Abstract
Vitamin D deficiency is common among kidney transplant (KT) recipients because of reduced sunlight exposure, low intake of vitamin D, the immunosuppressive drug regimen administered, and steroid therapy. Glucocorticoids regulate expression of genes coding for enzymes that catabolize vitamin D, further reducing its level in serum. Although vitamin D primarily regulates calcium homeostasis, vitamin D deficiency is associated with the risk of several diseases, such as diabetes mellitus and tuberculosis. Aim of this review is to highlight endocrine and metabolic alterations due to the vitamin D deficiency by evaluating the mechanisms involved in the development of KT-related disease (cardiovascular, bone mineral density, and new-onset diabetes after transplantation). Next, we review evidence to support a link between low vitamin D status and KT-related diseases. Finally, we briefly highlight strategies for restoring vitamin D status in KT patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D3 (cholecalciferol) is formed in the skin by photolysis of 7-dehydrocholesterol by ultraviolet radiation from sunlight. Cholecalciferol is transported in the bloodstream bound to vitamin D binding protein and is hydroxylated in the liver to 25-hydroxyvitamin D [25(OH)D or calcidiol]. In the kidney, 25(OH)D is further hydroxylated to form the active hormone, i.e., 1,25-dihydroxyvitamin D [1,25-(OH)2D, or calcitriol] [1].
Vitamin D deficiency is defined by the US Endocrine Society guideline as 25(OH)D less than 20 ng/ml (50 nmol/l), vitamin D insufficiency as 25(OH)D between 21 and 29 ng/ml, and the upper cutoff to minimize the risk of adverse effects as 25(OH)D equal to 100 ng/ml (250 nmol/l) [2]. Vitamin D deficiency is common in the general population and more frequent in subgroups such as the elderly and patients with chronic diseases, particularly kidney failure (CKD) [3].
It is estimated that 17 % of patients with CKD have vitamin D insufficiency, and approximately 80 % have vitamin D deficiency [4]. Production of 1,25-(OH)2D is also reduced in CKD owing to fibroblast growth factor 23–mediated inhibition of enzymatic 1α-hydroxylation and by lack of viable kidney tissue containing the active enzyme [5]. In addition, the higher risk of vitamin D deficiency in patients with CKD is due to decreased exposure to sunlight, hyperpigmentation, and reduced ingestion of foods that are natural sources of vitamin D [6]. Thus, many patients present hypovitaminosis D at kidney transplantation (KT) [7]. In addition, immunosuppressive therapy undertaken by these subjects was reported to have an effect on vitamin D metabolism. In particular, calcineurin inhibitors (tacrolimus and cyclosporine) were found to suppress vitamin D receptor (VDR), thus increasing renal calcium wasting [8]. Moreover, the administered glucocorticoids may activate the genes involved in the expression of enzymes that catabolize vitamin D, further reducing its level [9].
Although KT patients are generally healthier than patients with end-stage kidney disease, Sadlier and Magee [10] reported that only 12 % of KT patients had normal vitamin D status, whereas 29 % had vitamin D deficiency. Similarly, Stavroulopoulos et al. [6] showed that 25(OH)D insufficiency and deficiency was prevalent in more than 90 % of patients, particularly in the first year after KT. Tripathi et al. [11] reported 25(OH)D deficiency and insufficiency in 57.8 and 94.7 %, respectively, of KT patients.
Studying the vitamin D metabolites, Riancho et al. reported that patients with a graft duration longer than 6 months had a normal serum levels of 25(OH)D and a reduction of serum levels of 1,25-(OH)2D while patients transplanted 1-6 months before and those with a longer duration of the graft had no differences in terms of vitamin D metabolites [12]. Conversely Barros et al. reported that 25(OH)D levels remain low 6 months after KT, whereas 1,25-(OH)2D levels rapidly return to normal during the first 6 months after KT [13].
