Abstract
Purpose
Emerging clinical evidence has implied that alkaline phosphatase (ALP) may contribute to gestational diabetes mellitus (GDM). However, there were no studies to reveal the independent and prospective associations between ALP and GDM. Our aim was to explore the independent and prospective associations between early maternal ALP level and GDM risk and glucose regulation.
Methods
In a prospective cohort study with 2073 singleton mothers at four maternity units in China, maternal serum ALP levels were measured before 20 gestational weeks. Using logistic regression, we analyzed the relationship between maternal ALP level and risk of GDM. We further explored the relationships of ALP level to fasting blood glucose (FBG), 1-h and 2-h post-load blood glucose (1-h, 2-h PBG) with multiple linear regression. Finally, we analyzed the association between maternal ALP level and isolated impaired fasting glucose (i-IFG) and isolated impaired glucose tolerance (i-IGT) risk.
Results
The maximum value of maternal ALP level was 90 U/L, within the normal range. After adjustment for confounding factors, the odds ratio (ORs) of GDM increased linearly with ALP level (p for overall association = 0.002, p for nonlinear association = 0.799), with the OR comparing the highest versus lowest quartile of 2.47 (95% CI 1.47, 4.15). Moreover, each additional of 10 U/L ALP level was associated with a 2% higher FBG (p = 0.043) and a 12% higher 1-h PBG (p = 0.004). Higher ALP level also increased the risk of i-IFG (OR 3.73, 95% CI 1.17–11.86) and i-IGT (OR 2.03, 95% CI 1.07–3.84).
Conclusions
Even within the upper limit of normal, higher early maternal ALP level could increase the risk of GDM. Moreover, both FBG and PBG were increased with early maternal ALP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is one of the most complex and common endocrine-metabolic pregnancy disorders. GDM affects 3–21.2% of all pregnancies in Asian women, and its prevalence keeps increasing worldwide [1]. Worst of all, both the women with GDM and their offspring have a substantially increased risk to develop type 2 diabetes mellitus (T2DM) after pregnancy [2]. Thus, GDM is widely regarded as an initiator of a vicious circle between generations, which will make a big “contribution” to the global diabetes epidemic. Therefore, identifying women who are at high risk of GDM and in-depth understanding of the pathogenesis of GDM are of great clinical significances and crucial for public health.
Alkaline phosphatase (ALP) are a group of enzymes, which exists in almost all tissues of humans, with particularly high activities in the liver and bone in the general population [3, 4]. Although ALP has been studied for many years, its biological function remains to be understood. Conventionally, serum alkaline phosphatase activity is widely taken as an indicator of cholestasis and bone turnover in routine clinical practice [5]. But for pregnant women, ALP is quite a special enzyme, due to its time-dependent feature. It increases progressively throughout pregnancy and reaches peak levels at term in normal pregnancy, returning to normal values after delivery [6]. Therefore, ALP may play a vital role during pregnancy. But for a long time, studies could not find significant associations between maternal ALP and pathologic conditions of pregnancy [7]. However, emerging clinical evidence has implied that ALP may contribute to GDM.
Serum ALP activity has been observed to be elevated in type 2 diabetes (T2DM) patients for many years [8]. Studies also found that ALP correlated positively with liver insulin resistance that can lead to overproduction of glucose in the basal state [9, 10]. Moreover, a recent large prospective study further confirmed that high-serum ALP was an independent risk factor of T2DM [11]. These suggested that elevated serum ALP activity may be involved in the development of T2DM. Since there is similar pathogenesis between T2DM and GDM, we wonder the explicit association between maternal ALP activity and GDM pregnant women. In fact, two case-report studies have observed the extreme elevation of ALP in the GDM women [12, 13]. Lately, Anjum S et al. also found significantly higher ALP in GDM patients when compared with the non-GDM group in a cross-sectional study [14]. The most exciting thing was that we even found whatever the gestational week was, ALP was significantly higher in the GDM group than in the non-GDM group in our pilot study (Supplementary Fig. 1). These evidence all indicated that increased maternal ALP activity may be implicated in GDM. Despite the fact that the meaning of the association between alkaline phosphatase and subsequent gestational diabetes is not clear up to now, epidemiologic studies are also needed to explore the role of new biological and clinical markers as risk factors. In this context, we speculated that elevated maternal ALP activity may be associated with an increased risk of GDM. However, there were no studies to reveal the independent and prospective associations between ALP and GDM, to date. To demonstrate our hypothesis, we examined the association of early maternal ALP level with subsequent risk of GDM and glucose regulation in 2073 pregnant women from a prospective cohort study. The study will help for an in-depth understanding of the pathogenesis of GDM and for exploring the new physiological and pathological significance of ALP.
