Abstract
Purpose
Thyroid function is known to influence glucose metabolism, and thyroid-stimulating hormone is the most useful parameter in screening for thyroid dysfunction.
Therefore, the aim of this study was to investigate the incidence of type 2 diabetes according to baseline thyroid-stimulating hormone level and thyroid-stimulating hormone change in euthyroid subjects.
Methods
We identified and enrolled 17,061 euthyroid subjects without diabetes among participants who had undergone consecutive thyroid function tests between 2006 and 2012 as a part of yearly health check-up program. Thyroid-stimulating hormone changes were determined by subtracting baseline thyroid-stimulating hormone level from thyroid-stimulating hormone level at 1 year before diagnosis of diabetes or at the end of follow-up in subjects who did not develope diabetes.
Results
During 84,595 person-years of follow-up, there were 956 new cases of type 2 diabetes. Cox proportional hazards models showed the risk of incident type 2 diabetes was significantly increased with each 1 μIU/mL increment in TSH after adjustment for multiple confounding factors (hazard ratio = 1.13, 95% confidence interval: 1.07–1.20, P < 0.001). Compared with individuals in the lowest tertile (−4.08 to 0.34 μIU/mL), those in the highest thyroid-stimulating hormone change tertile (0.41–10.84 μIU/mL) were at greater risk for incident type 2 diabetes (hazard ratio = 1.25, 95% confidence interval: 1.05–1.48, P for trend = 0.011). However, baseline thyroid-stimulating hormone level and tertile were not associated with the risk for diabetes.
Conclusions
Prominent increase in thyroid-stimulating hormone concentration can be an additional risk factor for the development of type 2 diabetes in euthyroid subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) and thyroid disease are the most common endocrinopathies encountered in clinical practice. It has long been recognized that these disorders are closely related, and that glucose metabolism is controlled by interactions between insulin and thyroid hormone. Whereas type 1 diabetes and thyroid disease share an autoimmune origin, insulin resistance is assumed to be a key feature of type 2 DM in either hypo—or hyperthyroidism [1, 2]. In hyperthyroidism, insulin resistance is mainly associated with the induction of hyperinsulinemia and glucose intolerance due to the enhanced hepatic gluconeogenesis [3]. On the other hand, prolonged peripheral glucose accumulation attenuates basal plasma insulin, and increased glucose-induced insulin secretion after glucose challenge leads to insulin resistance in hypothyroidism [4]. The relationship between type 2 DM and thyroid dysfunction is important, as it could provide insights into the pathophysiological processes of metabolic syndrome, atherosclerosis, and related cardiovascular outcomes [5].
Although positive correlations are present in subjects with overt thyroid dysfunction, there are still controversies over whether the same positive associations are present in the euthyroid population. Moreover, there have been few longitudinal studies to evaluate the effect of normal thyroid hormones and/or changes in their levels during specific periods of time on the incidence of diabetes. Thyroid-stimulating hormone (TSH) has been recommended as the single best screening test for detecting thyroid dysfunction [6]. However, our longitudinal study that examined the association between changes in each thyroid hormone and incident type 2 diabetes in 6235 euthyroid subjects showed changes in TSH had a modest effect on the development of DM, compared to T3 change or free T4 change only after adjustment for all confounders [7]. Therefore, the aim of this study was to clarify the association between TSH levels within the normal reference range and/or its changes over time and the development of type 2 DM according to different metabolic status in a large population.