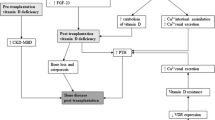
Vitamin D status in KT patients is an emerging theme. Although vitamin D primarily maintains calcium homeostasis, low vitamin D levels are associated with many diseases, such as breast, prostate, and colon cancer as well as diabetes mellitus and tuberculosis (Fig. 1) [14]. Of note, prostate, colon, breast, β cells of pancreas, monocytes, and macrophages, express both the VDR and 1α hydroxylase enzyme (CYP27B1). Extrarenal production of calcitriol controls directly or indirectly more than 200 genes, involved in the regulation of cellular proliferation and differentiation, apoptosis, angiogenesis, and insulin and renin secretion [15]. Three recent reviews have extensively examined the effects of vitamin D deficiency in the development of post-KT diseases [16–18]. In this work, we aimed to provide our contribution on this issue, mainly focusing on cardiovascular, metabolic, and endocrine outcomes [cardiovascular and bone mineral density (BMD) diseases and new-onset diabetes after transplantation (NODAT)]. We also briefly present strategies for restoring normal vitamin D status in KT patients.
Vitamin D and cardiovascular disease
Cardiovascular disease (CVD) accounts for 40–60 % of all-cause mortality and is the leading cause of death and allograft loss among KT recipients [19]. Although improved cardiac function and amelioration of cardiovascular morbidity and mortality are observed in kidney recipients with preserved kidney function, annual risk of death from CVD is 3.5–5 %—up to 50-fold higher than the risk in the age-matched general population [20].
Multiple CVD risk factors prevalent among dialysis patients persist or even increase after transplantation. Along with traditional CVD risk factors, nontraditional risk factors associated with reduced glomerular filtration rate include 25(OH)D deficiency, hyperhomocysteinemia, or factors unique to transplantation itself, including direct effects of maintenance immunosuppressive drugs [21]. General population studies have associated 1,25-(OH)2D levels with incident heart disease [22, 23]. The Framingham offspring study, involving 1,739 participants, associated 25(OH)D deficiency (<15 ng/ml) with higher incidence of cardiovascular events, with an adjusted hazard ratio of 1.62 (95 % confidence interval (CI), 1.11–2.36, p = 0.01) [22]. Moreover, a very recent observational study with longitudinal design considering 435 stable renal transplant recipients has reported that low 25(OH)D is independently associated with an increased risk of all-cause mortality [23]. Therefore, a potential role of 25(OH)D on excess CVD risk in KT recipients is not surprising.
Furthermore, levels of 25(OH)D in serum are strongly and inversely associated with very-low-density lipoprotein, triglyceride levels, serum renin activity, and brachial artery vasodilatation—key factors in atherosclerosis [24]. Also, in stable renal transplant recipients, 25(OH)D <12 ng/ml was associated with a rapid estimated glomerular filtration rate decline [23]. In addition, both VDRs and the CYP27B1 enzyme are found in vascular smooth-muscle cells, endothelial cells, and cardiomyocytes, potentially affecting growth and proliferation of these cells [15, 25]. It has been demonstrated that subjects deficient in 25(OH)D showed greater expression of proinflammatory transcription factor NF-κB in vascular smooth-muscle cells [26]. Conversely, inhibition of NF-κB by oral salsalate improved flow-mediated dilation in the patients with lower 25(OH)D levels. These findings make hypothesize that vascular endothelial dysfunction is mediated in part by NF-κB-related inflammation [26]. Consequently, a beneficial effect on CVD risk by vitamin D supplementation in KT recipients is plausible.
A recent meta-analysis on vitamin D supplementation concluded from limited evidence that moderate to high doses of vitamin D supplements may reduce CVD risk [27]. However, that analysis included studies that assessed active vitamin D analogs and calciferols in dialysis or the general population, not in kidney recipients. Moreover, no benefit of vitamin D supplementation was apparent in analysis restricted to randomized controlled trials (RCTs). Several lines of evidence suggest that KT patients manifest increased CVD risk and 1,25-(OH)2D deficiency, which is prevalent in these patients and might be involved in atherosclerosis. Future studies need to evaluate whether such supplementation yields better cardiovascular outcomes.
Vitamin D and NODAT
NODAT has been related to several risk factors. Corticosteroids and the commonly used immunosuppressive medications (e.g., cyclosporine and tacrolimus) play an important role on its pathogenesis [28]. The incidence of NODAT varies with the type of organ involved. The estimated rates are 9–18 % after kidney, 20–33 % after liver, 26–40 % after lung, and 29 % after heart transplants [28–30]. NODAT increases risk also for renal allograft loss, infection, cardiovascular events, and total mortality—particularly among KT patients [31].