Materials and methods
Study participants
The multicenter Tongji Maternal and Child Health Cohort (TMCHC) study enrolled 8000 pregnant women from September 2013 to April 2016 at four maternity centers in Wuhan, in central China, to investigate the impacts of maternal diet and other lifestyle factors before and/or during pregnancy on pregnancy outcomes [15, 16]. All study participants were informed of the study protocol, and signed informed consent before inclusion. All procedures performed in studies involving participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The TMCHC study was approved by the Ethics Review Committee of Tongji Medical College, Huazhong University of Science and Technology (No. 201302).
For this study, we excluded women with pre-diagnosed diabetes mellitus, and/or liver diseases. Finally, 2073 pregnant women from the TMCHC study who had their ALP measured prior to 20 gestational weeks were included to analyze the associations between early maternal ALP level and GDM, as well as glucose regulation.
Maternal characteristics
The demographic and sociological characteristics presented were based on data from a structured questionnaire as part of a face-to-face interview by a trained investigator upon enrollment. This questionnaire included recognized and putative GDM risk factors, such as maternal age, educational and income level, parity, alcohol and cigarette abuse, presence of insomnia prior to the current pregnancy, and family history of diabetes. Educational levels were categorized as ≤9, 10–15, and ≥16 years of schooling completed. Income levels were categorized as ≤4999, 5000–9999, and ≥10,000 Chinese Yuan (CNY) according to monthly household income. Smoking (or drinking) before pregnancy was defined as smoking (or drinking) no less than three times per week for more than half of 1 year before pregnancy; otherwise, the status was defined as no-smoking (or no-drinking). Insomnia, which was the subjective feeling of pregnant women, was defined as difficulty initiating or maintaining sleep, or early awakening with an inability to return to sleep, together with the associated impairment of daytime functioning. Gestational weeks at the liver function test (LFT) and oral glucose tolerance test (OGTT) were both calculated based on the self-reported last menstrual period. The weight and height at the time of LFT and OGTT were measured by an automatic weight and height scale with light indoor clothing and bare feet.
Exposure assessment
After an overnight fasting of more than 10 h, all the participants had their fasting serum ALP level as well as other LFT parameters, such as alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase (γ-GT), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), total protein (TP), albumin (Alb), and globulin (Gb) levels, measured prior to 20 weeks of gestation by the professional laboratory technicians at the clinical laboratory. We obtained the above data from the medical records. The normal local physiologic amount of maternal ALP was 35–100 U/L.
Diagnosis of GDM
The 75-g 2-h OGTT test was conducted to identify GDM (and its subgroups) cases. It including fasting blood glucose concentration (FBG) and blood glucose concentrations at 1- and 2 h after the glucose load (1-, 2-h PBG), was implemented in the morning in all subjects after more than 10 h of fasting at 24–28 weeks of gestation by professional laboratory technicians. The presence of one or more abnormal serum glucose levels (i.e., FBG ≥ 5.1 mmol/L, 1-h PBG ≥ 10.0 mmol/L, and/or 2-h PBG ≥ 8.5 mmol/L) was defined as GDM, in accordance with the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criterion [17]. In addition, one previous study has indicated that there were different mechanisms of abnormal glucose metabolism at different OGTT time points and have different effects on pregnancy outcomes [18]. Based on this study, we subgrouped GDM according to the OGTT time point in the study. Isolated impaired fasting glucose (i-IFG) was defined as FBG ≥ 5.1 mmol/L, 1-h glucose < 10.0 mmol/L, and 2-h glucose < 8.5 mmol/L. Isolated impaired glucose tolerance (i-IGT) was defined as 1-h PBG ≥ 10.0 mmol/L and/or 2-h PBG ≥ 8.5 mmol/L and FPG < 5.1 mmol/L. The combination of IFG and IGT (IFG + IGT) was defined as FBG ≥ 5.1 mmol/L, 1-h PBG ≥ 10.0 mmol/L, and/or 2-h PBG ≥ 8.5 mmol/L.
