Abstract
Obstructive sleep apnea (OSA) has been associated with dysregulation of the hypothalamic–pituitary–adrenal axis and alterations in glucose metabolism with increased risk for type 2 diabetes. The aim of the current study was to compare morning plasma cortisol levels and glucose metabolism parameters between moderate (apnea–hypopnea index (AHI): 15–30 events/h) and severe OSA patients (AHI >30 events/h), with respective controls. A total of 56 male OSA patients, 24 moderate (AHI = 21.1 ± 5.3) and 32 severe (AHI = 49.7 ± 18.1), underwent a full-night polysomnography, oral glucose tolerance test (OGTT), and measurement of morning plasma cortisol levels. These groups were compared to 20 matched subjects in a control group. Morning plasma cortisol levels were statistically lower in severe OSA group than in moderate OSA and control groups (303.7 ± 93.5 vs. 423.9 ± 145.1 vs. 417.5 ± 99.8 pmol/L, P < 0.001). Significant negative correlations were found between morning plasma cortisol levels and AHI (r = −0.444, P = 0.002), as well as oxygen desaturation index (r = −0.381, P = 0.011). Fasting plasma glucose (5.0 ± 0.5 vs. 5.4 ± 0.7 vs. 4.9 ± 0.6 mmol/L, P = 0.009) was higher in the severe OSA group compared to moderate OSA and controls. Homeostasis model assessment insulin resistance (HOMA-IR) was higher in the severe OSA group compared to moderate OSA and controls (4.6 ± 3.7 vs. 2.7 ± 2.0 and 2.2 ± 1.8, respectively, P = 0.006). In conclusion, our study showed that morning plasma cortisol levels measured at 8 a.m. were significantly lower in severe OSA patients than those in moderate OSA group and controls. Morning plasma cortisol levels showed a negative correlation with AHI and oxygen desaturation index. Additionally, this study confirmed the evidence of glucose metabolism impairment in moderate and severe OSA patients, with more pronounced effect in the severe OSA patients group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is an increasingly common sleep-related breathing disorder characterized by repetitive pharyngeal collapse during sleep with associated airflow obstruction, intermittent hypoxemia, sympathetic excitation, and sleep fragmentation [1–3]. Sleep fragmentation leads to excessive daytime sleepiness often leading to the increased risk for motor vehicle and occupational accidents [1–3].
OSA patients are more likely to develop hypertension and experience cardiovascular morbidity and mortality. Even mild oxyhemoglobin desaturations (>2 %) are independently associated with metabolic dysfunction which has been proposed as a potential important predictor of increased cardiovascular risk [4–6].
Intermittent hypoxemia and sleep fragmentation in OSA are associated with alterations in hypothalamic–pituitary–adrenal (HPA) activity, specifically increased pulsatile cortisol release [4, 7, 8]. Excessive cortisol secretion is associated with numerous adverse consequences throughout the body. It can increase hepatic glucose production and insulin resistance that may result in altered glucose metabolism [9]. Furthermore, sleep deprivation may increase sympathetic activity and levels of plasma cortisol, although the available studies do not provide clear evidence of relationship between OSA and alterations in the levels of cortisol [7, 8, 10, 11]. The role of HPA axis in glucose metabolism alterations is, however, still largely debated since OSA features numerous pathophysiological mechanisms that can interfere with glucose metabolism. Therefore, in this study, we tried to integrate the relationship of HPA axis and glucose metabolism in OSA patients.
Chronic intermittent hypoxemia accelerates development of fasting hyperglycemia, glucose intolerance, and insulin resistance [12, 13]. In addition, it is independently associated with increased serum levels of triglycerides as well as several metabolic and inflammatory parameters such as low-density lipoprotein, uric acid, and C-reactive protein [4, 8].
There is increasing evidence suggesting a connection between OSA and glucose intolerance, insulin resistance, and type 2 diabetes [14–16]. Several recent studies have shown that the prevalence of altered glucose metabolism and type 2 diabetes are increased in OSA patients, independent of age and central obesity [11, 17–19].