Materials and methods
Study design
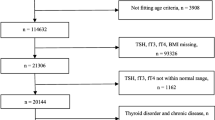
This retrospective longitudinal study included Asian adults aged ≥18 years who participated in medical health check-up programs at the Health Promotion Center of Samsung Seoul Hospital, Korea. Data collected during annual or biannual check-up programs included anthropometric data, laboratory results, and questionnaires about medical history and lifestyle. Among 24,184 participants who attended at least four follow-up visits between January 2006 and December 2012, a total of 7123 participants were excluded for the following reasons (Fig. S1): diagnosed with type 1 or type 2 diabetes at baseline (n = 1737), lacked complete results of thyroid function tests (TSH, total T3 [T3], total T4 [T4] or free T4 [FT4]) at baseline (n = 271), presumed to have overt or subclinical thyroid dysfunction from the baseline thyroid function tests (n = 3666); developed diabetes within 1 year from the first visit (n = 324), history of taking levothyroxine or anti-thyroidal drug at baseline and during follow-up period (n = 314); missing clinical variables at baseline (n = 160) and during follow-up period (n = 315); eGFR calculated by the Chronic Kidney Disease Epidemiology Collaboration formula of <60 ml/min/1.73 m2 (n = 119); and elevated total bilirubin or liver enzyme more than twice the upper normal limit (n = 217). Finally, 17,061 subjects (10,318 men and 6743 women) were enrolled in the study. All participants responded that they had no thyroid disease in their questionnaires, and no one took thyroid hormone or antithyroid medications according to electrical patient records. The observation period was the interval between the date of the first examination and the date of diagnosis of diabetes or the date of the last visit for participants who were free of diabetes until the end of the study.
This study was approved by the Institutional Review Board of Samsung Seoul Hospital and carried out in accordance with recommendations from the Declaration of Helsinki.
Definitions
Euthyroidism was defined as TSH within the reference range of 0.4–4.2 μIU/mL, T3 within the reference range of 76–190 ng/dL, FT4 within the reference range of 0.78–1.85 ng/dL, and T4 within the reference range of 4.7–12.5 ng/dL [8]. We defined the cut-off value for normal TSH as 0.4–4.2 μIU/mL in order to exclude subjects with slight subclinical hypothyroidism at baseline. The normal TSH range was defined as 0.3–6.5 μIU/mL for females and 0.3–6.0 μIU/mL for males, according to the laboratory reference ranges in commercial immunoradiometric kits.
TSH change was determined by subtracting baseline TSH level from the TSH level measured 1 year before the last date of diabetes diagnosis or the final level measured at the end of follow-up in subjects without incident DM. Type 2 DM was diagnosed by fasting glucose ≥126 mg/dL or HbA1c ≥ 6.5% based on American Diabetes Association criteria. Subjects taking anti-diabetic medications were also considered to have DM. Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg, a diastolic blood pressure (DBP) ≥ 90 mmHg, or taking anti-hypertensive medications due to a history of hypertension.
Clinical and biochemical measurements
Demographic characteristics and lifestyle factors were assessed by a structured questionnaire at the first medical check-up. Serum TSH level was measured using an immunoradiometric assay kit (Immunotech, Marseille Cedex, France). Serum T3, T4, and FT4 levels were measured using a commercialized radioimmunoassay kit (FT4 RIA KIT; Immunotech). TSH and thyroid hormone levels were assessed in the morning (8–9 am) to minimize the effect of diurnal variation. Analytical sensitivity and functional sensitivity for TSH were 0.01 and 0.14 μIU/mL, respectively. The minimum detectable concentration of TSH was estimated to be 0.01 μIU/mL. The intra-assay coefficient of variation was 3.7% and inter-assay coefficient of variation was 8.6%.
BMI was calculated by dividing the body weight by the square of the height (kg/m2). Total body fat and abdominal fat percent were measured using a foot-to-foot bioimpedance analysis scale (InBody body composition analyzer, InBody [720], Biospace, Seoul, Korea). The homeostasis model assessment index for insulin resistance (HOMA-IR) was calculated from the following formula: fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5 [9].
