Abstract
Ghrelin is an endogenous growth hormone secretagogue (GHS) causing release of GH from pituitary somatotropes through the GHS receptor. Secretion of GH is linked directly to intracellular free Ca2+ concentration ([Ca2+]i), which is determined by Ca2+ influx and release from intracellular Ca2+ storage sites. Ca2+ influx is via voltage-gated Ca2+ channels, which are activated by cell depolarization. The mechanism underlying the effect of ghrelin on voltage-gated Ca2+ channels is still not clear. In this report, using whole cell patch-clamp recordings, we assessed the acute action of ghrelin on voltage-activated Ca2+ currents in GH3 rat somatotrope cell line. Ca2+ currents were divided into three types (T, N, and L) through two different holding potentials (−80 and −40 mV) and specific L-type channel blocker (nifedipine, NFD). We demonstrated that ghrelin significantly and reversibly decreases all three types of Ca2+ currents in GH3 cells through GHS receptors on the cell membrane and down-stream signaling systems. With different signal pathway inhibitors, we observed that ghrelin-induced reduction in voltage-gated Ca2+ currents in GH3 cells was mediated by a protein kinase G-dependent pathways. As ghrelin also stimulates Ca2+ release and prolongs the membrane depolarization, this reduction in voltage-gated Ca2+ currents may not be translated into a reduction in [Ca2+]i, or a decrease in GH secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ghrelin, a 28-amino acid peptide hormone, activates the growth hormone secretagogue (GHS) receptor and increases GH levels both in vivo and in vitro in a number of species [1, 2]. Ghrelin stimulates GH secretion from somatotropes in vitro through the activation of multiple signaling cascades, including phospholipase C (PLC)/protein kinase C (PKC), cAMP/PKA, and through intra- and extra-cellular Ca2+-dependent mechanisms [3]. The Ca2+ influx through L-type voltage-gated Ca2+ channels is involved in the action of ghrelin on rat somatotropes [4]. The mechanism underlying the effect of ghrelin on all voltage-gated Ca2+ channels is still not clear. It has long been recognized that voltage-gated Ca2+ channels play a key role in the control of GH secretion from the pituitary [5, 6]. The Ca2+ influx through voltage-gated Ca2+ channels contributes predominantly to the elevation of the intracellular free Ca2+ concentration ([Ca2+]i) in somatotropes, leading to GH release [7, 8]. In ovine somatotropes, it has been shown that GH-releasing hormone (GHRH) and synthetic ghrelin agonists GH-releasing peptides (GHRPs) significantly influenced GH secretion by modifying T- and L-type currents through activation of the adenylate cyclase-dependent pathway or PKC pathways [6, 9]. Dominguez et al. reported that high voltage-activated Ca2+ channel activity was enhanced in GC rat somatotrope cell line after long-term treatment (96 h) of cells by ghrelin and GHRP-6 [10]. Short-term modification of Ca2+ channels by ghrelin in somatotropes is still unknown.
This study aimed to test the acute effect of ghrelin on the voltage-gated Ca2+ currents in the GH3 rat somatotrope cell line, a rat pituitary tumor cell line secreting GH with demonstrated expression of the GHS receptor (GHS-R) [11, 12]. Furthermore, the involvement of second messenger systems in ghrelin-induced effects on Ca2+ currents were determined.
Materials and methods
Chemicals
DMEM medium powder, F12 powder, Hepes, and sodium bicarbonate (NaHCO3) solution were purchased from Thermo Electron Corporation (Melbourne, Australia). Penicillin–streptomycin antibiotic solution and trypsin–EDTA solution were from Gibco (Gaithersburg, MD, USA). Fetal calf serum (FCS) was purchased from Thermo Trace Ltd (Melbourne, Australia). Tetrodotoxin (TTX) and KT5823 were purchased from Alomone Laboratories (Jerusalem, Israel). Nifedipine (NFD), nystatin, dimethyl sulfoxide (DMSO) and all general salts for recording solutions were purchased from Sigma (St. Louis, MO, USA). Ghrelin was obtained from Auspep (Parkville, Australia). Thapsigargin and 2-APB were obtained from Merck (Kilsyth, Australia). Chelerythrine chloride and H89 were purchased from CalBiochem-Novabiochem Pty Ltd (Alexandria, NSW, Australia).