Patient with OSA are divided into three categories of severity according to apnea–hypopnea index (AHI). An AHI of 5–14.9 is mild; 15–29.9 is moderate; and more than 30 events/h characterizes severe OSA [3, 20, 21]. Many studies showed that patients with moderate to severe OSA had higher risk for different cardiometabolic abnormalities [6], so the aim of the current study was to compare morning plasma cortisol levels and glucose metabolism parameters between moderate and severe OSA patients, and in comparison with healthy control subjects. Also, we wanted to explore the association of morning cortisol levels and glucose parameters with polysomnographic variables. The clinical consequences of such approach will be a better definition of preventive and therapeutic strategies in OSA patients. We hypothesized that subjects with severe OSA would have a higher prevalence of impaired glucose metabolism and increased morning plasma cortisol levels in comparison with moderate OSA patients.
Patients and methods
The study was approved by the Ethics Committee of the University of Split School of Medicine. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its latter amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in this study.
Subjects
Subjects with newly diagnosed OSA at the Split Sleep Medicine Center between June 2013 and December 2014 were prospectively recruited in this study. Patients were divided in two groups according to the severity of OSA. A diagnosis of OSA was defined in accordance with the guidelines established by the American Academy of Sleep Medicine (AASM) and European Sleep Research Society (ESRS) [3, 20, 21]. Patients were classified into two groups: moderate OSA group (15 ≤ AHI ≤ 30 events/h, 24 patients) and severe OSA group (AHI > 30 events/h, 32 patients). Exclusion criteria were (1) diagnosed diabetes mellitus, severe cardiovascular, neurological, psychiatric, respiratory or renal disease, and active malignant disease; (2) regular use of drugs that could interfere with glucose metabolism or HPA axis, sedatives or narcotics, and alcohol and drugs abuse; (3) history of any OSA treatment prior to the study enrollment; and (4) female patients. We decided to exclude females in our study due to the fact that OSA is significantly less prevalent among women. Likewise, due to possible cortisol level differences and other parameters that are sex-intrinsic, we managed to secure a more homogenous group with less potential discrepancies.
Our control group consisted of 20 subjects from a pool of healthy male volunteers matched with the OSA patients for age and BMI. Potential subjects were excluded if they had ESS score >9, which was used to evaluate daytime sleepiness. The Snoring, Tiredness, Observed apnea and high blood Pressure (STOP) questionnaire, a concise and easy-to-use screening tool for OSA with high sensitivity and specificity for determining the risk for OSA, has been used to identify subjects with high risk for OSA [22]. Subjects with a STOP questionnaire score ≥2 were excluded from this study due to the risk for the development of OSA [22]. Sleep studies were not performed for the control subjects due to the additional expenses and time limitations. Same exclusion criteria were applied in the control group. All other assessments were performed following the same protocol as in the study group.
Subjects included in this study underwent a detailed initial medical history interview, physical examination, and anthropometric measurements. Body weight and height were measured using a calibrated scale (Seca, Birmingham, UK) while the subjects wore light clothes. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2). Waist circumference was measured at the mid-point between the inferior tip of the ribcage and the superior aspect of the iliac crest, with the subjects standing in the upright position. Neck circumference was measured in the mid-way of the neck, between mid-cervical spine and mid-anterior neck (below laryngeal prominence), with the subjects standing in the upright position. Arterial blood pressure was measured in a sitting position at least twice between 8 and 9 a.m., after 10 min of rest, with a standard mercury sphygmomanometer and appropriate cuff size.
Sleep assessment
Full-night attended polysomnography (PSG) was performed, recording electroencephalography, electrooculography, mental and tibial electromyography, electrocardiography, nasal airflow, pulse oximetry, thoracic and abdominal movements, and snoring intensity (Alice 5LE, Philips Respironics, Eindhoven, The Netherlands). All data were stored on a personal computer and manually scored and evaluated in accordance with the published AASM and ESRS guidelines by the same certified sleep physician [20, 23, 24]. Sleep studies lasting less than 6 h were not accepted, and in such cases, a second PSG was undertaken. The self-administrated questionnaire, the Epworth Sleepiness Scale (ESS), validated in Croatian language [22], was used to measure excessive daytime sleepiness.