Statistical analysis
Normally distributed data were expressed as mean ± standard deviation, whereas unevenly distributed data were presented as median (interquartile range [IQR]: 25th–75th percentile) for continuous variables, and percentages were used for categorical variables. T-tests or Mann–Whitney U tests were used to analyze differences between subjects with incident DM and without DM. ANOVA for continuous variables and the χ 2-square test for categorical variables were used to assess baseline characteristics according to tertiles of baseline TSH level or TSH change. Multivariate Cox proportional hazards analysis was used to assess the relative risk of developing DM according to tertile of TSH level and TSH change as well as a continuous value. The validity of the proportional hazards assumption was evaluated by inspection of Schoenfeld residuals vs. time. No associations between residuals and time were present, so we could not reject the proportionality assumption. Each Cox regression model was adjusted for potential confounders affecting TSH level, and for traditional risk factors of diabetes such as HbA1c and fasting glucose. All variables had a variable inflation factor less than 2.0 indicating no relevant multicollinearity among the covariates in each regression models [10]. Model 1 was adjusted for baseline age and gender, while Model 2 was additionally adjusted for smoking status and use of lipid drugs at baseline. Model 3 was additionally adjusted for baseline HbA1c, TG, LDL-C, HDL-C, and hypertension, which were not associated with baseline TSH in our study. Model 4 was further adjusted for BMI and fasting glucose, which were significantly associated with baseline TSH. Model 5 was finally adjusted for family history of type 2 DM. Correlation analysis was performed to evaluate the relationships between changes in TSH level and changes in metabolic parameters. Linear regression analysis was used to evaluate the trends of TSH change from consecutive TSH measurements during the observation period.
Statistical Package for the Social Sciences (SPSS) version 22.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. A value of P < 0.05 was considered statistically significant.
Results
Characteristics of study population
During 84,595 person-years of follow-up, there were 956 (720 men and 236 women) incident cases of type 2 diabetes. Subjects who subsequently developed DM were more likely to be older, current smokers, hypertensive, and obese at baseline. They also had less favorable metabolic parameters such as high levels of LDL-C, TG, fasting glucose, HbA1c, insulin, and HOMA-IR with low levels of HDL-C (Table S1). There were no significant differences in baseline TSH, T4, or FT4 levels between two groups, whereas T3 level was higher in the DM group (Table S1).
Baseline TSH level and incident type 2 diabetes
The baseline characteristics for all participants according to baseline TSH tertiles are presented in Table 1. As TSH tertile increased, the subjects were older and the proportion of men decreased. There were no differences between TSH tertiles and lipid profiles, HbA1c, insulin resistance, or the prevalence of hypertension. However, levels of fasting glucose, T4 and FT4 were significantly lower in the highest TSH tertile group.
The proportion of participants who developed DM was not significantly different across baseline TSH tertiles (6.0% in the first, 5.6% in the second, 5.2% in the third tertile). Table 2 shows the hazard ratios (HR) and 95% confidence interval (CI) values for the incidence of type 2 DM according to baseline TSH tertiles and also as a continuous variable for each additional 1 μIU/mL of TSH. In both crude and adjusted models (Models 1–5), baseline TSH level did not have any significant effects on the risk of type 2 DM. When we analyzed TSH as a continuous variable, this association remained non-significant before and after adjustment for possible confounders.
TSH change during the follow-up period and incident type 2 diabetes in all participants
A probability density plot of TSH change (Fig. S2) demonstrated a positively skewed distribution for final TSH levels with some flattening of the curve compared to baseline TSH levels in subjects who developed type 2 DM.
Subjects with the highest TSH change were relatively older, but they had more favorable metabolic parameters at baseline such as lower BMI, fat percent, TC, TG, and insulin resistance (Table 3). Baseline TSH, T3, T4, and FT4 levels were all lower in the highest tertile of TSH change (Table 3). The proportion of subjects with incident DM significantly increased across tertiles of TSH change (5.0% in the first, 5.7% in the second, 6.1% in the third tertile). Together with baseline TSH levels, increasing proportion of subjects with incident type 2 DM remained apparent across tertiles of TSH change, regardless of baseline TSH level (Fig. S3).