Cell culture and preparation
The GH3 cells were obtained from the American Type Culture Collection (ATCC, Rockville. MD, USA) and grown as monolayer in 80 cm2 surface area plastic disposable culture flasks (Nunc, Roskilde, Denmark) with culture medium at 37°C in a humidified atmosphere (95% air–5% CO2). The culture medium contained 45% DMEM, 45% F12, 10% FCS, and 1% (v/v) penicillin–streptomycin antibiotic solution.
Cells were harvested during the logarithmic phase of growth, when they were visibly confluent in the flasks. Cell suspensions were obtained by removing culture medium and adding 3 ml trypsin–EDTA solution to the flasks. The cells were then detached from the surface of the flasks and 10 ml of DMEM medium added to cancel the trypsin activity. The cells were gently washed from the flasks and centrifuged (1500 rpm for 5 min). The supernatant was decanted and the pellet of cells were re-suspended in culture medium and seeded into 35 mm culture dishes. The medium was changed three times a week, and the electrophysiological recordings were performed after 2–5 days in culture dishes.
Electrophysiological recording
In patch-clamp experiments, the bath solution was composed of the following: 40 mM tetraethyl ammonium chloride, 90 mM NaCl, 5 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, and 10 mM HEPES (pH 7.4, adjusted with NaOH; osmolarity of 310 mOsmol/l with sucrose). To exclude the contamination of Na+ current, TTX was added to the bath solution with a final concentration of 1 μM on the day of experimentation. The pipette solution was composed of the following: 120 mM CsAsp, 20 mM tetraethyl ammonium chloride, 10 mM EGTA, 10 mM glucose, and 10 mM HEPES at pH 7.4 and osmolarity of 300 mOsm. The electrode was backfilled with this solution containing nystatin (300 μg/ml in 0.1% DMSO). This concentration of DMSO alone had no effect on membrane conductance when applied to the cells.
After obtaining a high-resistance seal, the pipette potential was set to −80 mV, and voltage pulses (20 mV amplitude, 200 ms duration) were delivered periodically to monitor the access resistance. Access to the cell interior was confirmed by the appearance of a membrane capacitance transient current, which usually occurred within 3–5 min under our experimental conditions. Typically, whole cell capacitance and series resistance (using only cells with <35 MΩ) were compensated (85%) and leak current was routinely subtracted using Clampex 7.0 (Axon Instrument, USA). Axon Instrument pClamp 7.0 software was used to acquire and analyze the data. Cell culture dishes were placed on the stage of an Olympus inverted microscope. Ghrelin and drugs were applied by hand with pipette to the culture dishes during recordings. Control recording was performed by applying vehicle instead of ghrelin to recorded cell and this caused no change in Ca2+ currents. Time-response curves indicate that, in our experimental system, the maximum effect of ghrelin occurred within 2 min. A gravity pressure system was used to perfuse the cells at a rate of 1 ml/min to wash off the applied drugs. All experiments were performed at room temperature (20–22°C).
Statistical analysis
A pCLAMP 7.0 software (Axon Instruments) was used to acquire and analyze the data. Student’s paired t-test was used to evaluate the statistical significance of differences between the means of the different groups, and the effects were considered significant at P < 0.05. Group data represent at least five recordings under the same experimental conditions and are expressed as mean ± SEM.