Data analysis
First, all variables were checked for normality and transformed or analyzed using nonparametric methods. Basic characteristics were compared across ALP-level quartiles by analysis of variance test for continuous variables and χ2 test for categorical variables. Second, restricted cubic splines (RCS) were used to analyze the overall associations between ALP level and GDM. Logistic regression analysis was used to evaluate the independent associations between the respective quartiles of ALP level and GDM. Finally, the qualitative and quantitive relationship between ALP level and blood glucose concentration was explored by smoothing function and multiple linear regression, respectively. Moreover, we also evaluated the associations between maternal ALP level and i-IFG, i-IGT, and IFG + IGT.
Progressive models were used as follows. In the crude model, the odds ratio (OR) was not adjusted for any variable; Model I was adjusted for maternal age, maternal weight at LFT, maternal height, gestational weeks at LFT, gestational weeks at OGTT, the rate of weight gain from LFT to OGTT (GWR), maternal educational and income levels, primiparity, family history of diabetes and insomnia, and drinking and smoking status before pregnancy. Model II added ALT, AST, γ-GT, TBIL, IBIL, TP, and Gb based on model I. In each model, quartile 1 acted as the reference when computing odds ratios (ORs) and the corresponding 95% confidence intervals (95% CIs).
RCS and logistic regression analysis was conducted by using SAS version 9.4 (SAS Institute, Cary, NC, USA). The other analyses were conducted with R (http://www.R-project.org). Significance was set at two-tailed p values < 0.05.
Results
Characteristics of the study population
All study measures were carried out in 2073 pregnant women at a mean ± SD of 16.20 ± 2.55 weeks’ gestation. The mean maternal age was 28.62 ± 3.23 years, which ranged from 18 to 43 years old. Serum ALP levels ranged from 13 to 90 U/L with a mean of 44.87 ± 9.66 U/L, which were all within the upper limit of normal for ALP (100 U/L). The basic and clinical parameters were listed in Table 1 according to serum ALP level quartiles. The cutoff values of serum ALP were 13.0–38.0, 38.0–44.0, 44.0–50.0, and > 50.0 U/L, respectively. There were remarkable differences in gestational weeks, maternal weight at LFT, maternal height, family history of diabetes, serum ALT, AST, γ-GT, TBIL, IBIL, TP, and Gb, but not in the rest of the variables. The incidence rate of GDM was 8.15% and the incidence rates of i-IFG, i-IGT, and IFG + IGT were 8.15%, 1.83%, 4.82%, and 1.50%, respectively. Women with higher quartiles of ALP had higher levels of FBG (p = 0.002) and 1-h PBG (p < 0.001), but not higher levels of 2-h PBG (p = 0.191). Furthermore, women with higher quartiles of ALP had a higher incidence of GDM (p < 0.001)
Associations between maternal ALP level and the risk of GDM
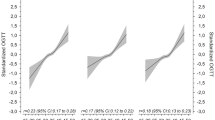
The continuous (overall) associations between ALP and risk of GDM are displayed in Fig. 1. It manifested that the odds ratio (OR) of GDM increased linearly with maternal ALP (p for overall association = 0.002, p for nonlinear association = 0.799). Moreover, the associations between ALP and risk of GDM are displayed in Table 2. In contrast with the bottom quartile, the highest quartile of ALP was significantly associated with the highest risk of GDM (unadjusted OR, 2.62 (95% CI 1.63, 4.21)) in all subjects (p for trend < 0.001). In mode I, the corresponding OR was 2.37 (95% CI 1.44, 3.89), p for trend of 0.001. In model II, the corresponding OR was 2.47 (95% CI 1.47, 4.15), p for trend of 0.001.