Vitamin D is important in regulating glucose homeostasis. Several human and animal studies have shown a positive correlation between vitamin D deficiency, glucose intolerance, and impaired insulin secretion [30]. Expression of the VDR and CYP27B1 in β pancreatic cells [15, 32], which secrete insulin, could partially explain the involvement of vitamin D in glucose homeostasis. Research increasingly recognizes vitamin D levels and downstream signaling as an important cofactor in regulating insulin secretion and insulin sensitivity, with potential implications in the pathophysiology of diabetes [33]. More specifically, vitamin D responsive elements have been identified in the promoter of human gene encoding for insulin. Also, in vitro studies showed that calcitriol stimulates insulin human gene transcription as well as expression of insulin receptor and glucose transport [15]. However, the precise mechanism by which VDR and CYP27B1 affect insulin secretion as well as how vitamin D is involved in insulin sensitivity, immune system activation, and inflammation need further study [30, 34]. In fact, human and animal studies supporting the potential usefulness of vitamin D supplementation are lacking, and secondary endpoint analyses from clinical trials have not consistently shown benefits [35]. In particular, no randomized clinical trials have yet studied how vitamin D supplementation affects incidence of NODAT. However, the Vitamin D Supplementation in Renal Transplant Recipients (VITALE) study in France will compare effects of high and low doses of vitamin D on several clinical outcomes that include NODAT, with a planned median follow-up of 2 years [36].
Vitamin D and bone mineral density in KT patients
Owing to the crucial role of vitamin D, impaired calcium metabolism and bone development are commonly observed in CKD. This derangement, known as CKD–mineral and bone disorder (CKD–MBD), has been widely reported in people with kidney disease and in most patients receiving dialysis [37].
Moreover, evidence has increasingly documented that CKD–MBD is associated with bone mass loss, as indicated by decreased BMD, particularly within the first 2 years after KT. In a prospective study involving 20 adults who received renal allograft, Julian et al. [37] reported a 7 % decrease in lumbar spine 6 months after transplantation and a 9 % decrease after 18 months. Factors existing before transplantation, such as bone disease (including osteitis fibrosa and osteomalacia), as well as post-transplantation factors, including use of immunosuppressive drugs, PTH status, and hypophosphatemia, may contribute to reduced BMD observed in KT patients [38]. These observations helped to make treating renal osteodystrophy before transplantation as well as preventing bone disease in the first year after transplantation essential. The most widely reported approach for preventing bone loss after transplantation involves using different vitamin D metabolites, calcium, or bisphosphonates [39, 40].
Although several studies have associated increased risk of fracture and lower cortical BMD with CKD in adults and children, reports of vitamin D administration’s beneficial effects for CKD patients are increasing [39, 41–44]. Several studies have investigated using vitamin D or its analogs to prevent bone loss after KT. For instance, in an RCT involving 40 KT patients, El-Agroudy et al. [45] reported that daily treatment with alfacalcidol (0.5 µg) or placebo in combination with 500 mg of calcium carbonate increased BMD at the lumbar spine, femoral neck, and forearm by 2.1, 1.8, and 3.2 % respectively. Similarly, Torres et al. [46] reported significant change in femoral neck BMD after treatment with calcitriol (0.5 µg, 48-h interval) and calcium (50 mg) in an RCT involving 90 KT patients. Table 1 shows selected RCTs that evaluated therapeutic benefits of supplementation with vitamin D (or its analogs) after renal allograft on BMD.
Vitamin D supplementation in KT
The National Kidney Foundation has developed clinical practice guidelines for treating vitamin D deficiency in patients with CKD [47]. For KT patients, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend using the treatment for the general population to correct vitamin D deficiency and insufficiency [48]. However, the supplementation now used for the general population does not restore normal vitamin D levels in KT patients [49].
Thus, several authors tried to identify the ideal dosage to restore healthy levels of vitamin D [50, 51].