Apnea was defined as a complete cessation of respiratory airflow for a minimum duration of 10 s, whereas hypopnea was defined as a decrease in airflow by more than 50 % from baseline for at least 10 s, combined with a reduction in hemoglobin oxygen saturation of at least 3 %. The AHI was defined as the average number of apneas plus hypopneas per hour of sleep. Oxygen desaturation index (ODI) was calculated as a number of significant oxygen saturation (SpO2) drops of 3 % or more per hour of sleep.
Blood sampling and laboratory analysis
Seven to fourteen days after the sleep studies were performed, patients underwent extensive laboratory analysis. After a 12-h fast, venous blood samples were collected at 8 a.m. through a polyethylene catheter inserted in a forearm vein. Blood sampling for all subjects included in this study was performed within the 90–120 min period after waking up. All blood samples were analyzed by the same experienced biochemist and at the same laboratory, with the same method for each assay following standard procedure. Biochemist was blinded to the subject’s assignment in the moderate or severe OSA group. Morning plasma cortisol and fasting plasma insulin were determined by electrochemiluminescence immunoassay (ECLIA) method (Roche Diagnostics GmbH, Mannheim, Germany). Fasting plasma glucose was measured using photometry with hexokinase method (Abbott, Chicago, USA) and HbA1c levels were measured by turbidimetric inhibition immunoassay (Roche Diagnostics GmbH, Mannheim, Germany). Other laboratory assays were performed by routine laboratory methods.
Each subject underwent a 75-g oral glucose tolerance test (OGTT) shortly after the collection of the first fasting blood sample, during which plasma glucose and serum insulin levels were determined at 0 and 120 min. Impaired fasting glucose (IFG) was defined as fasting plasma glucose from 5.6 to 6.9 mmol/L and impaired glucose tolerance (IGT) as plasma glucose in the range of 7.8–11 mmol/L 2 h after the glucose load. Pre-diabetes was defined as IFG and/or IGT and/or HbA1c in the range between 5.7 and 6.4 % [25, 26].
Insulin resistance was assessed by the homeostatic model assessment index of insulin resistance (HOMA-IR) calculated as the product of the fasting serum insulin concentration (mU/L) and fasting plasma glucose concentration (mmol/L) divided by 22.5 [27].
Statistical analysis
Statistical analyses were performed using statistical software MedCalc for Windows, version 11.5.1.0 (MedCalc Software, Mariakerke, Belgium). Continuous data were presented as mean ± standard deviation, whereas categorical variables were presented as whole numbers and percentages. The Kolmogorov–Smirnov test was used to assess normality of data distribution. We used Student t test analysis to measure differences in polysomnographic data between moderate and severe OSA groups. The comparison between OSA patients (moderate and severe) and the control group were tested using one-way ANOVA with post hoc Tukey HSD test in parameters of glucose metabolism, morning plasma cortisol levels, and other biochemical parameters. Furthermore, correlations between morning plasma cortisol levels, glucose metabolism parameters, and polysomnographic data were evaluated using Pearson’s correlation coefficients. The prevalence of glucose metabolism disorders between OSA patients and control were assessed by Chi-square analysis. The statistical significance was set at P < 0.05.
Results
The study population consisted of 56 male OSA patients (24 moderate and 32 severe) and 20 controls, without statistically significant differences in age. There were no statistically significant differences in anthropometric parameters between the groups except in neck circumference. Baseline characteristics of subjects included in the study are presented in Table 1.
The analyses of polysomnographic data and the Epworth Sleepiness Scale (ESS) scores are presented in Table 2. AHI was significantly higher in the severe than in the moderate OSA group. Severe OSA patients had lower mean and minimum arterial oxygen saturation, while oxygen desaturation index (ODI), number of obstructive, and central and mixed apnea events per total sleep time were higher in the severe OSA group. In addition, there was no significant difference in excessive daytime somnolence measured by the ESS score between the groups.
The results of biochemical parameters of OSA patients and control subjects are presented in Table 3. There were no statistically significant differences between the groups other than HDL cholesterol levels, which were the lowest in the severe OSA group, while there was no difference between the moderate OSA group and control subjects.