Overall association between early maternal ALP and risk of GDM. The overall association used RCS with three knots (reference value for ALP was 44 U/L) after adjustment for maternal age, maternal weight at LFT, maternal height, gestational weeks at LFT, gestational weeks at OGTT, GWR, maternal educational and income levels, primiparity, family history of diabetes and insomnia, drinking and smoking status before pregnancy, and maternal serum ALT, AST, γ-GT, TBIL, IBIL, TP, and Gb in 2073 participants
The relationships of maternal ALP with fasting and post-load blood glucose levels
To find out the reason that high ALP would increase the risk of GDM, we used smoothing function and multivariate linear regression analysis to reveal the relationships of maternal ALP to FBG, 1-h PBG, and 2-h PBG. These results were shown in Fig. 2 and Table 3, respectively. In Fig. 2, we found that there were significant positive associations between ALP and FBG and 1-h PBG, but not 2-h PBG. In Table 3, we further demonstrated that FBG increased with each additional of 10 U/L maternal ALP (β = 0.03, p < 0.001) in crude model, (β = 0.02, p = 0.016) in model I, and (β = 0.02, p = 0.043) in model II. One-hour PBG also increased with each additional of 10 U/L maternal ALP (β = 0.13, p < 0.001) in crude model, (β = 0.11, p < 0.001) in model I, and (β = 0.12, p = 0.004) in model II. However, the 2-h PBG was not increased with maternal ALP level. Therefore, we speculated that higher maternal ALP level may increase the risk of GDM, finally by inducing elevation of FBG and 1-h PBG.
The relationships between early maternal ALP and the FBG (a), 1-h PBG (b), and 2-h PBG (c). The relationships between early maternal ALP and FBG were adjusted for maternal age, maternal weight at LFT, maternal height, gestational weeks at LFT, and gestational weeks at OGTT in 2073 participants. The relationships between serum ALP concentrations and 1- and 2-h PBG were further adjusted for fasting glucose concentrations based on the former
The independent associations between maternal ALP level and the risk of i-IFG, i-IGT, and IFG and IGT
In order to further determine the effect of maternal ALP level on FBG and PBG, we explored the independent associations between maternal ALP and i-IFG, i-IGT, and IFG + IGT (displayed in Table 4). In contrast with the bottom quartile, the highest quartile of ALP was significantly associated with the highest risk of i-IFG (unadjusted OR 4.63 (95% CI 1.55, 13.76)) and p for trend was 0.005. In mode I, the association was still persistent. The OR was 4.18 (95% CI 1.35, 12.88) and p for trend was 0.011. In model II, the association was even more persistent. The OR was 3.73 (95% CI 1.17, 11.86) and p for trend was 0.041. Moreover, there was also significant association between ALP and i-IGT. The corresponding ORs were 1.89 (95% CI 1.05, 3.40) and p for trend was 0.030 in the crude model, 1.78 (95% CI (0.97, 3.27)) and p for trend of 0.043 in model I, and 2.03 (95% CI 1.07, 3.84) and p for trend of 0.017 in model II. However, there was no association between ALP and IFG + IGT. These results manifested that higher early maternal ALP could even increase the risk of i-IFG and i-IGT, but not IFG + IGT.