Table 4 shows HRs for type 2 DM according to TSH change during the follow-up period. Both crude and multivariate Cox regression models (Model 1–5) showed substantially increased risks for type 2 DM in individuals in the highest tertile of TSH change compared to those in the lowest, when each model was adjusted for baseline metabolic parameters. When TSH change was analyzed as a continuous variable, per 1 μIU/mL increase, the HR of incident DM was 1.13 (95% CI 1.07–1.20, P < 0.001) in fully adjusted model. These associations were still significant in both females (HR 1.14 [95% CI 1.04–1.25], P = 0.003) and males (HR 1.13 [95% CI 1.07–1.21], P < 0.001), when TSH change was analyzed as a continuous variable in a Cox regression model which was fully adjusted for baseline metabolic parameters (Data not shown). Even when we divided all participants into two groups according to median 2.0 μIU/mL of baseline TSH as a cut off value, higher TSH change as a continuous variable was associated with enhanced risk for type 2 DM in both groups (Table S2).
TSH change and incident type 2 diabetes in subjects maintaining euthyroid status
TSH levels at 1 year before diagnosis of diabetes in 956 subjects were mostly within the normal range (862 subjects, 90.2%), whereas biochemical hyperthyroidism (defined as TSH < 0.4 μU/mL) and hypothyroidism (defined as TSH > 4.2 μU/mL) were found in 11 (1.2%) and 83 (8.7%) subjects, respectively. Among the remaining 16,105 subjects who did not develop diabetes, TSH levels at the end of follow-up showed a significantly lower proportion of hypothyroidism: euthyroidism was present in 14,616 (90.8%) subjects, hyperthyroidism in 349 (2.2%) subjects, and hypothyroidism in 1140 (7.1%) subjects.
Since hypothyroidism itself may affect the development of diabetes [11], an additional Cox regression analysis was performed for subjects maintaining euthyroid status at final follow-up. In Table 5, the highest TSH increment was independently associated with increased risk for type 2 DM.
Baseline TSH and TSH change according to baseline metabolic risk factors
Since prediabetics are more susceptible to type 2 DM, total participants were classified into prediabetes and normal glucose tolerance (NGT) groups for additional Cox regression analysis. However, TSH increment was significantly associated with higher risk of type 2 DM, regardless of baseline glycemic status (Table S3).
More specifically, total participants with incident DM were divided into two groups according to the number of metabolic risk factors at baseline; Group 1 had less than two metabolic risk factors (n = 222), and Group 2 had two or more metabolic risk factors (n = 734). We defined metabolic risk factors as follows: BMI ≥ 25 kg/m2, prevalence of hypertension, TG ≥ 150 mg/dL, HDL-C ≤ 40 mg/dL in males or 50 mg/dL in females, fasting glucose ≥100 mg/dL, and HbA1c ≥ 5.7 %. While baseline TSH levels did not differ regardless of the number of metabolic risk factors, TSH change was significantly lower in Group 2, compared to Group 1 (0.21 ± 1.14 vs. 0.42 ± 1.44 μIU/mL, P = 0.030).
The correlation between changes in metabolic parameters and TSH change
Changes in metabolic parameters were determined by subtracting baseline value from the final value in the same way TSH change was calculated. When changes in BMI, TG, HDL-C, LDL-C, HbA1c and fasting glucose were adjusted in Cox regression models instead of baseline values (Data not shown), TSH increment as a continuous variable was still an independent risk factor for incident type 2 DM in total participants (HR 1.08 [95% CI 1.01–1.14], P = 0.015) and subjects maintaining euthyroid status (HR 1.10 [95% CI 1.01–1.20], P = 0.033). We especially analyzed the HR of TSH change for incident DM according to the BMI change tertiles (Table S4). Although TSH change could be the consequence of a change in BMI, TSH increment was independently associated with a higher risk for incident DM in both the crude and fully adjusted model.