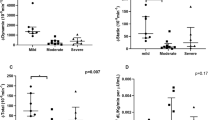
Morning plasma cortisol levels were statistically lower in severe OSA group than in moderate OSA and control groups (303.7 ± 93.5 vs. 423.9 ± 145.1 vs. 417.5 ± 99.8 pmol/L, respectively, P < 0.001) (Fig. 1). In the moderate OSA group, 5 of 24 patients had morning plasma cortisol levels above reference range, and none of them were below reference range. In the severe OSA group, there were 3 of 32 patients with morning plasma cortisol levels below the reference intervals, while none of them had morning plasma cortisol values above reference interval. All of the control subjects had morning plasma cortisol levels within the reference interval.
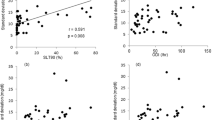
Significant negative correlations were found between morning plasma cortisol levels and AHI (r = −0.444, P = 0.002), as well as ODI (r = −0.381, P = 0.011) when we performed analysis on joined both groups of OSA patients. Also, there was a significant negative correlation between morning plasma cortisol levels and AHI in the severe OSA group (r = −0.356, P = 0.046), but not in the moderate OSA group of patients, where this correlation was positive, but not significant (r = 0.278, P = 0.187) (Fig. 2).
Glucose metabolism parameters are summarized in Table 4. Fasting plasma glucose, fasting plasma insulin, and HbA1c were significantly higher in severe OSA group in comparison with controls, while there was no significant difference between moderate OSA group and controls. In comparison to moderate OSA group, the severe OSA group displayed significantly higher values in above-mentioned glucose metabolism variables. HOMA-IR was significantly higher in the severe OSA group in comparison to moderate OSA group and controls. There were no significant differences between groups in the plasma glucose and insulin values after 120 min of OGTT (Table 4).
The correlations of morning plasma cortisol levels and metabolic parameters, as well as between OSA indices and parameters of glucose metabolism were not found statistically significant for all analyses (data not shown).
Prevalence of glucose metabolism disorders is presented in Table 5. In the severe OSA group, more patients had impaired fasting glucose, increased HbA1c, and insulin resistance than in the control and the moderate OSA group, respectively. There were no statistically significant differences in the prevalence of impaired glucose tolerance in all groups. Pre-diabetes defined as impaired fasting glucose and/or impaired glucose tolerance and/or increased HbA1c was significantly more prevalent in the severe OSA group (Table 5).
Discussion
This study provided data on differences in morning plasma cortisol levels and glucose metabolism parameters between moderate and severe OSA patients, in comparison with the healthy control subjects. Morning plasma cortisol levels were lower in severe OSA group in comparison to moderate OSA group and controls. Moreover, morning plasma cortisol levels were similar between moderate OSA group and controls. The respiratory variables, AHI and ODI, were significantly inversely correlated with morning plasma cortisol levels. The glucose metabolism was impaired in moderate and severe OSA groups, but more pronounced impairment was observed in severe OSA patients.
The impact of sleep-related breathing disorders on the HPA axis remains controversial [7]. The available studies do not provide clear evidence of the relationship between OSA and alterations in the plasma cortisol levels [10, 28–31]. The fluctuation of plasma cortisol throughout the night is complexly related to sleep, and thus it has been proposed as an important mechanism through which sleep disorders manifest some of their pathophysiological changes [7, 32].
Since nocturnal arousals are associated with alterations in the HPA axis activity, associated sympathetic activation, and increased pulsatile cortisol release, it was expected that plasma cortisol levels would be higher in OSA patients [7]. However, the empirical data do not always support this hypothesis; many previous studies have failed to find differences in cortisol levels between OSA patients and healthy subjects [10, 28, 30]. Inconsistences in timing of sample collection, age, body composition, and control of the variables that are known to be related with the HPA axis activity (smoking, depression, medications, and so on) may also contribute to the mixed findings [7]. Some authors suggested that OSA patients presented lower morning cortisol levels than control subjects [32, 39], while others showed no differences [10, 28, 30]. Interestingly, OSA patients maintained the physiological circadian activity of the HPA axis, with the highest cortisol levels in the morning and the lowest values in the evening [7, 29]. Some of the reported mixed findings could be explained by inconsistent inclusion criteria to the studies regarding BMI; patients with higher BMI showed impaired HPA axis sensitivity [33]. The potential confounding effects of BMI or abdominal obesity on cortisol production in our study could be ruled out because subjects in both groups have similar BMI and abdominal circumference.