Discussion
This is the first large prospective study that described the novel associations of maternal ALP level with the risk of GDM. We found that even within the normal range, higher early maternal ALP level could increase the risk of GDM. Moreover, both FBG and 1-h PBG were increased with early maternal ALP level. Higher early maternal ALP level could even increase the risk of i-IFG and i-IGT. It is possible that maternal serum ALP level continuously increases throughout pregnancy in normal pregnancy, the influence of higher early maternal ALP on abnormal pregnancy outcomes has often been neglected by researchers and clinicians for a long time. Our above-mentioned findings were consistent with two prospective studies concerning ALP and T2DM. Nannipieri et al. reported that ALP was remarkably associated with diabetes (p < 0.0004) [19]. In the study by Chen et al., ALP was proved to be a new independent risk factor of self-reported T2DM in both sexes [11]. However, in another mendelian randomization study, ALP was found not clearly associated with T2DM [20]. This discrepancy may due to its use of separate samples, which could not test whether the associations of ALP with the T2DM vary by level of other liver enzymes, by age or by sex. Moreover, our results were also in line with several previous studies concerning ALP and GDM. Of them, two case-report studies noted the extreme elevation of ALP in GDM pregnant women [12, 13]. Our results emphasized that even mild elevation of early maternal ALP levels could increase the risk of GDM based on a large cohort study. In a latest cross-sectional study in 2017, it was found that serum ALP levels were significantly higher in the 50 GDM patients when compared with 50 non-GDM women (p = 0.0038) [14]. We confirmed that maternal circulating ALP levels were prospectively and independently associated with the increased incidence of GDM.
Our research extended to explore the reason of positive associations between higher maternal ALP and risk of GDM. We found that both FBG and 1-h PBG were increased with maternal ALP. Concerning the result that FBG increased with maternal ALP, it was in accordance with several previous studies. In the study of Rao G M et al., they reported that there was a statistically significant positive correlation between the serum ALP levels and the FBG concentrations among T2DM patients (r = 0.35; p < 0.001) [8]. Another study from 320 T2DM patients with abnormal liver function also showed that ALP correlated positively with FBG in both sexes [21]. It was the first finding that 1-h PBG increased with ALP. Thus, a lot of research was needed to probe the related mechanism in the future. Accordingly, we found for the first time that higher early maternal ALP could even increase the risk of i-IFG and i-IGT, except for IFG+IGT. Taken together, these robust evidence indicated that the positive association between early maternal ALP and risk of GDM, i-IFG, and i-IGT was mainly due to the upregulation of FBG and 1-h PBG.
Although the biological mechanisms of the association between early maternal ALP and glucose regulation are unclear, there are several possibilities. First, a study found that ALP correlated positively with liver insulin resistance [9], and high hepatic insulin resistance often means overproduction of glucose in the basal state, despite fasting hyperinsulinemia [10]. That is, high ALP may mean high FBG. This is consistent with our result that high serum ALP levels correlated positively with FBG. Moreover, we have to point out that either in normal pregnancy or in GDM, insulin secretion increases steadily from the first trimester and reaches a maximum in the third trimester, returning to normal values after delivery [22, 23]. This is very consistent with the time-dependent feature of maternal ALP displayed in the previous study [6]. Thus, although no direct evidence, we will venture to guess that ALP may influence insulin metabolism, which finally may lead to GDM. This causality is worthy of study in the future. Second, lipid accumulation also is a possibility. ALP is often taken as a marker of visceral obesity or fatty liver [24], and many studies have proven that both visceral obesity [25, 26] and fatty liver [27,28,29] are independent risk factors of type 2 diabetes. In our study, higher maternal ALP levels with an increased risk of GDM were indeed observed in normal and high pre-pregnancy body mass index (pre-BMI) pregnant women, but not in low pre-BMI women (Supplementary Table 1). This finding was in line with the above speculation. The association that higher ALP levels with an increased risk of GDM were still robust, even though we had adjusted the maternal weight at baseline, maternal height, and GWR in normal and high pre-BMI groups. This suggested that some other mechanism may also be involved in the association, aside from lipid accumulation. Inflammation might be one of the potential mechanisms apart from lipid accumulation, finally. As we know, GDM is a state of chronic, low-grade subclinical inflammation. A previous study proved that serum ALP was positively associated with serum c-reactive protein, a marker for inflammation [30]. Furthermore, it has been demonstrated that maternal C-reactive protein was an independent risk factor of GDM [31]. But more studies are needed to confirm our speculation.
