Abstract
In both type 1 diabetes (T1D) and type 2 diabetes (T2D), there is a substantial β-cell mass loss. Residual β-cell mass is susceptible to cellular damage because of specific pancreatic β-cell characteristics. β cells have a low proliferation rate, being in human adults almost zero and a low antioxidant system that makes β cells susceptible to oxidative stress and increases their vulnerability to cell destruction. Different strategies have been addressed to preserve pancreatic β-cell residual mass and function in patients with diabetes. However, the effect of many compounds proposed in rodent models to trigger β-cell replication has different results in human β cells. In this review, scientific evidence of β-cell of two major regenerative approaches has been gathered. Regeneration proceedings for pancreatic β cells are promising and could improve β-cell proliferation capacity and contribute to the conservation of mature β-cell phenotypic characteristics. This evidence supports the notion that regenerative medicine could be a helpful strategy to yield amelioration of T1D and T2D pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the human pancreas, islets represent 1–1.5% of the organ’s volume, of which approximately 73.6 ± 1.7% corresponds to β-cells [1]. During the fetal stage, in both rodents and humans, β-cell mass is thought to be formed by cell division (10–20%) and from the differentiation of progenitor cells or from the conversion of non-endocrine cells into endocrine cells (80%) [2].
In humans, early childhood has been identified as the period with the most rapid β-cell mass expansion. In the first two years of life, β-cell mass is duplicated, and at five years of age β-cell volume is established [3]. After this proliferation peak, during the developmental stage, β cells undergo a period of apoptosis, which limits their availability to multiply [2]. Later in adulthood, the proliferation rate is very low, cell cycle progression markers have been found in less than 1% of β cells [4]. In addition, is worth mentioning that different than in the fetal stage, in adults β-cell mass increases mainly by replication of preexisting β cells [5].
In rodents, the highest proliferative peak of β cell occurs during the fetal stage and reaches 10%, then proliferation declines to 3% during the postnatal stage [6]. In rodents, during certain conditions such as exposition to mitogens, growth factors, and nutrients, the proliferative rate reaches 10–15% [7]. Hormones, metabolites, and growth factors are important regulators of endocrine cell mass [8]. However, many molecules that have been proposed as drivers of rodent β-cell replication, have proven not to be effective in human β cells [9]. Between rodents and humans, there are differences in islets composition, tissue organization, and proliferation capacity [10]. Consequently, is difficult to translate the experimental findings using murine models into insights about pancreatic human cell proliferation.
Another factor that modulates pancreatic β-cell mass is the number of progenitor cells arrested during G2 of the cell cycle, becoming quiescent cells [2, 6]. Additionally, underdeveloped fetal β-cell mass can also result from reduced differentiation and decreased expression of key transcription factors: pancreatic and duodenal homeobox 1 (Pdx1), NK6 homeobox 1 (Nkx6.1), and paired box protein 4 (Pax4) [11].
The generation of the endocrine cell type needs the expression of specific transcription and growth factors. During islets development and maturation this expression signature changes through the different stages as is required [12]. Mature differentiated islets exposed to conditions like nutrient overload, oxidative and endoplasmic reticulum (ER) stress or inflammation can undergo β-cell dedifferentiation. Dedifferentiation is characterized by the loss of β-cell mature characteristics with the upregulation of genes that are typically expressed during the embryonic stage of islets [13], the downregulation of β-cell-specific enriched genes, and the alteration of functional β-cell mass. These disturbances result in β-cell dysfunction, with the inability of β cell to produce and secrete insulin which in long term is associated with the development of pathologies such as diabetes [2].
In both T1D and T2D, there is a substantial β-cell loss [14]. Cell mass is reduced by around 70 to 90% in T1D and by 40 to 60% in T2D [15]. The proposed mechanism by which β-cell mass decreases in T1D involves the immunological destruction of β-cell, while in T2D includes increased secretory demand, inflammation, and oxidative and ER stress.
Since regenerative medicine could benefit both T1D and T2D patients, several research groups have contributed to the identification of compounds and molecules to stimulate human β-cell proliferation and that promote the maintenance of mature β-cells characteristics [16]. This review gathered scientific evidence of two major strategies in the regenerative approach: the enhancement of β-cell proliferative capacity and the prevention of the dedifferentiation of functional β-cells, considering that both could have important implications in diabetes treatment.
Dual-specificity tyrosine-regulated kinase-1A inhibitors are capable to induce β-cell proliferation in human cells
Recent discoveries have identified the dual-specificity tyrosine-regulated kinase-1a (DYRK1A) as the target of compounds to stimulate adult human β-cell proliferation. Has mentioned before β-cell proliferation rate is very low, and its induction is especially complicated in human cells. DYRK1A inhibitors are one of the few groups of compounds recognized for their ability to enhance the proliferation of β cells in humans [17].
The gene that encodes DYRK1A is located on chromosome 21 and its kinase is the most highly expressed in human β cells [17]. DYRK1A inhibitors are considered the principal human β-cell mitogenic target; although DYRK1B is also an important target, and the combination of DYRK1A and DYRK1B could possible yielded synergetic effects. In contrast, the silencing of DYRK2, DYRK3, and DYRK4 has proven no effect on proliferation [18].
DYRK1A inhibitors include harmine, 5-iodotubericidin (5-IT), INDY, leucettine-41, GNF4877, TG003, AZ191, CC-401, among others [18]. Drugs such as harmine, 5-IT, INDY, GNF4877, or CC-401 have demonstrated to induce human β-cell proliferation in a range of 1.5–3%; measured by Ki67, EdU, BrdU, and/or PCNA staining of insulin-positive cells [19], this proliferation rate is similar to the rate that occurs physiologically in human β cells during the first year of life [20]. The inhibitors, 5-IT and GNF4877 have proven to be the more potent drivers of human β-cell proliferation of the group [18]. Nowadays, a series of different treatments have emerged using combinations of these compounds to obtain a more potent effect on proliferation and specific toward β cell.
DYRK1A inhibitors activate cell cycle progression through a family of transcription factors, the nuclear factor activated in T cells (NFaT) (Fig. 1). NFaTs are de-phosphorylated by calcineurin to be able to translocate to the nucleus; then they bind to and transactivate, the cell cycle genes, CCNE, CCNA (encoding cyclins E and A), and CDK1, and repress the expression of the cell cycle inhibitors CDKN1C, CDKN2A, and CDKN2B [21], which encode the arresting proteins of G1 phase p57KIP2, p16INK4a, and p15INK4b, respectively [20].
NFaT signaling pathway. The intracellular calcium (Ca2+) activates calcineurin (CN), which then de-phosphorylates the family of transcription factors, nuclear factor activated in T cells (NFaT). In their de-phosphorylated state NFaT translocate into the nucleus, to bind and activate the cell cycle genes; cyclins E (CCNE) and A (CCDNA), and cyclin-dependent kinase 1 (CDK1) and repress the cell cycle inhibitors genes; CDKN1C, CDKN2A, CDKN2B which encodes p57KIP2, p16INK4a, and p15INK4b, respectively. NFaT, also modulate the expression of β-cell genes; pancreatic and duodenal homeobox 1 (PDX1), NK6 homeobox 1 (NKX6.1), MAF BZIP Transcription Factor A (MAFA), solute carrier family 2 member 2 (SLC2A2), chromogranin B (ChgB), islet amyloid polypeptide (IAPP), and insulinoma-associated protein 2 (IA2). The dual-specificity tyrosine-regulated kinase-1a (DYRK1A), glycogen synthase kinase-3 (GSK3), and casein kinase 1 (CK1) are able to re-phosphorylated NFaT members, which results in NFaT returning to the cytoplasm, the termination of its transcriptional activity, and in consequence cell quiescence. DYRK1A inhibitors restrain NFaT re-phosphorylation permitting proliferation to continue
Nuclear NFaTs can be phosphorylated by DYRK1A, glycogen synthase kinase-3 (GSK3), and casein kinase 1 (CK1) and return to the cytosol where its transcriptional activity is inactive [22]. DYRK1A inhibitors act by restraining NFaT re-phosphorylation, thus maintaining their stimulation [20]. Several reports have indicated that DYRK1A can phosphorylate a broad range of targets in addition to the NFaT family, including TP53, p27kip, and LIN52 which are involved in cell cycle regulation [19].