However, evidence from RCTs is lacking. The paucity of published studies does not allow to correctly examine the effect of vitamin D supplementation on outcomes, also due to the heterogeneity in vitamin D formulations prescribed in the different studies. The currently running VITA-D study aims to evaluate whether cholecalciferol administered at a dose of 6800 International Units daily over a time period of 1 year in KT recipients with vitamin D deficiency could have beneficial effects upon graft function, incidence of acute rejection episodes, and frequency and severity of post-transplant infection [52]. Also, the VITALE study has been designed to demonstrate that high doses of vitamin D (100000 International Units every 2 weeks for 2 months then monthly for 22 months) can reduce the risk of extra-osseous diseases (e.g., NODAT, major cardiovascular events, denovo malignancy) without inducing adverse effects, and also aims to determine the necessary levels of 25OHD to achieve these effects [36].
Hopefully, the results of these studies will provide the correct dose of cholecalciferol or ergocalciferol required in the renal transplant population and interesting suggestions on the vitamin D deficiency effects in different stages of kidney dysfunction as well as in the general population.
Conclusion and future perspectives
In addition to the emerging evidence suggesting a linkage between vitamin D and many non-skeletal conditions [53–58], many findings suggest an association between vitamin D deficiency and KT-related cardiovascular, metabolic, and endocrine diseases. However, data are missing regarding guidance for or against recommending vitamin D supplementation to prevent or treat KT-related disease, outside KDIGO recommendations. Further studies still need to investigate the role of vitamin D in KT-related disease and vitamin D supplementation’s beneficial effect.
References
A.M. Wootton, Improving the measurement of 25-hydroxyvitamin D. Clin. Biochem. Rev. 26, 33–36 (2005)
G. Muscogiuri, G. Tirabassi, G. Bizzaro, F. Orio, S.A. Paschou, A. Vryonidou, G. Balercia, Y. Shoenfeld, A. Colao, Vitamin D and thyroid disease: to D or not to D? Eur. J. Clin. Nutr. (2014). doi:10.1038/ejcn.2014.265
H. Taskapan, M. Wei, D.G. Oreopoulos, 25(OH) vitamin D3 in patients with chronic kidney disease and those on dialysis: rediscovering its importance. Int. Urol. Nephrol. 38, 323–329 (2006)
E.A. González, A. Sachdeva, D.A. Oliver, K.J. Martin, Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am. J. Nephrol. 24, 503–510 (2004)
C. Bosworth, I.H. de Boer, Impaired vitamin D metabolism in CKD. Semin. Nephrol. 33, 158–168 (2013)
A. Stavroulopoulos, M.J. Cassidy, C.J. Porter, D.J. Hosking, S.D. Roe, Vitamin D status in renal transplant recipients. Am. J. Transpl. 7, 2546–2552 (2007)
C.C. Hesketh, G.A. Knoll, A.O. Molnar, A. Tsampalieros, D.L. Zimmerman, Vitamin D and kidney transplant outcomes: a protocol for a systematic review and meta-analysis. Syst. Rev. 3, 64 (2014)
C.T. Lee, H.Y. Ng, Y.H. Lien, L.W. Lai, M.S. Wu, C.R. Lin, H.C. Chen, Effects of cyclosporine, tacrolimus and rapamycin on renal calcium transport and vitamin D metabolism. Am. J. Nephrol. 34, 87–94 (2011)
J.M. Pascussi, A. Robert, M. Nguyen, O. Walrant-Debray, M. Garabedian, P. Martin, T. Pineau, J. Saric, F. Navarro, P. Maurel, M.J. Vilarem, Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J. Clin. Investig. 115, 177–186 (2005)
D.M. Sadlier, C.C. Magee, Prevalence of 25(OH) vitamin D (calcidiol) deficiency at time of renal transplantation: a prospective study. Clin. Transplant. 21, 683–688 (2007)