In a correlation analysis (Table 6), TSH change was positively associated with a change in HbA1c, while baseline TSH was not associated with changes in fasting glucose and HbA1c. TSH change was also positively associated with changes in BMI, body fat percent, TC, TG, and LDL-C, whereas HDL-C change was inversely associated with TSH change in both total subjects and subjects maintaining euthyroid status.
Thyroid auto-immunity
Among 4998 subjects who tested for anti-microsomal antibodies (AMA), 290 subjects showed positive results (definition of positivity: >60 IU/mL). Both baseline TSH level (2.38 ± 0.96 μIU/mL, P < 0.001) and TSH change (0.18 ± 1.39 μIU/mL, P < 0.001) were significantly higher in AMA positive individuals, compared to antibody negative individuals (2.15 ± 0.90 and −0.06 ± 1.10 μIU/mL, respectively). However, antibody prevalence did not affect the relationship between TSH change and the risk for DM in Cox regression analysis (data not shown). Moreover, TSH increment as a continuous variable remained significant for AMA negative individuals (n = 4708) in a crude (HR 1.23 [95% CI 1.09–1.38], P = 0.001) and fully adjusted model (HR 1.21 [95% CI 1.05–1.38], P = 0.007).
Discussion
To the best of our knowledge, this is the first longitudinal study reporting modest effect of TSH change on the incidence of type 2 DM in a large euthyroid population. Whereas baseline TSH itself was not related to the risk for incident DM and insulin resistance, TSH increment over time was associated with the aggravation of metabolic parameters and increased risk for type 2 DM development. A small portion of the enrolled subjects were already prediabetics at baseline (n = 3851, 22.6%) or progressed to hypothyroidism during the follow-up period (n = 1140, 7.1%), who could be more likely to develop diabetes. However, TSH increment still remained a significant risk factor for incident type 2 DM in subjects with normal glucose tolerance and subjects maintaining euthyroid status until final follow-up in this study. In addition, upper part of the TSH distribution [12] or positive AMA [13] have been linked to an increased risk for hypothyroidism, and finally these two components could make synergistic effect to develop diabetes [14, 15]. However, our study showed that higher TSH increment were also associated with a higher risk of diabetes in subjects with lower baseline TSH (below the median value of TSH in whole population) or without AMA. The group with both low-normal TSH and negative AMA (n = 2256) had a 1.39-fold (95% CI 1.15–1.66, p = 0.001) and 1.49-fold (95% CI 1.18–1.89, p = 0.001) higher risk for incident diabetes in a crude and a fully adjusted Cox regression model.
It should be noted that TSH increments might not represent fluctuations in TSH during the observation period, as they were calculated from TSH levels at baseline and at the end of follow-up. Since TSH change was strongly associated with the slope of the linear regression model, which was calculated from TSH levels in sequence (median number of measurements, 6.0; IQR, 5.0–7.0) during the observation period (median, 5.2 years; IQR 4.2–6.0) in Figure S4, TSH level seemed to increase gradually before the onset of diabetes, instead of showing a dramatic spike.
The pathophysiological mechanisms underlying the interaction between TSH increment and deterioration of glucose homeostasis are still not clear. Although we observed that neither baseline levels nor changes in fasting glucose were correlated with TSH change, changes in HbA1c were associated with TSH change in this study. It may be because HbA1c might reflect the aggravation of glycemia for a certain period time better than fasting glucose level per se, since HbA1c is an indicator of average glycemic control over 2–3 months. In accordance with our study, one longitudinal study [16] demonstrated that the incidence of metabolic syndrome was associated with TSH increment over time, but not baseline TSH level, in a euthyroid sample. Moreover, TSH increment over a 3-year follow-up period was positively associated with changes in insulin level and HOMA-IR after adjustment for multiple confounders at baseline, whereas baseline TSH levels and HOMA-IR were not associated. Besides, the associations between favorable metabolic parameters such as, lower BMI, abdominal fat and basal insulin levels in the highest tertile of TSH change with an increased risk of DM also seems counterintuitive, but it suggests that even healthy subjects without metabolic risk factors may develop type 2 DM if their TSH levels increase drastically. In contrast, subjects having many metabolic risk factors were more susceptible to changes in TSH level, which indicated that they were more likely to develop type 2 DM when their TSH levels increased slightly compared to subjects without metabolic risk factors.