Additionally, in calcineurin phosphatase knockout mice has been shown a decreased β-cell mass and proliferation, with a reduction of Insulin-1 (INS1), PDX1, and 2 expression, which are all regulators of β-cell function [23]. Calcineurin/NFaT pathway also modulates the expression of chromogranin B (ChgB), islet amyloid polypeptide (IAPP), and insulinoma-associated protein 2 (IA2, also called ICA152) which are β-cell granule components and other proliferative regulators such as CcnD2 and Forkhead box (FOX) factor FoxM1 [24]. Several studies suggest that NFaT is a reasonable target of β-cell proliferation. Moreover, calcineurin and NFaT signaling are conserved across rodents and humans [17], and DYRK1A inhibitors have provided the most widely replicated effects [21].
In the next section, several DYRK1A inhibitors and combinatorial treatments are presented. Each of them has been demonstrated to increase β-cell proliferation through different mechanisms and to improve pancreatic function.
Harmine increase human β-cell proliferation and expression of genes associated with β-cell mature differentiation stage
In 2015, the DYRK1A inhibitor harmine was discovered [25]. Harmine action is related to its inhibitory function of kinases activities of DYRK1A, DYRK1B, DYRK2, DYRK3, monoamine oxidases (MAOs), and cdc-like kinases (CLKs) [23] and the reduction of SMAD proteins abundance [18].
Aamodt et al. demonstrated that harmine was the only compound that significantly increased human β-cell proliferation compared to other 13 compounds tested that include neurotransmitters, growth factors, hormones, proteins, and small molecules. They observed a limited increase in β-cell proliferation using gamma-aminobutyric acid (GABA), platelet-derived growth factor (PDGF), UK-432097, and A-134974 and a minimal or no effect using serotonin, myostatin, activin A, 5′-N ethylcarboxamidoadenosine (NECA), prolactin, erythropoietin, and exendin-4 [26].
Harmine expands β-cell mass without causing DNA damage or β-cell death [26]. Moreover, harmine also leads to increased β-cell differentiation markers (PDX1, NKX6.1, and MAFA) and has proven to improve glucose tolerance in immunodeficient mice transplanted with human islets [20].
5-IT exerts a potent effect on human β-cell proliferation and ameliorate pancreatic function
The mitogenic capability of 5-IT on β-cell is attributed to its DYRK1A and adenosine kinase inhibitory capacity. Ackeifi et al. explored the mitogenic efficacy of harmine, 5-IT, INDY, leucettine-41, and GNF4877 in human islets. Harmine, INDY, and leucettine-41 yielded a ~3.5% increase in proliferation rate; while 5-IT and GNF4877 shown a ~10-fold more potent effect than the other compounds [18]. According to Dirice et al., 5-IT displayed a far greater effect in stimulating β-cell proliferation than harmine. Harmine has optimal activity at 5–10 mmol/L, whereas 5-IT exhibits a similar effect at 0.5–1 mmol/L [17]. Additionally, it has been reported that lower doses of 5-IT are more selective [18].
In human islets, six days of treatment with 1 mmol/L of 5-IT leads to a 10–12-fold increase in β-cell proliferation. Immunohistochemical analysis has allowed proving the transition of all cell cycle phases in the 5-IT–treated human islets, with an ~5–6-fold increased incorporation of BrdU, pHH3, and Ki67. Importantly, it should be considered that 5-IT could exert effects in other endocrine pancreatic cells [17].
After 5-IT treatment, Dirice et al. observed an increase in insulin and C-peptide blood levels in mice transplanted with human islets. Human islets treated with 5-IT also demonstrated an enhanced glucose-stimulated insulin secretion (GSIS). Expression analysis has indicated that 5-IT strongly upregulated Solute Carrier Family 2 Member 2 (SLC2A2) gene expression, which encodes to GLUT2 and might contribute to GSIS improvement after 5-IT administration. Treatment with 5-IT also downregulated the expression of Regenerating Family Member 1 Beta (REG1B) which encodes an exocrine pancreas protein involved in pancreatic lithogenesis, of Regenerating Family Member 3 Alpha (REG3A) which encodes a secretory protein associated with pancreatic inflammation, and of Olfactomedin 4 (OLFM4) which has been related with the promotion of pancreatic cells cancer [17].
DYRK1A and transforming growth factor-β inhibitors have a synergistic effect on human β-cell proliferation and enhance the expression of genes associated with β-cell mature differentiation stage
The transforming growth factor-β (TGF-β) has been demonstrated to induce p16INK4a expression and to reduce β-cell replication through SMAD3. TGF-β1, -2, and -3 ligands are expressed endogenously in the human pancreas, among islets, ductal, and acinar cells [27]. TGF-β signaling results in phosphorylation and consequently activation of SMAD proteins. SMAD then translocate into the nucleus and modulate gene expression [28].
SB431542 is an inhibitor of TGF-β which blocks SMAD3 phosphorylation via ALK5. Adult mice β-cell treated with SB431542 showed enhanced replication rate and a reduction in p16INK4a levels. TGF-β and p16INK4a have been associated with β-cell senescence, resulting in declined β-cell replication during aging. Additionally, in adult human islets (40–60 years old) where the β-cell proliferation rate is almost zero, SB431542 treatment demonstrated an increased β-cell replication accompanied by a reduction of Mll1 and SMAD3 binding at the Ink4a/Arf locus. In mice transplanted with human islets, SB431542 showed a tenfold increase in β-cell proliferation [27].
The combined inhibition of DYRK1A and TGFβSF signaling induce a remarkable improvement of human β-cell proliferation in vitro and in vivo. Moreover, this effect was preserved in islets from cadaveric donors, islets from donors with T2D, and human stem cell-derived β cells [19].
DYRK1A inhibitors seem to modulate cell cycle activators activity, whereas synergistically, TGFβSF inhibitors have been shown to repress cell cycle inhibitors. It has been proposed that TGFβSF inhibitor effects are mediated by SMAD signaling and Trithorax chromatin remodeling [19].
Harmine in combination with TGF-β inhibition, produced a dramatic increase in human islets proliferation rate; with an average of 5–8% measured by Ki67-positive β cells labeling, achieving in some samples indices as high as 15–18%. Immunolabeling essays with TUNEL and γH2AX proved, no DNA damage nor β-cell death, respectively. Importantly, proliferation was also observed in α, δ, PP, and ductal cells, suggesting that mitogenic effects were not specific for β cells [19].
In addition, combined harmine-TGFβSF inhibitor treatment enhanced expression of PDX1, NKX6.1, MAF BZIP Transcription Factor (MAF)A, MAFB, SLC2A2, and Proprotein Convertase Subtilisin/Kexin Type 1 (PCSK1) on pancreatic islets [19].
