Abstract
Bone morphogenetic protein 7 (BMP7), also known as osteogenic protein-1 (OP-1) is a member of Transforming growth factor-β (TGF-β) family of proteins. Bone morphogenetic proteins were discovered in 1965 by Marshal Urist, of which BMP7 is of particular interest in this review being a leptin-independent anorexinogen and having role in energy expenditure in the brown adipose tissue, which makes it a potential target for preventing/treating obesity. As it has been established that Obesity displays a state of leptin-resistance, thus a protein-like BMP7 which acts through a leptin-independent pathway could give new therapeutic directions. This review will also discuss the synthesis and action of BMP7, along with its receptors and signal transduction. A brief note about BMP7-mediated brown fat development and energy balance is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone morphogenetic protein 7 (BMP7), which is a member of TGF-β superfamily [1–4], is well known for its osteogenic properties [5–7]. It is a glycosylated disulfide-linked 36 kDa homodimeric protein [8], having role in the induction, development, and regulation of adipocytes, especially brown adipose tissue (BAT) [5, 9, 10]. Neurotropic factors—brain-derived neurotropic factor (BDNF) and cilliary-derived neurotropic factor having role in appetite regulation and energy balance, [11–15] functionally resemble BMP7 [16]. Recently, the members of BMP family have been found to be associated with regulation of appetite. BMP7 is identified as an anorexinogen which acts through a leptin-independent central mTOR pathway in the hypothalamus [16]. This multifunctional cytokine [6] is involved in mitochondrial biogenesis [6, 9, 17] and energy expenditure in BAT [6, 16], which makes it a potential therapeutic agent for combating obesity [18]. OP1 (brand name for human recombinant BMP7) has clinical uses like bone fracture treatment, spinal fusions, etc [19]. In 2001, OP1 implant has been Food and Drug Administration (FDA) approved under humanitarian device exemption program as an alternative to autograft, though in 2008 some complications were reported in the cervical spine fusion [20, 21]. Although till date no clinical data are available for BMP7 in relation to obesity [19], but in near future eyeing BMP7 interventions could provide a hope for losing weight. The aim of this review is to summarize and explore the role of BMP7 in adipogenesis, energy expenditure, and this leptin-independent anorexinogen in treating diseases like obesity which is associated with leptin resistance.
Site of synthesis and action of BMP7
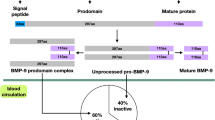
During embryogenesis and postnatal development, kidney is the major site of BMP7 synthesis [8]. In adults, collecting tubules and glomeruli of kidney, bone, cartilage, heart, urinary bladder, etc., express BMP7 [22–24]. BMP7, which is a secreted signaling protein is also synthesized in the adipose tissue by the stromal vascular cells [25, 26] and also expressed in various regions of brain including hypothalamus, cortex, hippocampus, leptomeninges, and habenular nucleus [27]. Cerebrospinal fluid (CSF) has an ample concentration of this bioactive neurotropic factor [16, 27]. BMP7 concentration in CSF is regulated in the choroid plexus as it has high amount of BMP7 mRNA [27–30]. Hypothalamus which is well known to be the major site involved in the modulation of feeding behavior and metabolism for homeostatic regulation [16, 31, 32], is the major site of action of BMP7 in brain [16, 27, 33].
BMP7 receptors and signal transduction
The TGF-β family ligand, BMP7 receptors are transmembrane heterodimers and are also known to regulate food intake [5, 16, 34]. They are serine/threonine kinase glycoproteins and consist of: (1) Three Type I receptors and (2) Three Type II receptors [5, 16]. BMPR1a, BMPRIb, and ALK2 are among Type I, and BMPRII, ActRIIa, ActRIIb among Type II receptors. BMPRII of the Type II receptors are widespread in the brain while Type I receptors are expressed in hypothalamus [3, 16]. BMPRIa mRNA has been found in visceral and subcutaneous adipose tissue [1].
BMPRIa and BMPRII receptors have been found to be correlated with human obesity. mRNA expression of both of these receptors in adipose tissue is relatively higher in obese and overweight individuals as compared to normal adults [1, 2]. BMPR2 is also associated with insulin sensitivity, adipogenesis, and glucose metabolism [2].