Karaca et al. reported reduced response to the stimulation tests in OSA subjects. The area under the curve of the cortisol response was negatively correlated with the AHI [34]. The stimulation tests of HPA axis in that study were performed in the morning and lower levels of cortisol were found in the OSA group compared with controls [34]. One of the possible explanations of morning cortisol levels discrepancies is that the mild increment in plasma cortisol levels during the nighttime due to intermittent hypoxia led to the increased negative feedback effect on the HPA axis [34]. Intermittent hypoxia, sleep fragmentation, and autonomic arousal during sleep may represent OSA-induced chronic stressors that can compromise the HPA axis activity. It has been shown that relative hypocortisolism may occur as the system loses its resiliency in response to the chronic recurring stress, reflecting in reduced HPA axis responding ability [29, 35]. According to this theory, one of the possible explanations for our findings may be that in severe OSA patients, HPA axis responding ability is reduced. Similar results were reported by Malakasioti et al. in the pediatric population. Among children with hypertrophic tonsils, moderate to severe OSA patients had significantly lower morning plasma cortisol levels than participants with mild OSA or control subjects. In addition, a negative association was demonstrated between the severity of OSA and morning plasma cortisol levels [31].
In our study, we did not find difference in morning plasma cortisol levels between patients with moderate OSA and control group. This finding is consistent with other similar studies [10, 28, 30]. Likewise, patients in severe OSA group had significantly lower morning cortisol levels in comparison to other groups.
Another finding of our study is the impairment of the glucose metabolism in moderate and severe OSA patients. Fasting plasma glucose and fasting plasma insulin levels were significantly higher in severe compared to moderate OSA patients, as well as HbA1c and HOMA-IR. Accordingly, those values were significantly higher in severe OSA group in comparison with controls, while there was no significant difference between moderate OSA group and controls. Additionally, there were more patients with impaired fasting glucose, insulin resistance, increased HbA1c levels, and pre-diabetes in the severe OSA group, respectively.
In our study, severe OSA patients had lower morning plasma levels with higher insulin resistance and other glucose metabolism alterations. It is important to highlight that pathophysiological mechanisms of insulin resistance and glucose metabolism alterations in OSA are of complex nature and cannot be approached linearly. This state could arise due to the fact that patients with OSA usually exhibit nocturnal hypercortisolism, and this dynamic might be responsible, at least partially, for the negative effects on glucose metabolism [4, 7, 8, 36, 37]. A study by Plat et al. showed that the metabolic effects of elevations in plasma cortisol are more pronounced in the evening than in the morning, thus implicating that the elevations in the evening and nocturnal cortisol can contribute to alterations in glucose tolerance, insulin sensitivity, and insulin secretion [38]. Moreover, these negative cortisol effects could occur even in the absence of high hormonal values by the local effects mediated by adipose 11b hydroxysteroid dehydrogenase type I (11b-HSD1), which regenerates cortisol from cortisone and whose expression is associated with obesity [36, 39, 40]. The studies examining the dynamics of the HPA axis are scarce, and there are no available data about the specific correlations of, e.g., 11β-HSD activity, circulating cortisol-binding globulin (CBG) levels and OSA, all of which might be implicated in the pathophysiology of cortisol dysregulation.
Therefore, we hold that a real question is what are the actual contributions of HPA axis and cortisol alterations to glucose metabolism alterations in OSA patients? This is a relevant question to ask since OSA exhibits a multitude of pathophysiological mechanisms that may contribute to the development of insulin resistance. Such mechanisms that are featured in OSA include autonomic activation, direct effects of intermittent hypoxemia on glucose regulation, and release of proinflammatory cytokines such as interleukin-6 and tumor necrosis factor-alpha [4, 8]. Several studies showed, despite controlling for obesity and other important confounding variables of insulin resistance, that AHI and/or minimum oxygen saturation were significant determinants of fasting insulin level and HOMA-IR [17, 18]. Based on previous elaboration, it is evident that insulin resistance and glucose metabolism in OSA most likely depends on the complex relationships between various pathophysiological constituents. Some of these discussed models could potentially provide the explanation for our finding that patients with severe OSA had lower morning cortisol plasma levels but had higher insulin resistance and higher glucose levels.