The proliferation rate in donors with T2D using harmine in combination with three different TGFβSF inhibitors (ALK5, LY364947, GW788388) led to synergistic increases in Ki67 labeling and a proliferation rate of ~5%. Particularly, harmine combined with ALK5 increased PDX1, NKX6.1, and MAFA gene expression, which are important markers of β-cell mature differentiation state and β-cell function [19].
Harmine and glucagon-like peptide-1 have a relatively selective effect on β-cell proliferation and the expression of genes associated with β-cell mature differentiation stage
Incretins have been reported to induce β-cell replication in rodents through diverse signaling pathways. Glucagon-like peptide-1 (GLP-1) has been demonstrated to stimulate PI3K-AKT, PKCζ, and cAMP-PKA-CREB pathways to induce β-cell proliferation in rodents, nonetheless, none of these findings have proven to be reproducible in human islets. In contrast, GLP-1 or its analog exendin-4 are well-known potentiators of GSIS in both rodents and humans [28].
Combined harmine (10 μM) and GLP-1 (5 nM) treatment-induced proliferation of adult human cadaveric islets at an average rate of 5%, whereas harmine alone induced proliferation at a rate of ~2%. In other series of experiments, human islets transduced with harmine–GLP-1 increased their cell mass by approximately 40%. No evidence of DNA damage nor β-cell death was found in harmine and GLP-1 combined treatment [15].
The mitogenic synergy of harmine–GLP-1 combined treatment, could be presumably explained by harmine action on DYRK1A inhibition and NFaT translocation to the nucleus and by GLP-1 action on cAMP and its effects downstream upon protein kinase A (PKA) and Exchange Factor Directly Activated by CAMP 2 (EPAC2) pathways [15].
In islets derived from human donors with T2D, harmine–GLP-1 treatment activated β-cell proliferation and insulin secretory response was augmented. Moreover, harmine–GLP-1 increased the expression of PDX1, NKX6.1, MAFB, SLC2A2, PCSK1, and glucagon-like peptide-1 receptor (GLP1R), thus it could contribute to maintaining the differentiation state of islets [15].
These synergistic effects have been replicated with several DYRK1A inhibitors (harmine, 5-IT, INDY, leucettine-41, and GNF4877) and with five GLP1R agonists (exendin-4, liraglutide, lixisenatide, and semaglutide). Further, the GLP-1 family is relatively specific to β cell. GLP-1 receptor exists in pancreatic β and ductal cells, central nervous system, cardiac muscle, gastric smooth muscle, and T cells. This is an improvement over the ubiquitous expression of the DYRK1A inhibitors and could provide specificity to human β-cell proliferation [15].
In NOD scid gamma (NSG) mice transplanted with human islets (500 IEQs), the harmine-exenatide treatment led to increased circulating human insulin levels and enhanced blood glucose levels. Besides, in transplanted NSG mice with human islets (500 IEQs), harmine (10 mg/kg) and exenatide (0.5 μg/kg) combinatory treatment achieved a 1.1% β-cell proliferation rate [15].
Repeatedly, the induction of human β-cell proliferation in models in vivo is lower than in the in vitro settings. Ackeifi et al. in their experiments demonstrated a ~5% proliferation rate in vitro, while a 1.1% in vivo [15]. Similarly, Wang et al. reached a proliferation rate of ~5–8% in vitro, while a ~2% rate in vivo [19]. Lower β-cell proliferation rate in vivo could reflect the presence of inhibitory molecules, the limited availability of drugs, or suboptimal dosing [15]. These considerations are needed for the screening of molecules that drive human β-cell proliferation.
Collectively, is worth considering that DYRK1A is ubiquitous, thus adverse effects of its inhibitors affect many organs [21]. Harmine is able to inhibit MAOs and has been reported to have psychoactive effects (producing hallucination episodes) and to induce anxiety, tremor, convulsion, and ataxia [23]. Harmine has also proven to have effects on hepatocyte survival [29], adipogenesis [30], immune cell function [31], and circadian rhythm [32]. One challenge at this moment is that there is no molecule able to act specifically in β cells. In this sense, there is an urgent need to specifically target DYRK1A inhibitors, in order to, prevent emerging adverse effects [19, 21]. Other considerations needed are the oncogenic consequences if regenerative pathways are driven too aggressively. Drugs-driven cell cycle induction must be activated in a specific and temporally restricted form [21].
DYRK1A inhibitors have been demonstrated to increase β-cell proliferation, enhance the expression of genes associated with β-cell mature differentiation state, ameliorate insulin secretion, and in consequence improve GSIS. DYRK1A inhibitors are not the only compounds able to induce human β-cell proliferation. In the following section, another two approaches are presented.
Other inducers of β-cell proliferation
GNF-9228 enhance β-cell proliferation and protects them against cytotoxic stress
A recently identified small compound, GNF-9228 has proven to have relevant effects on pancreatic islets, including the increase of insulin secretion and human β-cell proliferation, and the protection of β cells against cytotoxic stress [33].
Hohmeier et al. showed that GNF-9228 triggered human β-cell relative to α- or δ-cell proliferation; insulin, glucagon, and somatostatin co-staining were used to identify specific cell types. Moreover, GNF-9228 treatment also ameliorated insulin secretion, treatment for 72 h increased 75–140% insulin secretion in human islets. Human islets were treated with GNF-9228 (10 μM) or GNF-9228 (10 μM) and GNF4877(5 μM), all islets prep increased EdU incorporation by ~10-fold in both conditions. GNF4877 provides no additive effect since it increased only fourfold EdU incorporation when used alone. In these series of experiments, the authors proposed that GNF-9228 induces islet cell proliferation in a DYRK1A/NFaT independent fashion, but further studies are needed to better understand its mechanisms of action [33].
In addition, in a rat β-cell line GNF-9228 proved to protect cells against cytotoxicity. GNF-9228 demonstrated a cytoprotective effect against ER stress induced by thapsigargin (250 nM) or a mixture of IL-1β (1 ng/mL) and interferon-gamma (IFN-γ) (100 U/mL) [33].
S-phase kinase-associated protein 2 overexpression increase β-cell proliferation in islets from human T2D donors
S-phase kinase-associated protein 2 (SKP2) gene encodes a 40 amino acid motif called the F-box. The F-box proteins constitute one of the four subunits of the SKP1-cullin-F-box (SCFs) a ubiquitin-protein ligase complex that functions in phosphorylation-dependent ubiquitination [34].
SKP2 belongs to the Fbls class of proteins containing ten tandem leucine-rich repeats. SKP2 is an essential component of substrate recognition which then mediates ubiquitination and subsequent proteasomal degradation of target proteins involved in cell cycle progression (specifically involved in G1/S transition), signal transduction, and transcription [34].
SKP2 has been shown to be the major p27kip1-ubiquitin ligase and a regulator of p27kip1 expression. In islets from human T2D donors, p27kip1 mRNA is increased compared to non-diabetic donors [35]. SKP2 reduced p27kip1 expression and enhanced the proliferative response induced by cdk6 and cyclin D3. In β cells from T2D donors overexpressing SKP2, the proliferative response was relatively doubled (3.7 ± 1.4% versus 8.1 ± 1.0%), and consequently, the β-cell mass was higher [35].
Besides p27kip1 downregulation, SKP2 could activate other additional pathways. Uniquely, SKP2 stimulates c-Myc monoubiquitinylation and modulates its transcriptional activity. c-Myc has shown to play an important role in human β cells cell cycle entry and to be essential in rodent insulinoma development and expansion [35].