Signal transduction of TGF-β ligands requires both Type I and Type II receptors. Heteromeric complex formation of BMPR1 and BMPR2 [1, 2, 35–37], finally activate SMAD proteins which results in expression of a number of target genes [5, 38]. Apart from the SMAD protein pathway, the other downstream signaling cascade is p38 mitogen-activated protein kinase (p38 MAPK)/activating transcription factor 2 (ATF2) pathway [2, 5, 17, 38]. The involvement of these two pathways in the signaling of BMP7 is reported from the experiments where brown adipocytes were treated with BMP7, and there was an enhancement in the phosphorylation of SMAD1/5/8 and also p38MAPK and ATF2 [5, 6, 16, 17]. Further treatment with SMAD-inhibitor and a p38-MAPK inhibitor supported the results of involvement of these two pathways [17]. BMP7 treatment did not altered the phospho-AMPK levels, phospho-Akt, or total phospho ACC levels which suggest that these pathways are not activated by BMP7 [17].
BAT adipogenesis mediated by BMP7
In the body, adipose tissue is an important endocrine organ. Earlier known as a passive reservoir of energy storage is now being known to have multiple roles in integration of appetite regulation, energy balance, glucose homeostasis, and secrete various adipocytokines essential for metabolism [9, 39, 40]. Two types of adipose tissue are present: (1) white adipose tissue (WAT) and (2) brown adipose tissue (BAT) [41]. With WAT having main function in energy storage [9], brown adipose tissue in particular is of importance in energy balance for its thermogenic, energy expending function [9, 10, 16, 17, 42] and insulin sensitivity [25, 43]. Highly vascularized BAT is derived from differentiation of mesenchymal stem cell [9, 44, 45] and has a large number of mitochondria for carrying out β-oxidation [17]. BAT is highly innervated by sympathetic nervous system (SNS) which is activated by hypothalamus upon sensing of stimulus (like cold, food) which inturn activate β-adrenoreceptors in BAT and downstream cGMP which causes immobilization of free fatty acids and leads to uncoupling protein 1 (UCP-1) activation [18, 46]. UCP-1 located in the inner mitochondrial membrane is unique to brown adipocytes [6, 9, 17, 25, 47]. This UCP-1 uncouple mitochondrial proton gradient from ATP production and generates heat (Fig. 1) [10, 48–50].
BMP7, among other members of the BMP family is known for induction, differentiation, and development of brown fat in particular [6, 9, 25, 51]. On one hand, where BMP7 knockout significantly reduced the BAT mass in mice [25], on the other hand, BMP7 induction or treatment promoted brown fat development, fatty acid oxidation, mitochondrial activity [17], and reduce body weight gain [6, 9, 25] along with induction of peroxisome proliferator-activated receptor gamma (PPAR-γ), peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1-α), nuclear respiratory factor 1 (NRF1), and cytochrome C expression [6]. GDF8, also known as myostatin, is an inhibitor of BMP7 signaling and inhibit adipogenesis and myogenesis [52–54]. BAT marker UCP1 expression is also increased upon BMP7 treatment [6, 18, 25, 26]. BMP7 is involved in browning of white fat by inducing UCP1 expression and brite cell formation [10, 18]. BAT which is involved in body weight regulation [9] and considered to disappear after infancy [55], have been observed in humans during post mortem [9, 56]. Small amounts of functionally active BAT are found by positron-emission tomography and computed tomography (PET-CT) imaging studies in humans, which can induce BAT formation and thus control weight gain [9, 56, 57]. Hence, the property of BMP7 in promoting brown fat lineage can be exploited as a target counteracting obesity and associated morbidities.
BMP7 and energy expenditure
The balance between energy intake and energy expenditure regulates body weight [32, 58]. BMP7 increases energy expenditure in the brown adipose tissue [6, 9, 16, 18, 25]. Thus, BMP7 treatment both systemically and by adenoviral method resulted in an increase in body temperature, BAT-mediated energy expenditure, oxidative phosphorylation, fatty acid uptake, mitochondrial biogenesis and decrease in food intake, body weight gain, and the white fat content by inducing brite cell formation [6, 16–18, 25]. Brite cells in WAT play a key role in energy expenditure [18, 59]. BMP7 treatment also increased thermogenic capacity in BAT by activating SNS and thereby triggering a signal transduction cascade which leads to adaptive increase in brown fat marker UCP-1 expression in the inner mitochondrial membrane independent of environmental temperature, which generate heat instead of adenosine-ri-phosphate (ATP) by uncoupling reaction [6, 9, 16–18, 25]. According to a study by Boon et al. [18], sympathetic BAT activation by BMP7 occurs at subneutral temperature. p38 MAP kinase and PGC-1 play an important role in BMP7-induced thermogenic function [6, 60]. Upon intracerebroventricular (i.c.v) infusion of appetite-inhibiting peptides like POMC and α-MSH, thermogenesis increases [61]. Thus, it can be concluded that neuropeptides that inhibit food intake increases thermogenesis [62]. This property of BMP7 to induce thermogenesis and energy expenditure in brown adipose tissue can be exploited as a potential target for targetting obesity and diabetes in humans [6, 9, 17, 63].