Results
Characterization of Ca2+ currents in GH3 cells
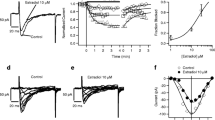
Voltage-gated calcium channels have been reported as L, T, N, P/Q, and R subtypes in different cell types. In GH3 cells, the voltage-gated calcium channel subtypes were reported differently. One study reported that T- and L-subtypes were found without P/Q and N subtypes [13]. Other report showed a significant proportion of total Ca2+ current as L-type and only a small part as T-type Ca2+ current in GH3/B6 cells [14]. In this study, we used two holding potentials (−80 and −40 mV) to isolate low-voltage-activated (T-type) and high voltage-activated (L, N, P/Q, and Rtype) Ca2+ currents. In addition, the L-type current blocker, NFD, was used to systemically examine the components of voltage-gated Ca2+ currents under different holding potentials. Figure 1 shows a group of recordings from one representative cell. The total Ca2+ currents shown in Fig. 1Aa were evoked by depolarizing test pulses from −50 up to +60 mV from a holding potential of −80 mV. NFD (10 μM) reduced about 30% of the total Ca2+ current (Fig. 1Ab), suggesting the presence of a significant portion of L-type current (comparing the difference between Fig. 1Aa and b). While using a holding potential of −40 mV to exclude T currents (Fig. 1Ac), total currents were reduced to 60% comparing to Fig. 1Aa. This suggested that the T-type current was significant in GH3 cells. Addition of NFD suppressed 37% of the total current (comparing Fig. 1Ac and Ad). The remaining currents were not T- or L-type currents (Fig. 1Ad). From Fig. 1A the results indicate that T-type current and L-type current are about 2/5 and 1/3 of the total Ca2+ current, respectively. The current–voltage (I–V) curves revealed two distinctive subtypes of Ca2+ currents recorded under holding potentials of −80 mV and −40 mV, respectively (Fig. 1B). These curves were made using the peak values measured from each of the Ca2+ current traces. The portions of different types in the same cell were shown in Fig. 1C.
Characterization of Ca2+ currents in GH3 cells. A The Ca2+ current following voltage steps from a holding potential of −80 and −40 mV and stepped to test pulses in increments of 10 to +60 mV and +50 mV as shown in the pulse protocols. a hp = −80 mV; b NFD treated with hp of −80 mV; c hp = −40 mV; d NFD treated with hp of −40 mV. B Current–voltage relationships of the Ca2+ currents shown in panel (A) measured at the peak of each current trace. In all I–V plots, the symbols represent a (filled circle), b (filled square), c (filled triangle) and d (filled diamond). C Results for the peak Ca2+ current measured following a depolarizing pulse to −10 and 0 mV from a holding potential of (a, b) −80 mV (c, d) −40 mV, respectively
Effect and time course of ghrelin on Ca2+ currents
Ghrelin was applied to the bath solution using the perfusion system, which gave an accurate and stable concentration of ghrelin in bath solution with possibility to include reagents affecting intracellular signaling systems in all following experiments. We first examined the effect of ghrelin on the total Ca2+ current with depolarizing pulses ranging from −50 to +60 mV at hp of −80 mV. Application of ghrelin (10 nM) significantly reduced Ca2+ currents following the application of depolarizing pulses up to +60 mV under hp of −80 mV. 100 and 10 nM doses of ghrelin had the same effect on Ca2+ currents, while 1 nM had no effect (data not shown). Therefore, the concentration of ghrelin used in all subsequent experiments was 10 nM. Figure 2A demonstrates the peak Ca2+ currents in controls (a) and after addition of ghrelin (10 nM) for 2 min (b), and the recovery of Ca2+ currents after ghrelin was washed away (c). Application of ghrelin significantly and reversibly decreased voltage-gated Ca2+ currents. The effect of ghrelin on total Ca2+ currents was also demonstrated in the I–V curves (Fig. 2B). The statistical data indicate that ghrelin significantly (**P < 0.01, n = 6) and reversibly decreased Ca2+ currents (Fig. 2C). We use normal bath solution as vehicle to show the stable total Ca2+ currents under control conditions with vehicle application (Fig. 3A). The time course of the response to ghrelin is also shown in Fig. 3B. The effect of ghrelin on the Ca2+ currents took place immediately and reached a maximum in about 2 min. The complete recovery of Ca2+ currents required approximately 4–6 min after the removal of ghrelin.