SKP2 overexpression caused an increased expression of CYCLIN E1, CYCLIN E2, CDC25A, E2F1, and E2F3 genes. The same genes were overexpressed when C-MYC expression was induced, and their abundance was also comparable. The only transcript differently regulated was E2F2 which was upregulated by c-Myc but not by SKP2. This suggests that SKP2 activates c-Myc ubiquitinylation and conducts the upregulation of cell cycle target genes. In brief, SKP2 downregulates p27kip1 and stimulates c-Myc monoubiquitinylation; through these effects, SKP2 enhanced β-cell proliferation and increased the expression of several genes involved in cell cycle progression [35].
The last treatments revised show two important characteristics (1) protection against cytotoxicity and (2) the capability to induce proliferation in β cells from T2D donors. In diabetes, residual β-cell mass is susceptible to cellular damage and dedifferentiation. The attenuation of the oxidative and ER stress damaging effects on β-cell and the mitigation of β-cell susceptibility to dedifferentiation could help to preserve pancreatic cell mass and function and yield amelioration of diabetes pathogenesis.
In summary, diverse molecules have proven to have a fundamental role in β-cell proliferation (Table 1). In order to enhance human β-cell proliferation in vivo, it is crucial to work on strategies considering the molecule specificity towards β cell, molecule availability in circulation, and the optimal doses. Moreover, it is essential to circumvent its interaction with inhibitory molecules, prevent adverse effects in other organs, and strictly avoid oncogenesis processes.
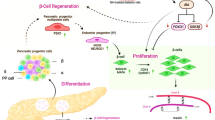
Redifferentiation
After undergoing all the differentiation processes, pancreatic cells reach their mature phenotypic state. The mature identity of β cells is tightly regulated by the expression of many essential transcription factors which allow β cell to have a suitable function [36]. During pancreas development, SRY-Box Transcription Factor 9 (SOX9), Forkhead Box A2 (FOXA2), and PDX1 are activated in pancreatic progenitor cells. Subsequently, Neurogenin-3 (NGN3) transcription factor specifies the endocrine cell lineage. The maturation of endocrine cells depends on additional transcription factors, such as PAX4 and aristaless-related homeobox (ARX). PAX4 and ARX mediate the specification of endocrine subtype destinies by repressing each other’s expression since they are essential for β and δ and α and PP cell lineages, respectively [37]. Many genes have been involved in β-cell maturation, such as Ins1, Ins2, Pdx1, MafA, and Slc2a2 [38]. Importantly, the maintenance of β-cell identity is regulated by transcription factors and/or chromatin modifications [39].
Mature β cells could degenerate from their differentiated state due to glucotoxicity, lipotoxicity, oxidative stress, ER stress, or inflammation this process is known as dedifferentiation. In dedifferentiation, β-cell reduces their expression of β-cell-enriched genes which are related to insulin synthesis, glucose metabolism, protein processing, and protein secretion [36, 40], and upregulate the expression of progenitor-cell-associated genes. This consequently leads to loss of the β-cell function and the inability of β cell to synthesize and secrete insulin properly [36]. Briefly, dedifferentiation is a process characterized by the loss of the expression of key β-cell maturation markers [41], while redifferentiation refers to the restoration of β-cell mature phenotypic characteristics.
Expression analysis on dedifferentiated β cells shown an increase of β-cell immaturity-associated markers, such as Ngn3, POU domain class 5 transcription factor 1 (Pou5f1), Nanog Homeobox (Nanog), and L-Myc; and a concomitantly decrease of key markers of β-cell maturity and functionality, including Ins2, Pdx1, Nkx6.1, MafA, and Slc2a2 [14, 38, 42].
In β-cell dedifferentiation, β cells return to a progenitor-like status [43]. Sachs et al. analyzed the cellular trajectory in which β cells transitioned from mature to immature, they observed that embryonic and streptozotocin (STZ)-diabetic β cells shared downregulation of insulin and FOXO1. Moreover, in STZ-diabetic β cells, they found the downregulation of genes involved in insulin and mitogen-activated protein kinase (MAPK) signaling and upregulation of ER stress-associated genes [41]. Likewise, stress response mediators and DNA binding inhibitors are increased during β-cell dedifferentiation [38].
Alteration of the FOXO1 function has been associated with β-cell dedifferentiation. FOXO1 is required to maintain β-cell identity and prevent β-cell conversion into non-β pancreatic endocrine cells [14, 44]. Several mediators of the NOTCH, WNT [45], and TGF-β pathways, such as HES1, β-catenin, PI3K-AKT, and SMAD3 have been involved in β-cell dedifferentiation [46]. Simultaneous inhibition of both WNT and Notch signaling pathways results in β-cell redifferentiation [47]. Besides, downregulation of miR-375, miR-141/miR-200 and miR-30 families, and miR-7 play an important role in the β-cell loss of phenotype [46].
In murine cells, it has been proposed that modulation of reactive oxygen species, ER stress, serum response factor (SRF), MAPK, TGF-β, WNT, and PDGF signaling factors, could be a critical strategy for reactivating the expansion and maturation of residual β cells in vitro and in vivo [48,49,50]. Additionally, Zhiyu et al. demonstrated that substantial hyperglycemia reduction (in their case with insulin therapy) permits the redifferentiation of Ngn3-positive cells into mature β cells. Consistently, expression levels of Pdx1, Nkx6.1, and MafA were restored and progenitor markers (Ngn3, Nanog, L-Myc, and Pou5f1) decreased [42].
In mouse islets, the co-existence of mature and immature β cells has been proven and in diabetic mouse models and patients with T1D and T2D [44]. In long periods of hyperglycemia β cells undergo dedifferentiation [38]. Besides, in human islets from patients with T2D, the β-cell dedifferentiation rate was positively correlated with disease progression [51].
Under obesity and insulin resistance conditions, pancreatic cells have demonstrated an adaptative β-cell mass expansion [52]. However, long-term hyperglycemia and β-cell functional overload conduce to an inflammatory state, oxidative and ER stress [53, 54], acceleration of apoptosis rate [55], and β-cell dedifferentiation into endocrine cells that express progenitor markers [44]. Lineage-tracing experiments attribute the loss of β-cell mass in metabolic stress to dedifferentiation rather than to an increased apoptosis rate; turning dedifferentiation into an important mechanism of β-cell failure [44].
Substantial experimental studies have strongly implied that β-cell regeneration is possible and that β-cell functional loss is reversible, thus redifferentiation could constitute an important approach for diabetes treatment [13]. Understanding how β cells maintain their mature functional status and how they can redifferentiate could be a helpful strategy in the preservation of the β-cell mass in both T1D and T2D patients [38, 56].
Anti-inflammatory treatment
β-cell dedifferentiation has shown to be promoted by pro-inflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor α (TNFα). In diabetes, inflammation could cause β-cells dedifferentiation and contribute to disease progression [38, 51].
β-cell exposure to IL-1β, IL-6, and TNFα causes β-cell dedifferentiation and impair insulin secretion capacity. Exposure to IL-1β, IL-6, or TNFα for 24 h decreased Ins2, Pdx1, Nkx6.1, Slc2a2, Foxo1, and glucokinase (Gck) mRNA expression in mouse islets. Considering, that IL-1β had the most potent outcome in mouse islets, its effects were also tested in human islets. Exposure of human islets to IL-1β for 24 h suppressed INS, PDX1, NKX6.1, SLC2A2, FOXO1, MAFA, MAFB, and NANOG expression and contrary increased SLC2A1 and POU5F1 expression [14].