Leptin-independent appetite regulation
Anorectic factors like leptin [64] reduce food intake by acting on hypothalamus by reducing the expression of anorexigenic neuropeptides like pro-opiomelanocortin (POMC), cocaine, and amphetamine transcript and decreasing expression of orexigenic neuropeptides like neuropeptide Y, agouti-related protein [31]. BMP7 is a novel anorectic factor which acts in hypothalamus and regulate feeding behavior [16]. Confocal microscopy showed that BMP7 and its receptors co-localized with appetite regulating neuropeptides in the brain. Peripheral treatment of mice with BMP7 adenovirus resulted in reduction of body weight [6, 16] and this reduction was more as compared to mice that received LacZ adenovirus [6]. In addition to the i.c.v administration of recombinant BMP7 to chow-fed mice [16], systemic treatment of BMP7 adenovirus to ob/ob mice [25] and administration to C57BL/6 mice, all individually resulted in a significant reduction in food intake [16]. Appetite regulatory effect of BMP7 is temperature dependent. BMP7 maintained its effect both in the DIO mice i.e., leptin-resistant mice plus in Ob mice i.e., leptin-deficient mice, which suggest that it mediate its signaling independent of leptin [16]. Additionally, for BMP7’s anorectic activity, melanocortin pathway is not essential [16]. mTOR (mammalian target of rapamycin) signaling is well known in the regulation of food intake [65–67] and BMP7 is able to reduce food intake by activating mTOR-p70S6 kinase pathway [16]. BMP7 treatment resulted in the phosphorylation of p70S6K (downstream target of mTOR) in the arcuate and paraventricular nucleus of hypothalamus, which are actively involved in appetite regulation [16]. The evidence for the role of mTOR signaling were reported by Townsend et al. [16] when i.c.v treatment with rapamycin, (mTOR pathway inhibitor) [68], completely rendered the food intake reducing capability of BMP7 in mice [16]. Myostatin (MSTN) gene expression is increased in the state of insulin resistance and obesity and soluble MSTN inhibitors could prevent these disorders [69, 70]. It has been proposed by a few lines of evidence that MSTN and BMP7 may compete each other in the hypothalamus [53, 71] and increased BMP7 signaling could be the cause of appetite loss in A-ZIP and Muscle-DN mice [72]. Studies reported so far indicate that BMP7’s anorectic effect is leptin-independent and inhibited by MSTN and mTOR inhibitor rapamycin.
Conclusion and future perspectives
BMP7 is a multifunctional ligand and anorexinogen of the TGF-β family with an important role in bone induction, brown fat adipogenesis and energy expenditure, which act through a central mTOR pathway in leptin-independent manner to reduce food intake and body weight. Having characteristics of reducing appetite, increasing thermogenesis, and energy expenditure in brown fat by increasing UCP1 mRNA, BMP7 is of particular therapeutic interest in treatment of obesity, diabetes, and other associated metabolic and degenerative diseases. Human recombinant BMP7 has been approved by FDA in 2001 and after that BMP7 containing osteogenic implants are being used. Since thermogenically active BAT traces have been found in the adult humans by landmark imaging techniques recently, thus developing a BMP7 molecular mimic/agonist or BMP7 spinal implant which could stimulate BAT formation, could lead to weight loss in humans. Since obese subjects generally have a state of reduced leptin-sensitivity, so the leptin-independent action of BMP7 in appetite loss could appear as a novel obesity treating breakthrough in near future with further research in this field.