There are two possible explanations for the association between TSH increment and incident diabetes. First, elevated TSH simply reflects the tendency to progress to hypothyroidism. TSH in the upper part of the reference range was known as an early sign of hypothyroidism [17–19]. FT4 level was significantly lower in the highest baseline TSH tertile, and FT4 change was inversely correlated with TSH change (r = −0.104, P < 0.001) in a subgroup [7], indicating that TSH increment actually reflected an underactive thyroid. However, baseline TSH and TSH change showed an inverse correlation (r = −0.254, P < 0.001) in this study. This is the opposite of what would be expected if TSH increment arose from occult thyroid disease. Therefore, TSH might be possible to directly affect metabolic parameters. TSH could stimulate leptin secretion in human adipose tissue in vitro [20], and leptin lowers insulin secretion and synthesis in pancreatic β-cells to cause an acute rise in blood glucose level [21]. TSH also directly accelerates cholesterol synthesis and gluconeogenesis in mouse liver via the TSH receptor [22, 23].
The strengths of this study include analysis of 7-year longitudinal data in a large number of subjects. We analyzed both baseline values and concurrent changes in variables to elucidate the associations between TSH and metabolic parameters, while TSH level was measured at the time of diagnosis of metabolic syndrome or diabetes in previous longitudinal studies. Our observations accurately reflect thyroid function during the early stage of diabetes by applying TSH levels measured 1 year before diagnosis, since thyroid function could be disturbed after onset of diabetes [24, 25], and patients had already started taking anti-diabetic medications such as metformin that could affect TSH at the time of diagnosis.
Nevertheless, there are several limitations. First, the study sample consisted of individuals who visited a university hospital for several medical check-ups and may not be representative of regional homogeneity. Missing values regarding the thyroid function test or clinical variables can result in the risk of selection bias as well. Second, the observation period was shorter in subjects with incident DM (median 3.3 years, IQR 2.5–4.6) than in subjects without incident DM (median 5.2 years, IQR 4.1–6.0). Therefore, we may have underestimated changes in TSH or other metabolic parameters in subjects with developing DM. Diabetes also might be underdiagnosed, because oral glucose tolerance test had not been performed. Third, FT4, T4, T3, and thyroid autoantibodies were not measured concurrently during the observation period. In addition, data for insulin resistance were only available in a sub-sample of all subjects. Fourth, seasonal variations in TSH concentration [26] or influences from external factors such as exercise, sleep deprivation [17] and iodine diet [27] were not considered. Fifth, the influence of drugs affecting thyroid function (e.g., amiodarone) and/or medications with a potential to increase the risk for DM (e.g., statins) was not thoroughly excluded. Finally, causal relatiopships between metabolic parameters and TSH change can not be established due to the retrospective nature of this study.
In conclusion, drastic increase in TSH concentration could be an additional risk factor for incident type 2 DM in euthyroid subjects regardless of baseline glycemic status, baseline TSH level, and BMI change. The development of type 2 DM is associated not with arbitrary cut-off values, but with relative increments in individual TSH. However, it remains unclear whether TSH changes induce unfavorable changes in metabolic parameters or whether they are consequences of such changes.
Abbreviations
- DM:
-
Diabetes mellitus
- TSH:
-
Thyroid-stimulating hormone
- T3:
-
Triiodothyronine
- T4:
-
Thyroxine
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TG:
-
Triglyceride
- LDL:
-
low-density lipoprotein
- HDL:
-
High-density lipoprotein
- HbA1c:
-
Glycated hemoglobin
- HOMA-IR:
-
The homeostasis model assessment of insulin resistance.