Effect of ghrelin on total voltage-gated Ca2+ currents. A Representative voltage-gated Ca2+ currents of GH3 cell at −10 mV evoked from a holding potential of −80 mV. The letters indicate control (a), during application of 10 nM ghrelin (b), and 2 min after the removal of ghrelin (c). B Current–voltage relationships of the peak and steady-state Ca2+ currents recorded with a holding potential of −80 mV stepped to the voltages indicated. Data were obtained from the same cell as in (A). In all I–V plots, the symbols represent control (filled circle), during application of ghrelin (filled square), and 2 min after the removal of ghrelin (filled triangle). C Results (mean ± SEM, n = 6) for the peak Ca2+ current measured following a depolarizing pulse to −10 mV from a holding potential of −80 mV. Ghrelin (10 nM) significantly (**P < 0.01, ghrelin vs. control) reduced the amplitude of the Ca2+ current with a full recovery 2 min after removal of ghrelin
Time-response relationship of the effect of ghrelin on voltage-gated Ca2+ currents. Voltage-gated Ca2+ currents were recorded every 2 min with a depolarizing pulse to 0 mV from a holding potential of −80 mV. Vehicle (recording medium, A) or ghrelin (10 nM, B) was applied to cells as indicated by the arrow. Data are shown as mean ± SEM (n = 5) of the peak current in each trace shown
Effect of ghrelin on the L-type Ca2+ currents
As L current is involved in the Ca2+ influx of ghrelin’s effect on somatotropes, we extended our studies by examining the effect of ghrelin on this current. The cells were recorded with a holding potential of −40 mV to exclude the T current. The remaining currents (non-T-type Ca2+ currents) may contain L, N, P/Q, and R types currents but are mainly the L-type current as shown in Fig. 1. The traces in Fig. 4A show the typical response to 10 nM ghrelin recorded at 0 mV (a, vehicle; b, ghrelin; c, recovery). Current–voltage relationships were obtained from the same cell as that recorded in panel A with depolarizing pulses ranging from −20 to +50 mV at hp of −40 mV (Fig. 4B). Ghrelin (10 nM) significantly (**P < 0.01, n = 6) decreased the non-T-type currents (Fig. 4C).
Effect of ghrelin on the non-T-type Ca2+ currents. A Representative voltage-gated Ca2+ currents of GH cell at 0 mV evoked from a holding potential of −40 mV. The letters indicate control (a), during application of 10 nM ghrelin (b), and 2 min after the removal of ghrelin (c). B Current–voltage relationships of the peak and steady-state Ca2+ currents recorded with a holding potential of −40 mV stepped to the voltages indicated. Data were obtained from the same cell as in (A). In all I–V plots, the symbols represent control (filled circle), during application of ghrelin (filled square), and 2 min after the removal of ghrelin (filled triangle). C Results (mean ± SEM, n = 6) for the peak Ca2+ current measured following a depolarizing pulse to 0 mV from a holding potential of −40 mV. Ghrelin (10 nM) significantly (**P < 0.01, ghrelin vs. control) reduced the amplitude of the Ca2+ current with a full recovery 2 min after removal of ghrelin
Effect of ghrelin on the T-type Ca2+ currents
When NFD was used to block the L-type Ca2+ current at hp of −80 mV, the currents obtained were excluded from the L-type Ca2+ current. The remaining currents are mainly the T-type current as shown in Fig. 1. The effect of ghrelin on the remaining currents was tested. Current–voltage relationships were obtained from the same cell as that recorded with depolarizing pulses ranging from −50 to +20 mV at hp of −80 mV (Fig. 5A). Ghrelin (10 nM) significantly (## P < 0.01, n = 6) decreased the non-L-type current (Fig. 5B).
Effect of ghrelin on the non-L-type Ca2+ currents. A Current–voltage relationships were obtained from the cells recorded with depolarizing pulses ranging from −50 to 20 mV at hp of −80 mV. The symbols represent control (filled circle), NFD (filled square) and ghrelin + NFD (filled triangle). B Results (mean ± SEM, n = 6) for the peak Ca2+ current measured following a depolarizing pulse to −10 mV from a holding potential of −80 mV. NFD (10 nM) significantly (**P < 0.01, NFD vs. control) decreased the total current and ghrelin significantly (## P < 0.01, ghrelin + NFD vs. NFD) decreased the non-L-type currents
Effect of ghrelin on the non-T and non-L-type Ca2+ currents
When the L-type Ca2+ current was blocked by NFD at hp of −40 mV, remaining currents were non-T (hp = −40 mV) and non-L-type (NFD) Ca2+ currents. The effect of ghrelin on the remaining Ca2+ currents (N, P/Q, and R type currents remained) was observed. Current–voltage relationships were obtained from the same cell as that recorded with depolarizing pulses ranging from −20 to +40 mV at hp of −40 mV (Fig. 6A). Ghrelin (10 nM) significantly (## P < 0.01, n = 6) decreased the non-T and non-L-type currents (Fig. 6B).