Mainly IL-1β, but also IL-6 and TNFα have proven to repress β-cell identity markers. Nordmann et al. reported that inhibition of IL-1β, IL-6, and TNFα or inhibition of NF-κB by sodium salicylate treatment improved insulin secretion and blood glucose levels. Treatment of diabetic animal models with anti-inflammatory agents improved insulin secretion in isolated islets. However, while IL-1β has a more prominently effect on β-cell dedifferentiation, only TNFα antagonism partially prevented the loss of β-cell identity [14].
Inhibition of inflammatory cytokines has been demonstrated to improved glycemia and augmented β-cell insulin secretion. However, only modestly improved β-cell dedifferentiation [14]. In rats, it has been proven that the anti-inflammatory treatment using salsalate diminishes β-cell dedifferentiation by the inhibition of the Notch pathway [57] and possibly through its effects on insulin secretion [14].
Betacellulin
Betacellulin (BTC) encodes a member of the epidermal growth factor family. BTC is a potent mitogen in different cell types and is highly expressed in the pancreas, liver, kidney, and small intestine [58]. BTC is able to stimulate β-cell proliferation across rodents and humans [59].
In mice expressing Neurogenic Differentiation Factor (Neurod) in liver cells, Btc treatment stimulated islet neogenesis. The combinatory gene therapy, Neurod-Btc induced liver expression of islet hormones, such as insulin-1, insulin-2, glucagon, and somatostatin; liver cells were able to produce insulin granules and STZ-diabetic mice maintained normal glucose and insulin levels during glucose tolerance test [60].
BTC positive effects on β-cell expansion are thought to be mediated by increased proliferation and neogenesis [58]. Several studies have proposed that BTC promotes β-cell neogenesis after selective β-cell destruction induce by alloxan and STZ treatments or by 90% pancreatectomy [58]. Also, it has been reported that combined treatment with activin A and BTC results in the regeneration of pancreatic β-cell in neonatal STZ-treated rats. BTC promotes pancreatic β-cell growth and differentiation and improves glucose metabolism by stimulating β-cell regeneration in diabetic animals [59].
In another series of experiments using two murine models of diabetes (chemical and autoimmune induced diabetic mice), rAd vector was used to constitutively express BTC. Shin et al. proved that a single injection of rAd-BTC produced remission of diabetes for >100 days by inducing the regeneration of insulin-producing β-cells in diabetic mice. Thus, BTC was able to regenerate a sufficient number of β cells to restore normoglycemia in diabetic mice [59].
BTC proved to induce β-cell regeneration in a model of severe diabetes induced with a high dose of STZ (400 mg/dL). The proposed mechanism implied that cells expressing PDX1 and somatostatin serve as precursors for β-cell regeneration. BTC effect on regeneration could result from the increase of β-cell mass, by increasing the precursor pool in islets or by their effect reducing hyperglycemia which is related to a increased number of insulin-positive cells. Also, blood glucose concentrations decrease may reduce glucose toxicity and thereby retard β-cell dedifferentiation [61].
In human islet-derived cells, BTC treatment reestablished the expression of insulin and 10.9% of cells shown an intense insulin staining. A similar percent of cells exhibited intense human C-peptide staining. Treatment for six days with BTC induced expression of β-cell genes, among them insulin, PDX1, NKX6.1, SLC2A2, NEUROD, NK2 Homeobox 2 (NKX2.2), and IAPP. These factors have been associated with the improvement of insulin biosynthesis, storage, sensing, and secretion. BTC was able to restore the β-cell phenotype, to improve insulin expression and storage, and to stimulate the expression of multiple factors involved in β-cell function [62].
BTC treatment mechanisms of action remain to be completely understood. BTC activity has been proposed to be mediated through the signaling pathway of erbB receptor family members. BTC could bind to the erbB receptor, trigger receptor phosphorylation, nuclear translocation, and consequently gene expression modulation [62]. In addition, BTC increased the expression of PDX1, an important transcription factor for the differentiation of progenitor cells into β-cell phenotype [63]. Also, in BTC-treated islets, it was observed an increased level of Akt suggesting that the proliferative effect of BTC could be mediated by phosphatidylinositide 3-kinase activation [64].
Redifferentiation cocktail
Redifferentiation cocktail (RC) has been demonstrated to stimulate β-cell redifferentiation. RC consists of soluble activin A, exendin-4, nicotinamide, and high glucose concentrations (25 mM of glucose) [38, 65]. Exposition to high glucose concentrations is controversial since it has been associated with glucotoxicity. However, this term should be reserved for nonphysiological, irreversible alterations in the cellular function of β cells. In this case, the high glucose concentration is used as a temporary pharmacological event to induce a determined effect, which is to increase cell sensitivity to glucose-stimulated insulin release and that is reversible upon glucose levels restoration [66].
Redifferentiation induced by RC leads to the downregulation of the WNT and TGF-β pathways and the upregulation of miR-375 [46]. Four days of RC treatment in combination with blocking the expression of ZEB1, a downstream effector of each of the signaling pathways, increased by 60% the redifferentiation of β-cell-derived (BCD) cells into insulin-producing cells. Moreover, redifferentiated cells were able to adequately respond to GSIS [67]. There is a possibility that BCD cells treated with RC acquire properties of multiple islet progenitor cells. RC contributes to the activation of several transcription factors associated with islet progenitor cells, including SOX9, NGN3, FOXA2, PAX4, and ARX [46, 65].
In BCD cells RC induced cell redifferentiation and proved to induced PAX4 and ARX expression [38, 65]. The activation of transcription factors by RC could be conducted more specifically by the inhibition of other factors associated with pancreatic endocrine cell maturation. ARX shRNA resulted in a 10-fold decrease of ARX transcript levels, a 12.8-fold increase in INS levels, and a 4.5-fold increase in PAX4 transcript levels, in accordance with the mutual inhibition between ARX and PAX4. Islet progenitor-cell transcription factors were also upregulated; NGN3 increased an 18.2-fold and PDX1, NKX6.1, MAFA, MAFB, NEUROD1, and NKX2.2 transcription factors were upregulated between 3.8–8.3-fold [37].
Treatment of BCD cells with ARX shRNA duplicated the number of insulin-producing cells. Friedman-Mazursky et al. found that in islet infected with ARX shRNA virus and treated with RC, ARX-positive cells reduced 70%, C-peptide-positive cells increased 2.6-fold, and PAX4-positive cells increased 2.7-fold. Moreover, RNA-seq analysis permitted the identification of 261 upregulated genes (among them INS, PDX1, and ABCC8) and 197 downregulated genes (among them SEPT5) in BCD cells treated with RC and ARX shRNA. Collectively, these results suggest that ARX inhibition in BCD cells treated with RC could potentiate β-cell redifferentiation [37].
Remarkably, in human cells, RC treatment-induced redifferentiation in 25% of BCD cells, which yield an 8–32-fold increase in newly generated insulin-producing cells. Additionally, it is worth mentioning that RC differentiation results were reproducible in all the donors included [65].