References
M. Mouradian, N. Aborizk, Diabetes mellitus and thyroid disease. Diabetes Care 6, 512–520 (1983)
G. Brenta, Why can insulin resistance be a natural consequence of thyroid dysfunction? J. Thyroid Res. 2011, 152850 (2011). doi:10.4061/2011/152850
V. Lambadiari, P. Mitrou, E. Maratou, A.E. Raptis, N. Tountas, S.A. Raptis, G. Dimitriadis, Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine. 39, 28–32 (2011). doi:10.1007/s12020-010-9408-3
A. Handisurya, G. Pacini, A. Tura, A. Gessl, A. Kautzky-Willer, Effects of T4 replacement therapy on glucose metabolism in subjects with subclinical (SH) and overt hypothyroidism (OH). Clin. Endocrinol. (Oxf). 69, 963–969 (2008). doi:10.1111/j.1365-2265.2008.03280.x
K.A. Iwen, E. Schroder, G. Brabant, Thyroid hormones and the metabolic syndrome. Eur. Thyroid J. 2, 83–92 (2013). doi:10.1159/000351249
L.M. Demers, C.A. Spencer, Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin. Endocrinol. (Oxf). 58, 138–140 (2003)
J.E. Jun, S.M. Jin, J.C. Bae, J.H. Jee, K.Y. Hur, M.K. Lee, T.H. Kim, S.W. Kim, J.H. Kim: The association between changes in thyroid hormones and incident type 2 diabetes: a 7-year longitudinal study. Thyroid (2016). doi:10.1089/thy.2016.0171
S.Y. Sohn, H.J. Kim, H.W. Jang, S.W. Kim, J.H. Chung, Lack of association between high serum thyroid-stimulating hormone level and risk of papillary thyroid microcarcinomas. Head Neck 36, 43–46 (2014). doi:10.1002/hed.23252
D.R. Matthews, J.P. Hosker, A.S. Rudenski, B.A. Naylor, D.F. Treacher, R.C. Turner, Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985)
S. Naf, X. Escote, M. Ballesteros, R.E. Yanez, I. Simon-Muela, P. Gil, G. Albaiges, J. Vendrell, A. Megia, Serum activin A and follistatin levels in gestational diabetes and the association of the Activin A-Follistatin system with anthropometric parameters in offspring. PLoS One 9, e92175 (2014). doi:10.1371/journal.pone.0092175
N. Gronich, S.N. Deftereos, I. Lavi, A.S. Persidis, D.R. Abernethy, G. Rennert, Hypothyroidism is a risk factor for new-onset diabetes: a cohort study. Diabetes Care 38, 1657–1664 (2015). doi:10.2337/dc14-2515
A.P. Bremner, P. Feddema, P.J. Leedman, S.J. Brown, J.P. Beilby, E.M. Lim, S.G. Wilson, P.C. O’Leary, J.P. Walsh, Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J. Clin. Endocrinol. Metab. 97, 1554–1562 (2012). doi:10.1210/jc.2011-3020
M.F. Prummel, W.M. Wiersinga, Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract. Res. Clin. Endocrinol. Metab. 19, 1–15 (2005). doi:10.1016/j.beem.2004.11.003
H. Ying, Y.P. Tang, Y.R. Bao, X.J. Su, X. Cai, Y.H. Li, D.F. Wang: Maternal TSH level and TPOAb status in early pregnancy and their relationship to the risk of gestational diabetes mellitus. Endocrine (2016). doi:10.1007/s12020-016-1022-6
T.I. Korevarr, High TSH and TPOAb positivity are associated with a higher risk of gestational diabetes mellitus in a synergistic manner. Clin. Thyroidol. 28, 267–269 (2016)