Effect of ghrelin on non-T and non-L-type Ca2+ currents. A Current–voltage relationships were obtained from the cells recorded with depolarizing pulses ranging from −20 to 40 mV at hp of −40 mv. The symbols represent control (filled circle), NFD (filled square) and ghrelin + NFD (filled triangle). B Results (mean ± SEM, n = 6) for the peak Ca2+ current measured following a depolarizing pulse to 0 mV from a holding potential of −40 mV. NFD (10 nM) significantly (**P < 0.01, NFD vs. control) decreased the total current and ghrelin significantly (## P < 0.01, ghrelin + NFD vs. NFD) decreased the non-T, non-L-type Ca2+ currents
Involvement of intracellular Ca2+ store in ghrelin-induced decrease in Ca2+ currents
To rule out the possible involvement of intracellular Ca2+ store in the ghrelin-mediated effect, we depleted the Ca2+ store by pretreatment of GH3 cells with thapsigargin (TSG, 1 μM, Fig. 7A) for 30 min or 2-APB (10 μM, Fig. 7B). Statistical data (Fig. 7) show that after any of these two pretreatments, ghrelin still reduced the Ca2+ current significantly (**P < 0.01, n = 5), suggesting that the intracellular Ca2+ store did not contribute to the ghrelin-induced decrease in Ca2+ currents.
Involvement of intracellular Ca2+ store in the ghrelin-induced decrease in the Ca2+ current. Statistical data show that thapsigargin (TSG, 1 μM for 30 min, A) or 2-APB (10 μM, B) do not prevent ghrelin from reducing the voltage-gated Ca2+ current in GH3 cells (mean ± SEM; n = 5, **P < 0.01, ghrelin + TSG vs. TSG or ghrelin + 2-APB vs. 2-APB)
Involvement of cAMP/PKA system in the Ca2+ current response to ghrelin
H89 is a selective PKA inhibitor. Incubation of cells with H89 (1 μM) for 10 min did not alter the Ca2+ current or the response to ghrelin (*P < 0.05) recorded with hp of −80 mV and depolarizing pulse to +10 mV (Fig. 8A, n = 5).
Antagonists of cAMP-PKA or PKC systems have no effect on the ghrelin-induced reduction in the Ca2+ current. Peak Ca2+ currents (mean ± SEM, n = 5) evoked by depolarizing pulses from a holding potential of −80 to 0 mV. The application of A H89 (1 μM) B chelerythrine (Chelery, 1 μM) did not modify the recorded Ca2+ current amplitude. Ghrelin significantly (*P < 0.05, ghrelin + H89 vs. H89 or ghrelin + Chelery vs. Chelery) and reversibly reduced the amplitude of Ca2+ currents in the presence of either of both compounds
Involvement of the PKC system in the Ca2+ current response to ghrelin
Chelerythrine is a specific blocker of PKC. Incubation with chelerythrine (1 μM) for 10 min did not significantly change the Ca2+ current or its response to ghrelin (Fig. 8B, n = 5, *P < 0.05).
Involvement of cGMP/PKG system in the Ca2+ current response to ghrelin
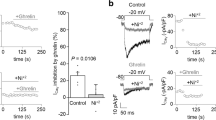
KT5823 is a specific blocker of cGMP-dependent PKG. Incubation with KT5823 (1 μM) for 10 min did not significantly change the Ca2+ current. The Ca2+ current response to ghrelin was, however, totally abolished when the PKG system was inhibited (Fig. 9, n = 8).