GLP-1–estrogen
GLP-1–estrogen conjugate was designed to investigate a pharmacological approach that combines the mitogenic actions of estrogen and the relative specificity of GLP-1 to β-cell [68]. Each of these molecules individually has been associated with the improvement of pancreatic function. Estrogen has been demonstrated to have a protective role in β-cell function and survival. It has been shown that estrogen protects β-cell from hypoxia and oxidative stress and that is able to stimulate β-cell proliferation. The proposed mechanisms for their action in the β-cell mass expansion include the induction of replication of existing β cells via ERα signaling and stimulation of neogenesis through activation of NGN3 or PDX1 [69]. On the other hand, GLP-1 results on β-cell proliferation have not been reproducible in humans. Nonetheless, GLP-1 is a strongly accepted potentiator of GSIS in both rodents and humans [28]. In this case, GLP-1 has been used as a carrier of estrogen; this strategy helps to prevent adverse side effects related to estrogens, such as uterus and tumor growth and increase β-cell. GLP-1 has been used to target the delivery of molecules towards β-cell, adding a relative selectivity to enhance pancreatic function [41, 69].
In STZ-diabetic mice, treatment with GLP-1–estrogen for 100 days proved to efficiently decrease fasting glucose, increased fasting C-peptide and insulin levels, and to improve islet architecture and β-cell mass [41]. To test a triple pharmacological perspective, Sachs et al. explored the effect of the insulin and GLP-1–estrogen combination. In STZ-diabetic mice, the combination therapy insulin (10 nmol kg−1) and GLP-1–estrogen (100 nmol kg–1), increased C-peptide levels and insulin content, normalized glycemia and glucose tolerance, and augmented the number of β cells. It is also worth mentioning that combination therapy enables a 60% reduction of insulin dose (10 nmol kg−1 versus 25 nmol kg−1) compared with monotherapy, which reduces the risk of hypoglycemia [41].
In insulin and GLP-1–estrogen-treated mice a substantially increased expression of β-cell maturity markers was observed and almost no β cells remained dedifferentiated. Insulin and GLP-1–estrogen has also shown to increase β-cell proliferation after 25 days of treatment, in the insulin and GLP-1–estrogen group 1.13% of cells corresponded to β-cell compared with 0.34% in the STZ group [41].
Insulin and GLP-1–estrogen could contribute to ER stress mitigation; this effect could protect β cells and promotes their regeneration capacity. In insulin and GLP-1–estrogen-treated mice it was found induction of the ER-associated degradation (ERAD) pathway measured by Sel1l and Hrd1 staining in islets after 25 days of cotreatment. Other ERAD-associated genes were also upregulated, such as Sdf2l1, Herpud1, Dnajb11, Dnajb9, Derl3, and Hspa5 which have been involved in the correct insulin folding and/or function. Additionally, an increased abundance of tRNA could be related to estrogen effects on proliferation [41].
In long-term hyperglycemia, β-cell functional overload can conduce to β-cell dedifferentiation. Dedifferentiation has been characterized by loss of β-cell mature characteristics and results in loss of β-cell mass and pancreatic cell dysfunction. During dedifferentiation β-cell expression profiles are altered with an increase of markers associated with immaturity and a decrease of β-cell maturity markers. Collectively, experimental studies have suggested that β-cell redifferentiation is a feasible strategy for the protection of pancreatic mass and function in diabetes pathogenesis.
Several compounds had been evaluated for their use in β-cell redifferentiation (Table 2), and with these, therapeutic ramifications have emerged. Among these compounds, we found anti-inflammatory agents, BTC, RC, and the triple pharmacological scheme Insulin + GLP-1–estrogen. All of them have demonstrated to partially restore the β-cell mature phenotype and most of them proved to improve insulin secretion and the GSIS response. Furthermore, the triple combinatory scheme was also able to diminish ER stress and to stimulate β-cell proliferation.
Pancreatic β cells had been characterized for their low proliferation rate and their susceptibility to oxidative stress that make them vulnerable to cellular damage. Since in both T1D and T2D there is substantial β-cell destruction, preservation of pancreatic mass is crucial.
Regeneration strategies could contribute to the amelioration of diabetes pathogenesis through the improvement of β-cell proliferation capacity and the restoration of the β-cell mature phenotype. Finally, despite investigation progress and, while regenerative strategies appear to be promising for both T1D and T2D, more studies are necessary considering safety, feasibility, and scalability to translate these findings to the clinical setting.
References
A. Pisania, G.C. Weir, J.J. O’Neil, A. Omer, V. Tchipashvili, J. Lei et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab. Investig. 90, 1661–75 (2011). https://doi.org/10.1038/labinvest.2010.124.Quantitative
H.I. Marrif, S.I. Al-Sunousi, Pancreatic β cell mass death. Front. Pharmacol. 7, 83 (2016). https://doi.org/10.3389/fphar.2016.00083
B.E. Gregg, P.C. Moore, D. Demozay, B.A. Hall, M. Li, A. Husain et al. Formation of a human β-cell population within pancreatic islets is set early in life. J. Clin. Endocrinol. Metab. 97, 3197–206 (2012). https://doi.org/10.1210/jc.2012-1206
I. Cozar-Castellano, N. Fiaschi-Taesch, T.A. Bigatel, K.K. Takane, A. García-Ocaña, R. Vasavada et al. Molecular control of cell cycle progression in the pancreatic β-cell. Endocr. Rev. 27, 356–70 (2006). https://doi.org/10.1210/er.2006-0004
Y. Dor, J. Brown, O.I. Martinez, D.A. Melton, Adult pancreatic β-cell are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–6 (2004)
I. Swenne, Effects of aging on the regenerative capacity of the pancreatic β-cell of the rat. Diabetes 32, 14–9 (1983)
R.N. Kulkarni, E.-B. Mizrachi, A. García-Ocaña, A.F. Stewart, Human β-cell proliferation and intracellular signaling driving in the dark without a road map. Diabetes 61, 2205–13 (2012). https://doi.org/10.2337/db12-0018
T. Mezza, R.N. Kulkarni, The regulation of pre- and post-maturational plasticity of mammalian islet cell mass. Diabetologia 57, 1291–303 (2014). https://doi.org/10.1007/s00125-014-3251-7
N. Fiaschi-Taesch, T.A. Bigatel, B. Sicari, K.K. Takane, F. Salim, S. Velazquez-Garcia et al. Survey of the human pancreatic β-cell G1/S proteome reveals a potential therapeutic role for Cdk-6 and cyclin D1 in enhancing human β-cell replication and function in vivo. Diabetes 58, 882–93 (2009). https://doi.org/10.2337/db08-0631.N.F.-T
L.U.C. Bouwens, I. Rooman, Regulation of pancreatic beta-cell mass. Physiol. Rev. 85, 1255–70 (2005). https://doi.org/10.1152/physrev.00025.2004
B. Reusens, C. Remacle, Programming of the endocrine pancreas by the early nutritional environment. Int J. Biochem Cell Biol. 38, 913–22 (2006). https://doi.org/10.1016/j.biocel.2005.10.012
D.J. Hill, Development of the endocrine pancreas. Rev. Endocr. Metab. Disord. 6, 229–38 (2005). https://doi.org/10.1016/B978-0-323-18907-1.00030-5
Q. Zhou, D.A. Melton, Pancreas regeneration. Nature 557, 351–8 (2018). https://doi.org/10.1038/s41586-018-0088-0
T.M. Nordmann, E. Dror, F. Schulze, S. Traub, E. Berishvili, C. Barbie et al. The role of inflammation in β-cell dedifferentiation. Sci. Rep. 2017:6285. https://doi.org/10.1038/s41598-017-06731-w.