References
Y. Bottcher et al., Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes. 58(9), 2119–2128 (2009)
D. Schleinitz et al., Genetic and evolutionary analyses of the human bone morphogenetic protein receptor 2 (BMPR2) in the pathophysiology of obesity. PLoS. ONE. 6(2), e16155 (2011)
S. Kishigami, Y. Mishina, BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 16(3), 265–278 (2005)
Y. Yamamoto, M. Oelgeschlager, Regulation of bone morphogenetic proteins in early embryonic development. Naturwissenschaften. 91(11), 519–534 (2004)
N. Zamani, C.W. Brown, Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr. Rev. 32(3), 387–403 (2011)
Y.H. Tseng et al., New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 454(7207), 1000–1004 (2008)
E. Ozkaynak et al., OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J. 9(7), 2085–2093 (1990)
M. Tanaka et al., Expression of BMP-7 and USAG-1 (a BMP antagonist) in kidney development and injury. Kidney Int. 73(2), 181–191 (2008)
A.M. Cypess, C.R. Kahn, Brown fat as a therapy for obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17(2), 143–149 (2010)
B. Cannon, J. Nedergaard, Brown adipose tissue: function and physiological significance. Physiol. Rev. 84(1), 277–359 (2004)
C.M. Vacher et al., A putative physiological role of hypothalamic CNTF in the control of energy homeostasis. FEBS Lett. 582(27), 3832–3838 (2008)
G. Perides et al., Neuroprotective effect of human osteogenic protein-1 in a rat model of cerebral hypoxia/ischemia. Neurosci. Lett. 187(1), 21–24 (1995)
J.K. Sabo, T.J. Kilpatrick, H.S. Cate, Effects of bone morphogenic proteins on neural precursor cells and regulation during central nervous system injury. Neurosignals. 17(4), 255–264 (2009)
B. Lebrun et al., Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton. Neurosci. 126–127, 30–38 (2006)
J.W. Cordeira et al., Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J. Neurosci. 30(7), 2533–2541 (2010)
K.L. Townsend et al., Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 26(5), 2187–2196 (2012)
K.L. Townsend et al., Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxid. Redox Signal. 19(3), 243–257 (2013)
M.R. Boon et al., BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS. ONE. 8(9), e74083 (2013)
M. Bluher, Adipokines-removing road blocks to obesity and diabetes therapy. Mol. Metab. 3(3), 230–240 (2014)
K.L. Ong et al., Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 35(19), 1794–1800 (2010)
A.P. White et al., Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop. 31(6), 735–741 (2007)
S. Vukicevic, M.N. Helder, F.P. Luyten, Developing human lung and kidney are major sites for synthesis of bone morphogenetic protein-3 (osteogenin). J. Histochem. Cytochem. 42(7), 869–875 (1994)
M. Zeisberg, Bone morphogenic protein-7 and the kidney: current concepts and open questions. Nephrol. Dial. Transplant. 21(3), 568–573 (2006)
A. Divoux, K. Clement, Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes. Rev. 12(5), e494–e503 (2011)
T.J. Schulz, Y.H. Tseng, Systemic control of brown fat thermogenesis: integration of peripheral and central signals. Ann. N. Y. Acad. Sci. 1302, 35–41 (2013)
T.J. Schulz et al., Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. U S A 108(1), 143–148 (2011)
B. Dattatreyamurty et al., Cerebrospinal fluid contains biologically active bone morphogenetic protein-7. Exp. Neurol. 172(2), 273–281 (2001)
M.N. Helder et al., Expression pattern of osteogenic protein-1 (bone morphogenetic protein-7) in human and mouse development. J. Histochem. Cytochem. 43(10), 1035–1044 (1995)
C.E. Johanson et al., Choroid plexus recovery after transient forebrain ischemia: role of growth factors and other repair mechanisms. Cell. Mol. Neurobiol. 20(2), 197–216 (2000)
S. Soderstrom, T. Ebendal, Localized expression of BMP and GDF mRNA in the rodent brain. J. Neurosci. Res. 56(5), 482–492 (1999)
A.P. Coll, I.S. Farooqi, S. O’Rahilly, The hormonal control of food intake. Cell. 129(2), 251–262 (2007)
M.W. Schwartz, D. Porte Jr, Diabetes, obesity, and the brain. Science. 307(5708), 375–379 (2005)
K. Ohyama, R. Das, M. Placzek, Temporal progression of hypothalamic patterning by a dual action of BMP. Development. 135(20), 3325–3331 (2008)
J.L. Wrana et al., Mechanism of activation of the TGF-beta receptor. Nature. 370(6488), 341–347 (1994)
J. Massague, F. Weis-Garcia, Serine/threonine kinase receptors: mediators of transforming growth factor beta family signals. Cancer Surv. 27, 41–64 (1996)