Effect of blockade of cGMP/PKC systems on the ghrelin-induced reduction in the Ca2+ current. Peak Ca2+ current (mean ± SEM, n = 8) evoked by depolarizing pulses from a holding potential of −80 to 0 mV. The application of KT5823 (1 μM) did not modify the recorded Ca2+ current. Ghrelin application in the presence of KT5823 did not reduce the amplitude of the Ca2+ current but totally abolished the Ca2+ current response to ghrelin
Discussion
Present study was undertaken to examine the acute effect of ghrelin on Ca2+ channel function in GH3 cells. The results indicated that voltage-gated Ca2+ currents were rapidly and reversibly reduced by application of ghrelin onto GH3 cells. Such effect of ghrelin did not act upon PLC pathway and was independent of either the cAMP/PKA system or the PKC system. In addition, Ca2+ releasing from the intracellular Ca2+ store was not involved in this effect. This reduction in voltage-gated Ca2+ currents induced by ghrelin appeared to be mediated by the cGMP/PKG system in GH3 cells.
It is well established that GH secretion is under the reciprocal control of GHRH and SRIF at the level of the pituitary gland with additional regulation by ghrelin [1, 15]. Ghrelin, as an endogenous ligand for GHS-R, seems to be involved in as an additional neuroendocrine hormone contributing to the regulation of GH [1]. Indeed, ghrelin strongly stimulated GH release, both in vivo and in vitro, in a wide range of species including human and rodents [1, 2]. The effect of ghrelin on GH secretion is thought to be linked to multiple signaling pathways. In porcine somatotropes, GH release in response to ghrelin depends on the cAMP/PKA and PLC/PKC systems and through extracellular Ca2+ influx [3]. Ghrelin-stimulated GH release from isolated rat anterior pituitary cells is achieved through both intracellular Ca2+ release and extracellular Ca2+ influx. Furthermore, the L-type voltage-gated Ca2+ channel is the major channel responsible for Ca2+ influx induced by ghrelin [4]. Most of the existing data, however, are not obtained directly by electrophysiology. The action of ghrelin on voltage-gated Ca2+ currents is still unclear.
Recently, Dominguez et al. reported that high voltage-activated Ca2+ channel expression was increased and Ca2+ channel activity was enhanced in GC cells by long-term treatment (96 h) with ghrelin and GHRP-6[10]. There maybe difference between long-term and short-term treatments. Our previous study showed that voltage-gated K+ currents are reduced in GH3 cells by acute administration of ghrelin [16]. This reduction in K+ currents may contribute to a prolonged depolarization of cell membrane by ghrelin and an increase in Ca2+ influx during depolarization.
In this study we investigated the effect of ghrelin on the membrane voltage-gated Ca2+ channels. Ca2+ channels in most excitable cells can be classified into five main types (T, L, N, P/Q, and R) on the basis of their unique biophysical and pharmacological properties [17–21]. In this study, Ca2+ currents were roughly divided into three types (T, L, and Non-T/non-L) through two different holding potentials (−80 and −40 mV) and specific L-type channel blocker, NFD. Using whole cell patch-clamp techniques, we demonstrated that ghrelin significantly and reversibly decreases all types of Ca2+ currents in GH3 cells, indicating that ghrelin maybe coupled to Ca2+ channels via the GHS receptor on the cell membrane and down-stream signaling systems.
PLC pathway may play a role in the functional regulation of somatotropes [22]. GHRP-6 triggered the release of Ca2+ from the intracellular Ca2+ store and the activation of PKC systems in rat somatotropes [23]. It has also been demonstrated that the effect of ghrelin on GH secretion was through both intra- and extra-cellular Ca2+-dependent mechanisms [4]. In this experiment, thapsigargin (TSG) and 2-APB were used to deplete and block the intracellular InsP3 sensitive Ca2+ stores before the stimulation by ghrelin, and we found that the decrease in Ca2+ current produced by ghrelin was not affected. These results suggest that the decrease in the Ca2+ current induced by ghrelin is not depending on the Ca2+ release from the intracellular InsP3-sensitive Ca2+ stores.