C. Ackeifi, P. Wang, E. Karakose, J.E.M. Fox, B.J. González, H. Liu et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human β-cell regeneration. Sci. Transl. Med 12, eaaw9996 (2020)
D. Saunders, A.C. Powers, Replicative capacity of β-cells and type 1 diabetes. J. Autoimmun. 71, 59–68 (2016). https://doi.org/10.1016/j.jaut.2016.03.014
E. Dirice, D. Walpita, A. Vetere, B.C. Meier, S. Kahraman, J. Hu et al. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes 65, 1660–71 (2016). https://doi.org/10.2337/db15-1127
C. Ackeifi, E. Swartz, K. Kumar, H. Liu, S. Chalada, E. Karakose et al. Pharmacologic and genetic approaches define human pancreatic β cell mitogenic targets of DYRK1A inhibitors. JCI Insight 5, e132594 (2020)
P. Wang, E. Karakose, H. Liu, E. Swartz, V. Zlatanic, J. Wilson et al. Combined inhibition of DYRK1A, SMAD and trithorax pathways synergizes to induce robust replication in adult human beta cells. Cell Metab. 29, 638–52 (2020). https://doi.org/10.1016/j.cmet.2018.12.005.Combined
P. Wang, J. Alvarez-Perez, D.P. Felsenfeld, S. Sivendran, A. Bender, A. Kumar et al. Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase. Nat. Med. 21, 383–8 (2015). https://doi.org/10.1038/nm.3820.Induction
E. Karakose, C. Ackeifi, P. Wang, A.F. Stewart, Advances in drug discovery for human beta cell regeneration. Diabetologia 61, 1693–9 (2018). https://doi.org/10.1007/s00125-018-4639-6
Y. Gwack, S. Sharma, J. Nardone, B. Tanasa, A. Iuga, S. Srikanth et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441, 646–50 (2006). https://doi.org/10.1038/nature04631
J. Shirakawa, R.N. Kulkarni, Novel factors modulating human β-cell proliferation. Diabetes Obes. Metab. 18, 71–7 (2017). https://doi.org/10.1111/dom.12731.Novel
W.R. Goodyer, X. Gu, Y. Liu, R. Bottino, G.R. Crabtree, S.K. Kim, Neonatal β cell development in mice and humans is regulated by calcineurin/NFAT. Dev. Cell 23, 21–34 (2012). https://doi.org/10.1016/j.devcel.2012.05.014.Neonatal
W. Shen, B. Taylor, Q. Jin, S. Meeusen, Y. Zhang, A. Kamireddy et al. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat. Commun. 6, 8372 (2015). https://doi.org/10.1038/ncomms9372
K.I. Aamodt, R. Aramandla, J.J. Brown, N. Fiaschi-Taesch, P. Wang, A.F. Stewart et al. Development of a reliable automated screening system to identify small molecules and biologics that promote human β-cell regeneration. Am. J. Physiol. Endocrinol. Metab. 311, E859–68 (2016). https://doi.org/10.1152/ajpendo.00515.2015
S. Dhawan, E. Dirice, R.N. Kulkarni, A. Bhushan, Inhibition of TGF-β signaling promotes human pancreatic β-cell replication. Diabetes 65, 1208–18 (2016). https://doi.org/10.2337/db15-1331
G. Basile, R.N. Kulkarni, N.G. Morgan, How, when, and where do human β-cells regenerate? Curr. Diab Rep. 19, 48 (2020). https://doi.org/10.1007/s11892-019-1176-8.How
Y. Nakagawa, T. Suzuki, H. Ishii, A. Ogata, D. Nakae, Mitochondrial dysfunction and biotransformation of β-carboline alkaloids, harmine and harmaline, on isolated rat hepatocytes. Chem. Biol. Interact. 188, 393–403 (2010). https://doi.org/10.1016/j.cbi.2010.09.004
H. Waki, K.W. Park, N. Mitro, L. Pei, R. Damoiseaux, D.C. Wilpitz et al. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPAR g expression. Cell Metab. 5, 357–70 (2007). https://doi.org/10.1016/j.cmet.2007.03.010
B. Khor, J.D. Gagnon, G. Goel, M.I. Roche, K.L. Conway, K. Tran et al. The kinase DYRK1A reciprocally regulates the differentiation of Th17 and regulatory T cells. Elife 4, e05920 (2015). https://doi.org/10.7554/eLife.05920
D. Kondoh, S. Yamamoto, T. Tomita, K. Miyazaki, N. Itoh, Y. Yasumoto et al. Harmine lengthens circadian period of the mammalian molecular clock in the suprachiasmatic nucleus. Biol. Pharm. Bull. 37, 1422–7 (2014)
H.E. Hohmeier, L. Zhang, B. Taylor, S. Stephens, D. Lu, P. Mcnamara et al. Identification of a small molecule that stimulates human β-cell proliferation and insulin secretion, and protects against cytotoxic stress in rat insulinoma cells. PLoS ONE 15, e0224344 (2020). https://doi.org/10.1371/journal.pone.0224344
Y. Li, B. Hao, Structural basis of dimerization-dependent ubiquitination by the SCF Fbx4 ubiquitin ligase. J. Biol. Chem. 285, 13896–906 (2010). https://doi.org/10.1074/jbc.M110.111518
S. Tiwari, C. Roel, T. Mansoor, R. Wills, N. Perianayagam, P. Wang et al. Definition of a Skp2-c-Myc Pathway to Expand Human Beta-cells. Sci. Rep. 6, 28461 (2016). https://doi.org/10.1038/srep28461
P.P. Khin, J.H. Lee, H.S. Jun, A brief review of the mechanisms of β-cell dedifferentiation in type 2 diabetes. Nutrients 2021;13. https://doi.org/10.3390/nu13051593.
O. Friedman-Mazursky, R. Elkon, S. Efrat, Redifferentiation of expanded human islet β cells by inhibition of ARX. Sci. Rep. 6, 20698 (2016). https://doi.org/10.1038/srep20698
J. Zhang, F. Liu, The De-, Re-, and trans-differentiation of β-cells: Regulation and function. Semin Cell Dev. Biol. 103, 68–75 (2020). https://doi.org/10.1016/j.semcdb.2020.01.003
G. Domínguez Gutiérrez, A.S. Bender, V. Cirulli, T.L. Mastracci, S.M. Kelly, A. Tsirigos et al. Pancreatic β cell identity requires continual repression of non–β cell programs. J. Clin. Investig 127, 244–59 (2017). https://doi.org/10.1172/JCI88017.NEUROD1
M. Bensellam, J.C. Jonas, D.R. Laybutt, Mechanisms of β-cell dedifferentiation in diabetes: Recent findings and future research directions. J. Endocrinol. 236, R109–43 (2018). https://doi.org/10.1530/JOE-17-0516
Sachs S., Bastidas-Ponce A., Tritschler S., Bakhti M., Böttcher A., Sánchez-Garrido M.A., et al. Targeted Pharmacological Therapy Restores β-Cell Function for Diabetes Remission. 2 (Springer US, 2020). https://doi.org/10.1038/s42255-020-0171-3.