A strength of this study was its large sample size, which derived from an organized multicenter cohort study and use of the standardized and uniform protocol that measured fresh serum ALP immediately. Moreover, the substantial associations between ALP and GDM were robust even after adjusting for nearly all the traditional risk factors for GDM and a variety of other confounders. These findings are crucial for in-depth understanding of the pathogenesis of GDM and the pathophysiological significance of ALP. Another strength of this study is its meritorious clinical significance. On the one hand, maternal serum ALP continuously increases throughout pregnancy in normal pregnancy. The influence of higher serum ALP on abnormal pregnancy outcomes has been neglected for a long time. However, maternal serum ALP > 50 U/L (the clinical reference range was 30–100 U/L) was associated with 2.47 times greater risk of GDM, 3.73 times greater risk of i-IFG, and 2.03 times greater risk of i-IGT, when compared with <38.0 U/L in our study. The result indicated that even mild elevation of early maternal circulating ALP level could increase the subsequent risk of GDM significantly. Thus, early maternal circulating ALP should not be ignored any more in future clinical practice from then on. On the other hand, maternal ALP has been widely applied in the clinic as the regular liver function parameter. It is easy to obtain, cost-effective, and could be well-applied in clinical practices. Thus, maternal ALP may be promising in identifying women who are at a higher risk of GDM in early pregnancy, which is of great importance for preventing and curbing GDM. We acknowledge that the inclusion of Han Chinese women entirely may be a strength from the standpoint of data homogeneity, but at the same time a limitation to generalizability.
This study just heralds a new beginning, because limit studies focus on the associations between early ALP and GDM. Thus, more human studies are needed to confirm our results. Moreover, the placental alkaline phosphatase isoenzyme is known to comprise the majority of the great increase of maternal ALP during the third trimester and early maternal ALP comes mainly from bone and hepatic synthesis in common pregnant women. But the exact source of increased early ALP in GDM patients is still unknown, which needs to be figured out in future experiments [32, 33].
Conclusion
In conclusion, our results revealed for the first time that higher early maternal ALP, even within the upper limit of normal, could increase the risk of GDM in pregnant women. Moreover, both fasting and post-load glucose could increase with early maternal ALP. Maternal ALP may be promising in identifying women who are at high risk of GDM, which is of great importance for preventing and curbing GDM. Finding the major source of increased early ALP in GDM patients is of great concern in future studies. Early elevated maternal ALP should not be ignored anymore. In the future, more experimental studies are also needed to explore the specific pathogenic mechanism that elevated maternal ALP increase GDM risk, which will be very helpful for in-depth understanding of the pathogenesis of GDM and for exploring the new physiological and pathological significance of ALP.
References
E. Chiefari, B. Arcidiacono, D. Foti, A. Brunetti, Gestational diabetes mellitus: an updated overview. J. Endocrinol. Invest 40(9), 899–909 (2017)
P.M. Catalano, H.D. McIntyre, J.K. Cruickshank, D.R. McCance, A.R. Dyer, B.E. Metzger, L.P. Lowe, E.R. Trimble, D.R. Coustan, D.R. Hadden, B. Persson, M. Hod, J.J. Oats; Group, H.S.C.R., The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35(4), 780–786 (2012)