Using specific PKA and PKC pathway blockers (H89 and chelerythrine, respectively), we further examined the involvement of second messenger systems in this ghrelin-induced decrease in Ca2+ currents. It was previously shown that K+, Na+, and Ca2+ channels were modified by GHRH, GHRP, and somatostatin, through both PKA-cAMP and PKC systems in somatotropes through specific receptors of these peptides [24, 25]. Results in this experiment, however, indicated that the reduction of Ca2+ current induced by ghrelin was not affected by either H89 or chelerythrine. It is concluded therefore that PKA and PKC pathways are not involved in the effect of ghrelin on the voltage-gated Ca2+ current.
In addition to the cAMP/PKA and PLC/PKC systems, the cGMP-dependent protein kinases (PKGs) system also plays a potential role in regulating GH secretion from somatotropes [26, 27]. GHRH induces a robust increase in cGMP production via an extracellular Ca2+- and NO- independent mechanism [28]. Eight-bromo-cGMP, a cell-permeable agonist of cGMP, stimulated a fourfold increase in GH release from rat pituitary cells [26]. C-type natriuretic peptide (CNP) stimulates secretion of GH from GH3 cells via a cyclic GMP-mediated pathway [27]. NO donors S-nitroso-N-acetylpenicillamine and sodium nitroprusside (SNP), as well as a cGMP analog (dibutyryl guanosine 3′-5′-cyclic monophosphate), significantly increase GH secretion from cultured goldfish pituitary cells [29]. Cyclic GMP may directly modulate ion channels or indirectly act via PKG activation [16, 30, 31]. Rodriguez-Pacheco et al. reported that ghrelin-induced GH secretion through nitric oxide (NO)/cGMP signaling pathway from cultured pig somatotropes [32, 33]. Our previous studies indicate that ghrelin reduces voltage-gated K+ currents via the cGMP/PKG signaling pathway [16]. In this experiment, the specific PKG blocker, KT5823, has been demonstrated to have no effect on the recorded basal Ca2+ current but totally abolished the Ca2+ current response to ghrelin. It is therefore suggested that the reduction of voltage-gated Ca2+ currents by ghrelin is mediated by the cGMP/PKG system in GH3 cells.
In summary, we have shown that voltage-gated Ca2+ currents are decreased by acute administration of ghrelin through the cGMP/PKG signaling pathway in GH3 cells. As ghrelin also stimulates Ca2+ release and prolongs the membrane depolarization, this reduction in voltage-gated Ca2+ currents may not be translated into a reduction in [Ca2+]i, or a decrease in GH secretion. It should also be mentioned here that this observation is obtained in GH3 cell line, which maybe different to normal somatotroph cells, and further study is warranted.
References
M. Kojima, H. Hosoda, Y. Date, M. Nakazato, H. Matsuo, K. Kangawa, Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402(6762), 656–660 (1999)
K. Takaya, H. Ariyasu, N. Kanamoto, H. Iwakura, A. Yoshimoto, M. Harada, K. Mori, Y. Komatsu, T. Usui, A. Shimatsu, Y. Ogawa, K. Hosoda, T. Akamizu, M. Kojima, K. Kangawa, K. Nakao, Ghrelin strongly stimulates growth hormone release in humans. J. Clin. Endocrinol. Metab. 85(12), 4908–4911 (2000)
M.M. Malagon, R.M. Luque, E. Ruiz-Guerrero, F. Rodriguez-Pacheco, S. Garcia-Navarro, F.F. Casanueva, F. Gracia-Navarro, J.P. Castano, Intracellular signaling mechanisms mediating ghrelin-stimulated growth hormone release in somatotropes. Endocrinology 144(12), 5372–5380 (2003)
M. Yamazaki, H. Kobayashi, T. Tanaka, K. Kangawa, K. Inoue, T. Sakai, Ghrelin-induced growth hormone release from isolated rat anterior pituitary cells depends on intracellullar and extracellular Ca2+ sources. J. Neuroendocrinol. 16(10), 825–831 (2004)