B.L. Hogan, Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10(13), 1580–1594 (1996)
D.M. Kingsley, The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 8(2), 133–146 (1994)
A. Nohe et al., The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 277(7), 5330–5338 (2002)
E.D. Rosen, B.M. Spiegelman, Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 444(7121), 847–853 (2006)
N. Hosogai et al., Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 56(4), 901–911 (2007)
B.M. Spiegelman, J.S. Flier, Obesity and the regulation of energy balance. Cell. 104(4), 531–543 (2001)
K. Townsend, Y.H. Tseng, Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte. 1(1), 13–24 (2012)
K.I. Stanford et al., Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123(1), 215–223 (2013)
S. Gesta, Y.H. Tseng, C.R. Kahn, Developmental origin of fat: tracking obesity to its source. Cell. 131(2), 242–256 (2007)
H.E. Young et al., Mesenchymal stem cells reside within the connective tissues of many organs. Dev. Dyn. 202(2), 137–144 (1995)
M. Harms, P. Seale, Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19(10), 1252–1263 (2013)
M. Ghorbani, T.H. Claus, J. Himms-Hagen, Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem. Pharmacol. 54(1), 121–131 (1997)
D. Richard, F. Picard, Brown fat biology and thermogenesis. Front. Biosci. 16, 1233–1260 (2011)
D. Richard et al., Determinants of brown adipocyte development and thermogenesis. Int. J. Obes. 34(Suppl 2), S59–S66 (2010)
S. Cinti, The adipose organ. Prostaglandins Leukot. Essent. Fatty Acids. 73(1), 9–15 (2005)
D. Sellayah, P. Bharaj, D. Sikder, Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 14(4), 478–490 (2011)
J.N. Artaza et al., Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 146(8), 3547–3557 (2005)
A. Rebbapragada et al., Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol. Cell. Biol. 23(20), 7230–7242 (2003)
B.J. Feldman et al., Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc. Natl. Acad. Sci. U S A 103(42), 15675–15680 (2006)
V. Gilsanz, H.H. Hu, S. Kajimura, Relevance of brown adipose tissue in infancy and adolescence. Pediatr. Res. 73(1), 3–9 (2013)
J.M. Heaton, The distribution of brown adipose tissue in the human. J. Anat. 112(Pt 1), 35–39 (1972)
J. Nedergaard, T. Bengtsson, B. Cannon, Three years with adult human brown adipose tissue. Ann. N. Y. Acad. Sci. 1212, E20–E36 (2010)
B.B. Lowell, B.M. Spiegelman, Towards a molecular understanding of adaptive thermogenesis. Nature. 404(6778), 652–660 (2000)
P. Bostrom et al., A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 481(7382), 463–468 (2012)
E. Canalis, A.N. Economides, E. Gazzerro, Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 24(2), 218–235 (2003)
A. Voss-Andreae et al., Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 148(4), 1550–1560 (2007)
A.N. Verty, A.M. Allen, B.J. Oldfield, The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinology. 151(9), 4236–4246 (2010)
J.F. Tobin, A.J. Celeste, Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discov. Today. 11(9–10), 405–411 (2006)
I.S. Farooqi et al., Leptin regulates striatal regions and human eating behavior. Science. 317(5843), 1355 (2007)
D. Cota et al., Hypothalamic mTOR signaling regulates food intake. Science. 312(5775), 927–930 (2006)
H. Mori et al., Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 9(4), 362–374 (2009)
E.C. Villanueva et al., Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology. 150(10), 4541–4551 (2009)
D. Benjamin et al., Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 10(11), 868–880 (2011)
A. Koncarevic et al., A novel therapeutic approach to treating obesity through modulation of TGFbeta signaling. Endocrinology. 153(7), 3133–3146 (2012)
C. Zhang et al., Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia. 55(1), 183–193 (2012)
B. Fournier et al., Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol. Cell. Biol. 32(14), 2871–2879 (2012)
A.C. McPherron et al., Increasing muscle mass to improve metabolism. Adipocyte. 2(2), 92–98 (2013)
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saini, S., Duraisamy, A.J., Bayen, S. et al. Role of BMP7 in appetite regulation, adipogenesis, and energy expenditure. Endocrine 48, 405–409 (2015). https://doi.org/10.1007/s12020-014-0406-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0406-8