N.J. Fernandez, B.A. Kidney, Alkaline phosphatase: beyond the liver. Vet. Clin. Pathol. 36(3), 223–233 (2007)
U. Sharma, D. Pal, R. Prasad, Alkaline phosphatase: an overview. Indian J. Clin. Biochem. 29(3), 269–278 (2014)
E. Epstein, F.L. Kiechle, J.D. Artiss, B. Zak, The clinical use of alkaline phosphatase enzymes. Clin. Lab. Med. 6(3), 491–505 (1986)
F.A. Aleem, Total and heat-stable serum alkaline phosphatase in normal and abnormal pregnancies. Obstet. Gynecol. 40(2), 163–172 (1972)
F. Schiele, J. Henny, J. Hitz, C. Petitclerc, R. Gueguen, G. Siest, Total bone and liver alkaline phosphatases in plasma: biological variations and reference limits. Clin. Chem. 29(4), 634–641 (1983)
G.M. Rao, L.O. Morghom, Correlation between serum alkaline phosphatase activity and blood glucose levels. Enzyme 35(1), 57–59 (1986)
G. Sesti, T.V. Fiorentino, M.L. Hribal, A. Sciacqua, F. Perticone, Association of hepatic insulin resistance indexes to nonalcoholic fatty liver disease and related biomarkers. Nutr. Metab. Cardiovasc. Dis. 23(12), 1182–1187 (2013)
J. Vangipurapu, A. Stancakova, T. Kuulasmaa, J. Paananen, J. Kuusisto, E.-R.S. Group, E. Ferrannini, M. Laakso, A novel surrogate index for hepatic insulin resistance. Diabetologia 54(3), 540–543 (2011)
S.C. Chen, S.P. Tsai, J.Y. Jhao, W.K. Jiang, C.K. Tsao, L.Y. Chang, Liver Fat, hepatic enzymes, alkaline phosphatase and the risk of incident type 2 diabetes: a prospective study of 132,377 adults. Sci. Rep. 7(1), 4649 (2017)
J. Wojcicka-Bentyn, K. Czajkowski, J. Sienko, M. Grymowicz, M. Bros, Extremely elevated activity of serum alkaline phosphatase in gestational diabetes: a case report. Am. J. Obstet. Gynecol. 190(2), 566–567 (2004)
S. Lozo, A. Atabeygi, M. Healey, Extreme elevation of alkaline phosphatase in a pregnancy complicated by gestational diabetes and infant with neonatal alloimmune thrombocytopenia. Case Rep. Obstet. Gynecol. 2016, 4896487 (2016)
S.S. Siddiqi, A.G. Borse, A. Pervez, S. Anjum, A study of bone turnover markers in gestational diabetes mellitus. Indian J. Endocrinol. Metab. 21(1), 38–44 (2017)
H. Zhang, X. Qiu, C. Zhong, K. Zhang, M. Xiao, N. Yi, G. Xiong, J. Wang, J. Yao, L. Hao, S. Wei, N. Yang, X. Yang, Reproducibility and relative validity of a semi-quantitative food frequency questionnaire for Chinese pregnant women. Nutr. J. 14, 56 (2015)
T. Xiong, C. Zhong, X. Zhou, R. Chen, M. Xiao, Y. Wu, X. Hu, W. Wang, X. Li, C. Liu, G. Xiong, X. Yang, L. Hao, N. Yang, Maternal circulating transthyretin level is longitudinally associated with increased risk of gestational diabetes mellitus: it is not just an indicator of nutritional status. Diabetes Care 40(5), e53–e54 (2017)
B.E. Metzger, S.G. Gabbe, B. Persson et al. Consensus Panel International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33(3), 676–682 (2010). International Association of Diabetes and Pregnancy Study Groups
M.H. Black, D.A. Sacks, A.H. Xiang, J.M. Lawrence, Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care 33(12), 2524–2530 (2010)
M. Nannipieri, C. Gonzales, S. Baldi, R. Posadas, K. Williams, S.M. Haffner, M.P. Stern, E. Ferrannini; Mexico City diabetes, s., Liver enzymes, the metabolic syndrome, and incident diabetes: the Mexico City diabetes study. Diabetes Care 28(7), 1757–1762 (2005)
J. Liu, S.L. Au Yeung, S.L. Lin, G.M. Leung, C.M. Schooling, Liver enzymes and risk of ischemic heart disease and type 2 diabetes mellitus: a mendelian randomization study. Sci. Rep. 6, 38813 (2016)
K. Bora, M. Borah, H. Chutia, C.K. Nath, D. Das, A.A. Ruram, Presence of concurrent derangements of liver function tests in type 2 diabetes and their relationship with glycemic status: a retrospective observational study from Meghalaya. J. Lab. Physicians 8(1), 30–35 (2016)