S.S. Tripathi, E.M. Gibney, T.W. Gehr, A.L. King, M.J. Beckman, High prevalence of vitamin D deficiency in African American kidney transplant recipients. Transplantation 85, 767–770 (2008)
J.A. Riancho, A.L. de Francisco, C. del Arco, J.A. Amado, J.G. Cotorruelo, M. Arias, J. Gonzalez-Macias, Serum levels of 1,25-dihydroxyvitamin D after renal transplantation. Miner. Electrolyte Metab. 14, 332–337 (1988)
X. Barros, D. Fuster, N. Rodríguez, L. Rodas, M.J. Martínez-Osaba, J.M. Campistol, D. Rubello, P.M. Colletti, F. Campos, F. Pons, J.V. Torregrosa, Rapid calcitriol increase and persistent calcidiol insufficiency in the first 6 months after kidney transplantation. Nucl. Med. Commun. (2015). doi:10.1097/MNM.0000000000000265
R. Levi, J. Silver, Vitamin D supplementation after renal transplantation: how much vitamin D should we prescribe? Kidney Int. 75, 576–578 (2009)
M. Courbebaisse, J.C. Souberbielle, E. Thervet, Potential nonclassical effects of vitamin D in transplant recipients. Transplantation 89, 131–137 (2010)
R. McGregor, G. Li, H. Penny, G. Lombardi, B. Afzali, D.J. Goldsmith, Vitamin D in renal transplantation—from biological mechanisms to clinical benefits. Am. J. Transplant. 14, 1259–1270 (2014)
J. Bacchetta, S. Pelletier, G. Jean, D. Fouque, Immune, metabolic and epidemiological aspects of vitamin D in chronic kidney disease and transplant patients. Clin. Biochem. 47, 509–515 (2014)
C. Ponticelli, G. Sala, Vitamin D: a new player in kidney transplantation? Expert. Rev. Clin. Immunol. 10, 1375–1383 (2014)
A.O. Ojo, Cardiovascular complications after renal transplantation and their prevention. Transplantation 82, 603–611 (2006)
S. Aakhus, K. Dahl, T.E. Widerøe, Cardiovascular disease in stable renal transplant patients in Norway: morbidity and mortality during a 5-yr follow-up. Clin. Transplant. 18, 596–604 (2004)
E.M. Dimény, Cardiovascular disease after renal transplantation. Kidney Int. Suppl. 61, 78–84 (2002)
T.J. Wang, M.J. Pencina, S.L. Booth, P.F. Jacques, E. Ingelsson, K. Lanier, E.J. Benjamin, R.B. D’Agostino, M. Wolf, R.S. Vasan, Vitamin D deficiency and risk of cardiovascular disease. Circulation 117, 503–511 (2008)
C.A. Keyzer, I.J. Riphagen, M.M. Joosten, G. Navis, A.C. Muller Kobold, I.P. Kema, S.J. Bakker, M.H. de Borst, NIGRAM consortium, Associations of 25(OH) and 1,25(OH)2 Vitamin D with long-term outcomes in stable renal transplant recipients. J. Clin. Endocrinol. Metab. 100, 81–89 (2015)
J.L. Vacek, S.R. Vanga, M. Good, S.M. Lai, D. Lakkireddy, P.A. Howard, Vitamin D deficiency and supplementation and relation to cardiovascular health. Am. J. Cardiol. 109, 359–363 (2012)
D. Somjen, Y. Weisman, F. Kohen, B. Gayer, R. Limor, O. Sharon, N. Jaccard, E. Knoll, N. Stern, 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 111, 1666–1671 (2005)
K.L. Jablonski, M. Chonchol, G.L. Pierce, A.E. Walker, D.R. Seals, 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57, 63–69 (2011)
L. Wang, J.E. Manson, Y. Song, H.D. Sesso, Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann. Intern. Med. 152, 315–323 (2010)
G. Sarno, G. Muscogiuri, P. De Rosa, New-onset diabetes after kidney transplantation: prevalence, risk factors, and management. Transplantation 93, 1189–1195 (2012)
G. Sarno, R.J. Mehta, R. Guardado-Mendoza, L.M. Jimenez-Ceja, P. De Rosa, G. Muscogiuri, New-onset diabetes mellitus: predictive factors and impact on the outcome of patients undergoing liver transplantation. Curr. Diabetes Rev. 9, 78–85 (2013)