S.B. Park, H.C. Choi, N.S. Joo, The relation of thyroid function to components of the metabolic syndrome in Korean men and women. J. Korean. Med. Sci. 26, 540–545 (2011). doi:10.3346/jkms.2011.26.4.540
M.I. Surks, G. Goswami, G.H. Daniels, The thyrotropin reference range should remain unchanged. J. Clin. Endocrinol. Metab. 90, 5489–5496 (2005). doi:10.1210/jc.2005-0170
J.P. Walsh, A.P. Bremner, P. Feddema, P.J. Leedman, S.J. Brown, P. O’Leary, Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J. Clin. Endocrinol. Metab. 95, 1095–1104 (2010). doi:10.1210/jc.2009-1977
B.O. Asvold, L.J. Vatten, K. Midthjell, T. Bjoro, Serum TSH within the reference range as a predictor of future hypothyroidism and hyperthyroidism: 11-year follow-up of the HUNT Study in Norway. J. Clin. Endocrinol. Metab. 97, 93–99 (2012). doi:10.1210/jc.2011-1724
C. Menendez, R. Baldelli, J.P. Camina, B. Escudero, R. Peino, C. Dieguez, F.F. Casanueva, TSH stimulates leptin secretion by a direct effect on adipocytes. J. Endocrinol. 176, 7–12 (2003)
H.C. Denroche, F.K. Huynh, T.J. Kieffer, The role of leptin in glucose homeostasis. J. Diabetes Investig. 3, 115–129 (2012). doi:10.1111/j.2040-1124.2012.00203.x
T. Wang, J. Xu, T. Bo, X. Zhou, X. Jiang, L. Gao, J. Zhao, Decreased fasting blood glucose is associated with impaired hepatic glucose production in thyroid-stimulating hormone receptor knockout mice. Endocr. J. 60, 941–950 (2013)
L. Tian, Y. Song, M. Xing, W. Zhang, G. Ning, X. Li, C. Yu, C. Qin, J. Liu, X. Tian, X. Sun, R. Fu, L. Zhang, X. Zhang, Y. Lu, J. Zou, L. Wang, Q. Guan, L. Gao, J. Zhao, A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology 52, 1401–1409 (2010). doi:10.1002/hep.23800
F. Luna-Vazquez, R. Cruz-Lumbreras, J. Rodriguez-Castelan, M. Cervantes-Rodriguez, J. Rodriguez-Antolin, O. Arroyo-Helguera, F. Castelan, M. Martinez-Gomez, E. Cuevas, Association between the serum concentration of triiodothyronine with components of metabolic syndrome, cardiovascular risk, and diet in euthyroid post-menopausal women without and with metabolic syndrome. Springerplus 3, 266 (2014). doi:10.1186/2193-1801-3-266
J.M. Rondeel, W.J. de Greef, R. Heide, T.J. Visser, Hypothalamo-hypophysial-thyroid axis in streptozotocin-induced diabetes. Endocrinology 130, 216–220 (1992). doi:10.1210/endo.130.1.1530781
T.H. Kim, K.W. Kim, H.Y. Ahn, H.S. Choi, H. Won, Y. Choi, S.W. Cho, J.H. Moon, K.H. Yi, J. Park do, K.S. Park, H.C. Jang, S.Y. Kim, Y.J. Park, Effect of seasonal changes on the transition between subclinical hypothyroid and euthyroid status. J. Clin. Endocrinol. Metab. 98, 3420–3429 (2013). doi:10.1210/jc.2013-1607
P. Serog, M. Apfelbaum, N. Autissier, F. Baigts, L. Brigant, A. Ktorza, Effects of slimming and composition of diets on VO2 and thyroid hormones in healthy subjects. Am. J. Clin. Nutr. 35, 24–35 (1982)
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jun, J.E., Jin, SM., Jee, J.H. et al. TSH increment and the risk of incident type 2 diabetes mellitus in euthyroid subjects. Endocrine 55, 944–953 (2017). https://doi.org/10.1007/s12020-016-1221-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1221-1