is noteworthy. As shown in Table 4, the risk of thyroid nodules exhibited a 21% reduction in models 1 and 4 in the excessive iodine group, which indicated that TPO antibodies could play an important role in the prevalence of thyroid nodules [10, 27]. Furthermore, in models 1, 2, 3, and 4, the ORs of TAI patients almost remained unchanged after adjusting for the risk factors in the deficient iodine and excessive iodine groups, which suggested that the excessive iodine level could be fatal for TAI patients [22, 29]. However, the underlying mechanisms should be elucidated in future studies.
Limitations
This epidemiological study had some limitations. This cross-sectional study did not assess changes in the prevalence of thyroid diseases over time. Moreover, due to the minor population with thyroid diseases, the risk of bias should be considered to some extent. Additional factors, such as the levels of selenium, drugs, vitamin D, etc., might influence the prevalence of thyroid diseases. Finally, a limited number of cases with overt hyperthyroidism could be identified.
Conclusions
Adult inhabitants were in adequate iodine status in Jiangxi province in the TIDE study. The prevalence rates of thyroid dysfunction, thyroid nodules, and TAI were higher in women. Excessive iodine status was found as an independent risk factor for thyroid dysfunction or thyroid nodules. In addition, both iodine deficiency and excessive iodine were noted as independent risk factors for TAI in adults.
References
S. Kim et al. Association between iodine nutrition status and thyroid disease-related hormone in Korean adults: Korean national health and nutrition examination survey VI (2013-2015). Nutrients 11(11), 2757 (2019)
J. Santos et al. Iodine fortification of foods and condiments, other than salt, for preventing iodine deficiency disorders. Cochrane Database Syst. Rev. 2, CD010734 (2019)
H. Xu et al. Global, regional, and national burdens of common micronutrient deficiencies from 1990 to 2019: A secondary trend analysis based on the Global Burden of Disease 2019 study. EClinicalMedicine 44, 101299 (2022)
H. Chen, J. Lu, Y. Li, Secular trends in the prevalence of and disability-adjusted life years due to common micronutrient deficiencies in China from 1990 to 2019: an age-period-cohort study and joinpoint analysis. Front Nutr. 9, 754351 (2022)
Y. Zou et al. Iodine nutritional status after the implementation of the new iodized salt concentration standard in Zhejiang Province, China. BMC Public Health 14, 836 (2014)
Z. Yu et al. Iodine intake level and prevalence of thyroid disease in adults in Shaanxi province: a cross-sectional study. Ann. Transl. Med 9(20), 1567 (2021)
E. Frohlich, R. Wahl, Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 8, 521 (2017)
W. Teng et al. Effect of iodine intake on thyroid diseases in China. N. Engl. J. Med. 354(26), 2783–2793 (2006)
J. Bertinato, Iodine nutrition: Disorders, monitoring and policies. Adv. Food Nutr. Res 96, 365–415 (2021)
Y. Li et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of Mainland China. Thyroid 30, 568–579 (2020)
X. Sun, Z. Shan, W. Teng, Effects of increased iodine intake on thyroid disorders. Endocrinol. Metab. 29, 240–247 (2014)
B. Ren et al. Distributions of serum thyroid-stimulating hormone in 2020 thyroid disease-free adults from areas with different iodine levels: a cross-sectional survey in China. J. Endocrinol. Invest 44, 1001–1010 (2021)
P. Laurberg et al. The TSH upper reference limit: where are we at? Nature reviews. Endocrinology 7(4), 232–239 (2011)
J.M.B. Farebrother, Zimmermann, M. Andersson, Excess iodine intake: sources, assessment, and effects on thyroid function. Ann. NY Acad. Sci. 1446(1), 44–65 (2019)
S. Cui et al. Iodine Intake Increases IP-10 Expression in the Serum and Thyroids of Rats with Experimental Autoimmune Thyroiditis. Int J. Endocrinol. 2014, 581069 (2014)
Z. Shan et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a cross-sectional study in 10 cities. Thyroid 26(8), 1125–1130 (2016)
C. Reiners et al. Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96,278 unselected employees. Thyroid 14(11), 926–932 (2004)
B. Zou et al. The prevalence of single and multiple thyroid nodules and its association with metabolic diseases in Chinese: a cross-sectional study. Int J. Endocrinol. 2020, 5381012 (2020)
J. Song et al. Prevalence of thyroid nodules and its relationship with iodine status in Shanghai: a population-based study. Biomed. Environ. Sci. 29(6), 398–407 (2016)
X. Yu et al. A five-year follow-up study of goiter and thyroid nodules in three regions with different iodine intakes in China. J. Endocrinol. Invest 31(3), 243–250 (2008)
S. Gaengler et al. Iodine status and thyroid nodules in females: a comparison of Cyprus and Romania. Public Health 143, 37–43 (2017)
B. Wang et al. U-shaped relationship between iodine status and thyroid autoimmunity risk in adults. Eur. J. Endocrinol. 181(3), 255–266 (2019)
S. Wan et al. Autoimmune thyroid diseases after 25 years of universal salt iodisation: an epidemiological study of Chinese adults in areas with different water iodine levels. Br. J. Nutr. 124(8), 853–864 (2020)
F. Latrofa et al. Iodine contributes to thyroid autoimmunity in humans by unmasking a cryptic epitope on thyroglobulin. J. Clin. Endocrinol. Metab. 98(11), E1768–E1774 (2013)
Y. Luo et al. Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int J. Mol. Sci. 15(7), 12895–12912 (2014)
B. Deng et al. The relationship between metabolic parameters, age, and thyroid status: a cross-sectional study-based national survey of iodine nutrition, thyroid disease. Risk Manag Health. Policy 14, 1723–1730 (2021)
S. Shokri et al. Thyroid volume and nodular and diffuse thyroid diseases by ultrasonography in pregnant women: a case-control study. J. Res Med Sci. 25, 13 (2020)
Y. Li et al. Effect of the transition from more than adequate iodine to adequate iodine on national changes in the prevalence of thyroid disorders: repeat national cross-sectional surveys in China. Eur. J. Endocrinol. 186(1), 115–122 (2021)
Y. Li et al. Estimated change in prevalence of abnormal thyroid-stimulating hormone levels in China according to the application of the kit-recommended or NACB standard reference interval. EClinicalMedicine 32, 100723 (2021)
Acknowledgements
We sincerely thank professors Weiping Teng and Zhongyan Shan from the First Affiliated Hospital of China Medical University, as well as the study subjects who participated in this study.
Funding
This study was supported by the Research Fund for Public Welfare, the National Health and Family Planning Commission of China (Grant No. 201402005) and the Clinical Research Fund of Chinese Medical Association (Grant No. 15010010589).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, De., Hu, L., Shen, Yf. et al. Iodine status and its association with prevalence of thyroid diseases in adults from Jiangxi Province, China. Endocrine 82, 335–342 (2023). https://doi.org/10.1007/s12020-023-03413-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03413-8