A.R. Gosmanov, S. Dagogo-Jack, Predicting, managing and preventing new-onset diabetes after transplantation. Minerva Endocrinol. 37, 233–246 (2012)
P.T. Pham, P.M. Pham, S.V. Pham, P.A. Pham, P.C. Pham, New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab. Syndr. Obes. 4, 175–186 (2011)
S. Lee, S.A. Clark, R.K. Gill, S. Christakos, 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology 134, 1602–1610 (1994)
S. Ghavamzadeh, M. Mobasseri, R. Mahdavi, The effect of vitamin D supplementation on adiposity, blood glycated hemoglobin, serum leptin and tumor necrosis factor-α in type 2 diabetic patients. Int. J. Prev. Med. 5, 1091–1098 (2014)
E.O. Gosmanova, V. Tangpricha, A.R. Gosmanov, Endocrine-metabolic pathophysiologic conditions and treatment approaches after kidney transplantation. Endocr. Pract. 18, 579–590 (2012)
S. Abbas, J. Linseisen, S. Rohrmann, J.W. Beulens, B. Buijsse, P. Amiano, E. Ardanaz, B. Balkau, H. Boeing, F. Clavel-Chapelon, G. Fagherazzi, P.W. Franks, D. Gavrila, S. Grioni, R. Kaaks, T.J. Key, K.T. Khaw, T. Kühn, A. Mattiello, E. Molina-Montes, P.M. Nilsson, K. Overvad, J.R. Quirós, O. Rolandsson, C. Sacerdote, C. Saieva, N. Slimani, I. Sluijs, A.M. Spijkerman, A. Tjonneland, R. Tumino, D.L. van der A, R. Zamora-Ros, S.J. Sharp, C. Langenberg, N.G. Forouhi, E. Riboli, N.J. Wareham, Dietary vitamin D intake and risk of type 2 diabetes in the European prospective investigation into cancer and nutrition: the EPIC-interact study. Eur. J. Clin. Nutr. 68, 196–202 (2014)
M. Courbebaisse, C. Alberti, S. Colas, D. Prié, J.C. Souberbielle, J.M. Treluyer, E. Thervet, VITamin D supplementation in renAL transplant recipients (VITALE): a prospective, multicentre, double-blind, randomized trial of vitamin D estimating the benefit and safety of vitamin D3 treatment at a dose of 100,000 UI compared with a dose of 12,000 UI in renal transplant recipients: study protocol for a double-blind, randomized, controlled trial. Trials 15, 430 (2014)
B.A. Julian, D.A. Laskow, J. Dubovsky, E.V. Dubovsky, J.J. Curtis, L.D. Quarles, Rapid loss of vertebral mineral density after renal transplantation. N. Engl. J. Med. 325, 544–550 (1996)
R.J. Weisinger, R.G. Carlini, E. Rojas, E. Bellorin-Font, Bone disease after renal transplantation. Clin. J. Am. Soc. Nephrol. 1, 1300–1313 (2006)
R.G. De Sévaux, A.J. Hoitsma, F.H. Corstens, J.F. Wetzels, Treatment with vitamin D and calcium reduces bone loss after renal transplantation: a randomized study. J. Am. Soc. Nephrol. 13, 1608–1614 (2002)
T.J. Weber, L.D. Quarles, Preventing bone loss after renal transplantation with bisphosphonates: we can… but should we? Kidney Int. 57, 735–737 (2000)
M. Talalaj, L. Gradowska, E. Marcinowska-Suchowierska, M. Durlik, Z. Gaciong, M. Lao, Efficiency of preventive treatment of glucocorticoid-induced osteoporosis with 25-hydroxyvitamin D3 and calcium in kidney transplant patients. Transplant. Proc. 28, 3485–3487 (1996)
A.E. El-Agroudy, A.A. El-Husseini, M. El-Sayed, T. Mohsen, M.A. Ghoneim, A prospective randomized study for prevention of post renal transplantation bone loss. Kidney Int. 67, 2039–2045 (2005)
A. Ugur, N. Guvener, I. Isiklar, H. Karakayali, R. Erdal, Efficiency of preventive treatment for osteoporosis after renal transplantation. Transplant. Proc. 32, 556–557 (2000)
J.R. Jeffery, W.D. Leslie, M.E. Karpinski, P.W. Nickerson, D.N. Rush, Prevalence and treatment of decreased bone density in renal transplant recipients: a randomized prospective trial of calcitriol versus alendronate. Transplantation 76, 1498–1502 (2003)