An independent association between OSA, insulin resistance, and type 2 diabetes has been consistently demonstrated by a number of cross-sectional, observational, and large population-based studies [5, 17–19]. Moreover, the development of insulin resistance and β-cell dysfunction has been linked to the chronic intermittent hypoxia observed in OSA. Even mild recurrent oxyhemoglobin desaturations (>2 %) were independently associated with metabolic dysfunction [4–6]. A link between OSA and insulin resistance appears early, prior to the impaired glucose tolerance and the onset of diabetes. Impaired fasting glucose and impaired glucose tolerance represent intermediate states of abnormal glucose regulation that exists between glucose homeostasis and diabetes [41]. Although the natural history of IFG and IGT is variable, approximately 25 % of subjects with either disbalance will progress to type 2 diabetes over the period of 3–5 years [41]. In our study, the prevalence of pre-diabetes was very high, even 59.4 % in the severe, and 41.7 % in the moderate OSA group. In many previous studies, pre-diabetes has been found to be significantly higher in OSA patients (the estimates have ranged from 20 to 50 %) [9, 17].
There is emerging evidence that cardiovascular and metabolic complications tend to develop even if OSA does not worsen [42], so it is of great importance to treat OSA patients, predominantly with continuous positive airway pressure (CPAP). CPAP treatment as the current gold standard for OSA has shown beneficial effects in glucose metabolism [43]. Also, Galic et al. have shown similar improvement following mandibular advancement device (MAD) treatment in mild to moderate non-diabetic OSA patients [44].
Many studies correlated metrics of OSA severity with abnormalities in glucose homeostasis. This was not confirmed in our study since none of the OSA parameters correlated with glucose metabolism. In studies in which that link has been found, the AHI and the degree of chronic intermittent hypoxia have been consistently associated with the development of insulin resistance, glucose intolerance, and type 2 diabetes, independently of confounding factors such as obesity [9, 17, 45]. The association between OSA and abnormal glucose regulation may further increase the cardiovascular risk since both OSA and alterations in glucose metabolism are recognized as the risk factors for increased cardiovascular morbidity and mortality [18].
There are a few limitations in our study. Instead of 24-h cortisol profile, plasma cortisol levels were assessed at a single time point, which does not allow studying the precise alterations and dynamics of cortisol levels. However, precise blood sampling time (8 a.m.) was carried out with all patients sampled within the 90–120 min after waking up. Additionally, all the procedures were the same for all subjects included in this study. Although the sample sizes in the studied groups were large enough to demonstrate statistical significance between the groups, additional studies with larger number of studied patients, especially in the severe OSA group, would be beneficial to contribute to the findings of this study. Furthermore, different methodologies in the morning cortisol measurements may be of additional value to confirm these findings. Since our study population did not include female subjects, the results could not be applied to the whole OSA population.
In conclusion, our study showed that morning plasma cortisol levels (at 8 a.m.) were statistically lower in severe OSA group than those in moderate OSA and control groups, and these levels negatively correlated with AHI and ODI. Further studies with larger sample size and different approaches in cortisol measurements are needed to confirm these findings and to clarify precise alterations and dynamics of cortisol levels as a possible consequence of OSA in moderate and severe patient populations. Additionally, this study confirmed the evidence of glucose metabolism impairment in moderate and severe OSA patients, with more pronounced effect in the severe OSA patients group.