W. Zhiyu, N.W. York, C.G. Nichols, M.S. Remedi, Pancreatic β-cell dedifferentiation in diabetes and re-differentiation following insulin therapy. Cell Metab. 19, 872–82 (2014). https://doi.org/10.1016/j.cmet.2014.03.010.Pancreatic
X.N. Téllez, M. Vilaseca, Y. Martí, A. Pla, E. Montanya, β-Cell dedifferentiation, reduced duct cell plasticity, and impaired β-cell mass regeneration in middle-aged rats. Am. J. Physiol. Endocrinol. Metab. 311, E554–63 (2016). https://doi.org/10.1152/ajpendo.00502.2015
C. Talchai, S. Xuan, H.V. Lin, L. Sussel, D. Accili, Pancreatic β-cell dedifferentiation as mechanism of diabetic β-cell failure. Cell 150, 1223–34 (2012). https://doi.org/10.1016/j.cell.2012.07.029.Pancreatic
Y. Bar, H.A. Russ, S. Knoller, L. Ouziel-Yahalom, E. Shimon, HES-1 is involved in adaptation of adult human β-cells to proliferation in vitro. Diabetes 57, 2413–20 (2008). https://doi.org/10.2337/db07-1323
S. Efrat, Mechanisms of adult human β-cell in vitro dedifferentiation and redifferentiation. Diabetes Obes. Metab. 18, 97–101 (2016). https://doi.org/10.1111/dom.12724
A. Lenz, G. Toren-Haritan, S. Efrat, Redifferentiation of adult human β cells expanded in vitro by inhibition of the WNT pathway. PLoS ONE 9, e112914 (2014). https://doi.org/10.1371/journal.pone.0112914
M. Bakhti, A. Böttcher, H. Lickert, Modelling the endocrine pancreas in health and disease. Nat. Rev. Endocrinol. 15, 155–71 (2019). https://doi.org/10.1038/s41574-018-0132-z
W.L. Qiu, Y.W. Zhang, Y. Feng, L.C. Li, L. Yang, C.R. Xu, Deciphering pancreatic islet β cell and α cell maturation pathways and characteristic features at the single-cell level. Cell Metab. 25, 1194–1205.e4 (2017). https://doi.org/10.1016/j.cmet.2017.04.003
C. Zeng, F. Mulas, Y. Sui, T. Guan, N. Miller, F. Liu et al. Pseudotemporal ordering of single cells reveals metabolic control of postnatal beta-cell proliferation. Cell Metab. 25, 1160–75 (2017).https://doi.org/10.1016/j.cmet.2017.04.014.Pseudotemporal
J. Sun, Q. Ni, J. Xie, M. Xu, J. Zhang, J. Kuang et al. β-cell dedifferentiation in patients With T2D With adequate glucose control and nondiabetic chronic pancreatitis. J. Clin. Endocrinol. Metab. 104, 83–94 (2019). https://doi.org/10.1210/jc.2018-00968
W. Ying, Y.S. Lee, Y. Dong, J.S. Seidman, M. Yang, R. Isaac et al. Expansion of islet-resident macrophages leads to inflammation affecting β cell proliferation and function in obesity. Cell Metab. 29, 457–474.e5 (2019). https://doi.org/10.1016/j.cmet.2018.12.003
W. He, T. Yuan, K. Maedler, Macrophage-associated pro-inflammatory state in human islets from obese individuals. Nutr. Diabetes 9, 36 (2019). https://doi.org/10.1038/s41387-019-0103-z
M. Ghodsi, B. Larijani, A.A. Keshtkar, E. Nasli-Esfahani, S. Alatab, M.R. Mohajeri-Tehrani, Mechanisms involved in altered bone metabolism in diabetes: a narrative review. J. Diabetes Metab. Disord. 15, 52 (2016). https://doi.org/10.1186/s40200-016-0275-1
R.A. DeFronzo, E. Ferrannini, L. Groop, R.R. Henry, W.H. Herman, J.J. Holst et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 1, 1–22 (2015). https://doi.org/10.1038/s41574-019-0286-3
S. Efrat, Beta-cell dedifferentiation in type 2 diabetes: concise review. Transl. Clin. Res. 37, 1267–72 (2019). https://doi.org/10.1002/stem.3059
F. Han, X. Li, J. Yang, H. Liu, Y. Zhang, X. Yang et al. Salsalate prevents β-cell dedifferentiation in OLETF rats with type 2 diabetes through Notch1 pathway. Aging Dis. 10, 719–30 (2019). https://doi.org/10.14336/AD.2018.1221
Y.S. Oh, S. Shin, Y.J. Lee, E.H. Kim, H.S. Jun, Betacellulin-induced beta cell proliferation and regeneration is mediated by activation of ErbB-1 and ErbB-2 receptors. PLoS ONE. 2011;6. https://doi.org/10.1371/journal.pone.0023894.
S. Shin, N. Li, N. Kobayashi, J.W. Yoon, H.S. Jun, Remission of diabetes by β-cell regeneration in diabetic mice treated with a recombinant adenovirus expressing Betacellulin. Mol. Ther. 16, 854–61 (2008). https://doi.org/10.1038/mt.2008.22
H. Kojima, M. Fujimiya, K. Matsumura, P. Younan, H. Imaeda, M. Maeda et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 9, 596–603 (2003). https://doi.org/10.1038/nm867
L. Li, M. Seno, H. Yamada, I. Kojima, Betacellulin improves glucose metabolism by promoting conversion of intraislet precursor cells to β-cells in streptozotocin-treated mice. Am. J. Physiol. Endocrinol. Metab. 285, 577–83 (2003). https://doi.org/10.1152/ajpendo.00120.2003
L. Ouziel-Yahalom, M. Zalzman, L. Anker-kitai, S. Knoller, Y. Bar, M. Glandt et al. Expansion and redifferentiation of adult human pancreatic islet cells. Biochem. Biophys. Res. Commun. 341, 291–8 (2006). https://doi.org/10.1016/j.bbrc.2005.12.187
Y.S. Lee, G.J. Song, H.S. Jun, Betacellulin-induced α-cell proliferation is mediated by ErbB3 and ErbB4, and may contribute to β-cell regeneration. Front Cell Dev. Biol. 8, 1–11 (2021). https://doi.org/10.3389/fcell.2020.605110
M.Y. Song, U.J. Bae, K.Y. Jang, B.H. Park, Transplantation of betacellulin-transduced islets improves glucose intolerance in diabetic mice. Exp. Mol. Med. 46, e98–8 (2014). https://doi.org/10.1038/emm.2014.24
H.A. Russ, E. Sintov, L. Anker-Kitai, O. Friedman, A. Lenz, G. Toren et al. Insulin-producing cells generated from dedifferentiated human pancreatic beta cells expanded in vitro. PLoS ONE 6, e25566 (2011). https://doi.org/10.1371/journal.pone.0025566
R.P. Robertson, L.K. Olson, H.-J. Zhang, Differentiating glucose toxicity from glucose desensitization: A new message from the insulin gene. Diabetes 43, 1085–9 (1994). https://doi.org/10.2337/diab.43.9.1085
E. Sintov, G. Nathan, S. Knoller, M. Pasmanik-Chor, H.A. Russ, E. Shimon, Inhibition of ZEB1 expression induces redifferentiation of adult human β cells expanded in vitro. Sci. Rep. 5, 13024 (2015). https://doi.org/10.1038/srep13024
B. Finan, B. Yang, N. Ottaway, K. Stemmer, T.D. Müller, C.-X. Yi et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 18, 1847–56 (2012). https://doi.org/10.1038/nm.3009.Targeted
F. Mauvais-Jarvis, C. Le May, J.P. Tiano, S. Liu, G. Kilic-Berkmen, J.H. Kim, The role of estrogens in pancreatic islet physiopathology. Adv. Exp. Med Biol. 1043, 385–99 (2017). https://doi.org/10.1007/978-3-319-70178-3_18
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Desentis-Desentis, M.F. Regenerative approaches to preserve pancreatic β-cell mass and function in diabetes pathogenesis. Endocrine 75, 338–350 (2022). https://doi.org/10.1007/s12020-021-02941-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02941-5