E. Sivan, X. Chen, C.J. Homko, E.A. Reece, G. Boden, Longitudinal study of carbohydrate metabolism in healthy obese pregnant women. Diabetes Care 20(9), 1470–1475 (1997)
P.M. Catalano, E.D. Tyzbir, R.R. Wolfe, J. Calles, N.M. Roman, S.B. Amini, E.A. Sims, Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am. J. Physiol. 264(1 Pt 1), E60–E67 (1993)
A.T. Ali, C.B. Penny, J.E. Paiker, G. Psaras, F. Ikram, N.J. Crowther, The relationship between alkaline phosphatase activity and intracellular lipid accumulation in murine 3T3-L1 cells and human preadipocytes. Anal. Biochem. 354(2), 247–254 (2006)
E.J. Boyko, W.Y. Fujimoto, D.L. Leonetti, L. Newell-Morris, Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 23(4), 465–471 (2000)
T. Hayashi, E.J. Boyko, D.L. Leonetti, M.J. McNeely, L. Newell-Morris, S.E. Kahn, W.Y. Fujimoto, Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care 26(3), 650–655 (2003)
M. Shibata, Y. Kihara, M. Taguchi, M. Tashiro, M. Otsuki, Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 30(11), 2940–2944 (2007)
H. Yki-Jarvinen, Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2(11), 901–910 (2014)
T. Fukuda, M. Hamaguchi, T. Kojima, K. Mitsuhashi, Y. Hashimoto, A. Ohbora, T. Kato, N. Nakamura, M. Fukui, Transient remission of nonalcoholic fatty liver disease decreases the risk of incident type 2 diabetes mellitus in Japanese men. Eur. J. Gastroenterol. Hepatol. 28(12), 1443–1449 (2016)
A. Kerner, O. Avizohar, R. Sella, P. Bartha, O. Zinder, W. Markiewicz, Y. Levy, G.J. Brook, D. Aronson, Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb. Vasc. Biol. 25(1), 193–197 (2005)
M. Wolf, L. Sandler, K. Hsu, K. Vossen-Smirnakis, J.L. Ecker, R. Thadhani, First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 26(3), 819–824 (2003)
G.J. Valenzuela, L.A. Munson, N.M. Tarbaux, J.R. Farley, Time-dependent changes in bone, placental, intestinal, and hepatic alkaline phosphatase activities in serum during human pregnancy. Clin. Chem. 33(10), 1801–1806 (1987)
A.B. Okesina, D. Donaldson, P.T. Lascelles, P. Morris, Effect of gestational age on levels of serum alkaline phosphatase isoenzymes in healthy pregnant women. Int J. Gynaecol. Obstet. 48(1), 25–29 (1995)
Acknowledgements
The authors gratefully acknowledge the cooperation of the pregnant women who took part in this study. We also thank the staff at the maternity clinics of Hubei Maternal and Child Health Hospital and The Central Hospital of Wuhan for their considerable assistance with many aspects of this study. We are sincerely grateful to everyone in Tongji Maternal and Child Health Cohort Study Group.
Author contributions
N.Y., X.Y., and L.H. designed the study; T.X., C.Z., G.S., X.Z., R.C., Q.L., Y.W., Q.G., L.H., X.H., and M.X. researched the data; T.X. drafted the paper; L.H. and N.Y. reviewed/edited the paper and contributed to discussion. N.Y. was the guarantor of this work.
Funding
This work was supported by the National Program on Basic Research Project of China (No. 2013FY114200) and China Postdoctoral Science Foundation (No. 2018M632877).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Review Committee of Tongji Medical College, Huazhong University of Science and Technology (No. 201302).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Liping Hao, Nianhong Yang
Supplementary information
Rights and permissions
About this article
Cite this article
Xiong, T., Zhong, C., Sun, G. et al. Early maternal circulating alkaline phosphatase with subsequent gestational diabetes mellitus and glucose regulation: a prospective cohort study in China. Endocrine 65, 295–303 (2019). https://doi.org/10.1007/s12020-019-01954-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-01954-5