References
A.S. Jordan, D.G. McSharry, A. Malhorta, Adult obstructive sleep apnoea. Lancet 383, 736–747 (2014)
C. Guilleminault, L. Eldridge, A. Tilkian, B. Simmons, W. Dement, Sleep apnoea syndrome due to upper airway obstruction. Arch. Intern. Med. 137, 296–300 (1997)
W.T. McNicholas, Sleep-related breathing disorders: nosological classification, definitions, epidemiology, in Sleep Medicine Textbook, ed. by C. Bassetti, Z. Dogas, P. Peigneux (European Sleep Research Society, Regensburg, 2014), pp. 215–220
J.A. Dempsey, S.C. Veasey, B.J. Morgan, C.P. O’Donnell, Pathophysiology of sleep apnea. Physiol. Rev. 90, 47–112 (2010)
K. Stamatakis, M.H. Sanders, B. Caffo et al., Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep 31, 1018–1024 (2008)
J.L. Pepin, J.C. Borel, A.L. Borel, P. Levy, R. Tamisier, Sleep-related breathing disorders: comorbidities and special populations, in Sleep Medicine Textbook, ed. by C. Bassetti, Z. Dogas, P. Peigneux (European Sleep Research Society, Regensburg, 2014), pp. 251–258
M.L. Tomfohr, K.M. Edwards, J.E. Dimsdale, Is obstructive sleep apnea associated with cortisol levels? A systematic review of the research evidence. Sleep Med. Rev. 16, 243–249 (2012)
N.A. Dewan, F.J. Nieto, V.K. Somers, Intermittent hypoxemia and OSA: implications for comorbidities. Chest 147, 266–274 (2015)
S. Khani, J.A. Tayek, Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin. Sci. 101, 739–747 (2001)
Z. Lattova, M. Keckeis, E. Maurovich-Horvat et al., The stress hormone system in various sleep disorders. J. Psychiatr. Res. 45, 1223–1228 (2011)
E. Crawford-Achour, M. Saint Martin, F. Roche, Stress hormones in obstructive sleep apnea complications: the role of cortisol. Sleep Med. 15, 3–4 (2014)
S.L. Appleton, A. Vakulin, R.D. McEvoy et al., Nocturnal hypoxemia and severe obstructive sleep apnea are associated with incident type 2 diabetes in a population cohort of men. J. Clin. Sleep Med. 11, 609–614 (2015)
N.H. Kim, N.H. Cho, C.H. Yun et al., Association of obstructive sleep apnea and glucose metabolism in subjects with or without obesity. Diabetes Care 36, 3909–3915 (2013)
S. Seicean, H.L. Kirchner, D.J. Gottlieb et al., Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals. Diabetes Care 31, 1001–1006 (2008)
N.M. Punjabi, V.Y. Polotsky, Disorders of glucose metabolism in sleep apnea. J. Appl. Physiol. 99, 1998–2007 (2005)
A.A. Tahrani, A. Ali, M.J. Stevens, Obstructive sleep apnoea and diabetes: an update. Curr. Opin. Pulm. Med. 19, 631–638 (2013)
X. Wang, Y. Bi, Q. Zhang, F. Pan, Obstructive sleep apnea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirology 18, 140–146 (2013)
L.F. Drager, S.M. Togeiro, V.Y. Polotsky, G. Lorenzi-Filho, Obstructive sleep apnea: a cardiovascular risk in obesity and metabolic syndrome. J. Am. Coll. Cardiol. 62, 569–576 (2013)
B.D. Kent, L. Grote, S. Ryan et al., Diabetes mellitus prevalence and control in sleep disordered breathing: the European sleep apnea cohort (ESADA) study. Chest 146, 982–990 (2014)
The Report of an American Academy of Sleep Medicine Task Force, Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22, 667–689 (1999)
L.J. Epstein, D. Kristo, P.J. Strollo Jr et al., Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 5, 263–276 (2009)
R. Pecotic, I.P. Dodig, M. Valic, N. Ivkovic, Z. Dogas, The evaluation of the Croatian version of the Epworth sleepiness scale and STOP questionnaire as screening tools for obstructive sleep apnea syndrome. Sleep Breath. 16, 793–802 (2012)
J. Fischer, Z. Dogas, C.L. Bassetti et al., Standard procedures for adults in accredited sleep medicine centres in Europe. J. Sleep Res. 21, 357–368 (2012)
C.A. Kushida, M.R. Littner, T. Morgenthaler et al., Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 28, 449–521 (2005)
American Diabetes Association, Diagnosis and classification of diabetes mellitus. Diabetes Care 35, 64–71 (2012)
American Diabetes Association, Standards of medical care in diabetes—2014. Diabetes Care 37, 14–80 (2014)