A.E. El-Agroudy, A.A. El-Husseini, M. El-Sayed, M.A. Ghoneim, Preventing bone loss in renal transplant recipients with vitamin D. J. Am. Soc. Nephrol. 14, 2975–2979 (2003)
A. Torres, S. Garcia, A. Gomez, A. Gonzalez, Y. Barrios, M.T. Concepcion, D. Hernandez, J.J. Garcia, M.D. Checa, V. Lorenzo, E. Salido, Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney Int. 65, 705–712 (2004)
B.L. Kasiske, M.G. Zeier, J.R. Chapman, J.C. Craig, H. Ekberg, C.A. Garvey, M.D. Green, V. Jha, M.A. Josephson, B.A. Kiberd, H.A. Kreis, R.A. McDonald, J.M. Newmann, G.T. Obrador, F.G. Vincenti, M. Cheung, A. Earley, G. Raman, S. Abariga, M. Wagner, E.M. Balk, Kidney disease: improving global outcomes. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 77, 299–311 (2010)
Y. Obi, N. Ichimaru, T. Hamano, K. Tomida, I. Matsui, N. Fujii, M. Okumi, J.Y. Kaimori, K. Yazawa, Y. Kokado, Y. Tsubakihara, N. Nonomura, H. Rakugi, S. Takahara, Y. Isaka, Orally active vitamin D for potential chemoprevention of posttransplant malignancy. Cancer Prevent. Res. (Phila) 5, 1229–1235 (2012)
K.M. Wissing, N. Broeders, R. Moreno-Reyes, C. Gervy, B. Stallenberg, D. Abramowicz, A controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation 79, 108–115 (2005)
J.K. Berga, J.C. Albiach, S.B. Catalan, E.G. Martinez, A.S. Calabuig, A.A. Bernabeu, L.M.P. Mateu, Vitamin D deficiency in a renal transplant population: safe repletion with moderate doses of calcidiol. Transplant. Proc. 42, 2917–2920 (2010)
M. Courbebaisse, E. Thervet, J.C. Souberbielle, J. Zuber, D. Eladari, F. Martinez, M.F. Mamzer-Bruneel, P. Urena, C. Legendre, G. Friedlander, D. Prié, Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 75, 646–651 (2009)
U. Thiem, G. Heinze, R. Segel, T. Perkmann, F. Kainberger, F. Mühlbacher, W. Hörl, K. Borchhardt, VITA-D: cholecalciferol substitution in vitamin D deficient kidney transplant recipients: a randomized, placebo-controlled study to evaluate the posttransplant outcome. Trials 10, 36 (2009)
T. Yasuda, Y. Okamoto, N. Hamada, K. Miyashita, M. Takahara, F. Sakamoto, T. Miyatsuka, T. Kitamura, N. Katakami, D. Kawamori, M. Otsuki, T.A. Matsuoka, H. Kaneto, I. Shimomura, Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine 43, 230–232 (2013)
A. Colao, G. Muscogiuri, M. Rubino, L. Vuolo, C. Pivonello, P. Sabatino, M. Pizzo, G. Campanile, R. Fittipaldi, G. Lombardi, C. Di Somma, Hypovitaminosis D in adolescents living in the land of sun is correlated with incorrect life style: a survey study in Campania region. Endocrine (2014). doi:10.1007/s12020-014-0483-8
N. Sharifi, R. Amani, E. Hajiani, B. Cheraghian, Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine 47, 70–80 (2014)
M. Redzic, D.K. Powell, D.T. Thomas, Vitamin D status is related to intramyocellular lipid in older adults. Endocrine 47, 854–861 (2014)
D.D. Bikle, Vitamin D and cancer: the promise not yet fulfilled. Endocrine 46, 29–38 (2014)
R.H. Chen, X.H. Zhao, Z. Gu, P.L. Gu, B. Zhou, Z.H. Zhu, L.Y. Xu, Y.F. Zou, X.Z. Jiang, Serum levels of 25-hydroxyvitamin D are associated with cognitive impairment in type 2 diabetic adults. Endocrine 45, 319–324 (2014)
Acknowledgments
This manuscript was neither prepared nor funded in any part by a commercial organization. We thank Ivo Giovannini, MD, for his kind assistance.
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarno, G., Daniele, G., Tirabassi, G. et al. The impact of vitamin D deficiency on patients undergoing kidney transplantation: focus on cardiovascular, metabolic, and endocrine outcomes. Endocrine 50, 568–574 (2015). https://doi.org/10.1007/s12020-015-0632-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0632-8