D.R. Matthews, J.P. Hosker, A.S. Rudenski et al., Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985)
H. Raff, S.L. Ettema, D.C. Eastwood, B.T. Woodson, Salivary cortisol in obstructive sleep apnea: the effect of CPAP. Endocrine 40, 137–139 (2011)
C.M. Ghiciuc, L.C. Dima Cozma, R.M. Bercea et al., Restoring the salivary cortisol awakening response through nasal continuous positive airway pressure therapy in obstructive sleep apnea. Chronobiol. Int. 30, 1024–1031 (2013)
B. Panaree, M. Chantana, S. Wasana, N. Chairat, Effects of obstructive sleep apnea on serum brain-derived neurotrophic factor protein, cortisol, and lipid levels. Sleep Breath. 15, 649–656 (2011)
G. Malakasioti, E.I. Alexopoulos, V. Varlami et al., Low morning serum cortisol levels in children with tonsillar hypertrophy and moderate-to-severe OSA. Sleep 36, 1349–1354 (2013)
K.M. Edwards, R. Kamat, L.M. Tomfohr, S. Ancoli-Israel, J.E. Dimsdale, Obstructive sleep apnea and neurocognitive performance: the role of cortisol. Sleep Med. 15, 27–32 (2014)
G. Carneiro, S.M. Togeiro, L.F. Hayashi et al., Effect of continuous positive airway pressure therapy on hypothalamic–pituitary–adrenal axis function and 24-h blood pressure profile in obese men with obstructive sleep apnea syndrome. Am. J. Physiol. Endocrinol. Metab. 295, E380–E384 (2008)
Z. Karaca, S. Ismailogullari, S. Korkmaz et al., Obstructive sleep apnoea syndrome is associated with relative hypocortisolemia and decreased hypothalamo–pituitary–adrenal axis response to 1 and 250 μg ACTH and glucagon stimulation tests. Sleep Med. 14, 160–164 (2013)
C. Heim, U. Ehlert, D.H. Hellhammer, The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25, 1–35 (2000)
G. Tirabassi, M. Boscaro, G. Arnaldi, Harmful effects of functional hypercortisolism: a working hypothesis. Endocrine 46, 370–386 (2014)
T.M. Buckley, A.F. Schatzberg, On the interactions of the hypothalamic–pituitary–adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J. Clin. Endocrinol. Metab. 90, 3106–3114 (2005)
L. Plat, R. Leproult, M. L’Hermite-Baleriaux et al., Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J. Clin. Endocrinol. Metab. 84, 3082–3092 (1999)
S. Dube, B.J. Norby, V. Pattan, R.E. Carter, A. Basu, R. Basu, 11β-Hydroxysteroid dehydrogenase types 1 and 2 activity in subcutaneous adipose tissue in humans: implications in obesity and diabetes. J. Clin. Endocrinol. Metab. 100, E70–E76 (2014)
E.A. Lucassen, G. Cizza, The hypothalamic–pituitary–adrenal axis, obesity, and chronic stress exposure: sleep and the HPA axis in obesity. Curr. Obes. Rep. 1, 208–215 (2012)
D.M. Nathan, M.B. Davidson, R.A. DeFronzo et al., Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30, 753–759 (2007)
D. Fisher, G. Pillar, A. Malhotra, N. Peled, P. Lavie, Long-term follow-up of untreated patients with sleep apnoea syndrome. Respir. Med. 96, 337–343 (2002)
A. Dawson, S.L. Abel, R.T. Loving et al., CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J. Clin. Sleep Med. 4, 538–542 (2008)
T. Galic, J. Bozic, N. Ivkovic, T. Kurir Ticinovic, Z. Dogas, Effects of mandibular advancement device treatment on arterial stiffness and glucose metabolism in patients with mild to moderate obstructive sleep apnea: a prospective 1 year study. Sleep Breath. (2015). doi:10.1007/s11325-015-1186-y
E. Tasali, B. Mokhlesi, E. Van Cauter, Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133, 496–506 (2008)
Funding
This work was funded by the Croatian Science Foundation Grant #2013-11-5935. This study was performed at the University of Split School of Medicine, Split, Croatia. The funding sources did not have any involvement in study design, writing of the manuscript, and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bozic, J., Galic, T., Supe-Domic, D. et al. Morning cortisol levels and glucose metabolism parameters in moderate and severe obstructive sleep apnea patients. Endocrine 53, 730–739 (2016). https://doi.org/10.1007/s12020-016-0925-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0925-6