R.W. Holl, M.O. Thorner, G.L. Mandell, J.A. Sullivan, Y.N. Sinha, D.A. Leong, Spontaneous oscillations of intracellular calcium and growth hormone secretion. J. Biol. Chem. 263(20), 9682–9685 (1988)
C. Chen, J.D. Vincent, I.J. Clarke, Ion channels and the signal transduction pathways in the regulation of growth hormone secretion. Trends. Endocrinol. Metab. 5(6), 227–233 (1994)
A.P. Naumov, J. Herrington, B. Hille, Actions of growth-hormone-releasing hormone on rat pituitary cells: intracellular calcium and ionic currents. Pflugers Arch. 427(5–6), 414–421 (1994)
B.T. Lussier, M.B. French, B.C. Moor, J. Kraicer, Free intracellular Ca2+ concentration and growth hormone (GH) release from purified rat somatotrophs III. Mechanism of action of GH-releasing factor and somatostatin. Endocrinology 128(1), 592–603 (1991)
C. Chen, D. Wu, I.J. Clarke, Signal transduction systems employed by synthetic GH-releasing peptides in somatotrophs. J. Endocrinol. 148(3), 381–386 (1996)
B. Dominguez, T. Avila, J. Flores-Hernandez, G. Lopez–Lopez, H. Martinez-Rodriguez, R. Felix, E. Monjaraz, Up-regulation of high voltage-activated Ca(2+) channels in GC somatotropes after long-term exposure to ghrelin and growth hormone releasing peptide-6. Cell. Mol. Neurobiol. 28(6), 819–831 (2008)
E.F. Adams, B. Huang, M. Buchfelder, A. Howard, R.G. Smith, S.D. Feighner, L.H. van der Ploeg, C.Y. Bowers, R. Fahlbusch, Presence of growth hormone secretagogue receptor messenger ribonucleic acid in human pituitary tumors and rat GH3 cells. J. Clin. Endocrinol. Metab. 83(2), 638–642 (1998)
A.M. Nanzer, S. Khalaf, A.M. Mozid, R.C. Fowkes, M.V. Patel, J.M. Burrin, A.B. Grossman, M. Korbonits, Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen-activated protein kinase pathway. Eur. J. Endocrinol. 151(2), 233–240 (2004)
A. Lievano, A. Bolden, R. Horn, Calcium channels in excitable cells: divergent genotypic and phenotypic expression of alpha 1-subunits. Am. J. Physiol. 267(2 Pt 1), C411–C424 (1994)
G. Glassmeier, M. Hauber, I. Wulfsen, F. Weinsberg, C.K. Bauer, J.R. Schwarz, Ca2+ channels in clonal rat anterior pituitary cells (GH3/B6). Pflugers Arch 442(4), 577–587 (2001)
S. Conway, S.M. McCann, L. Krulich, On the mechanism of growth hormone autofeedback regulation: possible role of somatostatin and growth hormone-releasing factor. Endocrinology 117(6), 2284–2292 (1985)
X.F. Han, Y.L. Zhu, M. Hernandez, D.J. Keating, C. Chen, Ghrelin reduces voltage-gated potassium currents in GH3 cells via cyclic GMP pathways. Endocrine 28(2), 217–224 (2005)
D.R. Matteson, C.M. Armstrong, Properties of two types of calcium channels in clonal pituitary cells. J. Gen. Physiol. 87(1), 161–182 (1986)
U. Meza, G. Avila, R. Felix, J.C. Gomora, G. Cota, Long-term regulation of calcium channels in clonal pituitary cells by epidermal growth factor, insulin, and glucocorticoids. J. Gen. Physiol. 104(6), 1019–1038 (1994)
Y. Mori, G. Mikala, G. Varadi, T. Kobayashi, S. Koch, M. Wakamori, A. Schwartz, Molecular pharmacology of voltage-dependent calcium channels. Jpn. J. Pharmacol. 72(2), 83–109 (1996)
A.K. Ritchie, Estrogen increases low voltage-activated calcium current density in GH3 anterior pituitary cells. Endocrinology 132(4), 1621–1629 (1993)
S.M. Simasko, G.A. Weiland, R.E. Oswald, Pharmacological characterization of two calcium currents in GH3 cells. Am. J. Physiol. 254(3 Pt 1), E328–E336 (1988)
T. Lei, E.F. Adams, M. Buchfelder, R. Fahlbusch, Relationship between protein kinase C and adenylyl cyclase activity in the regulation of growth hormone secretion by human pituitary somatotrophinomas. Neurosurgery 39(3), 569–575 (1996). Discussion 575–566
K. Cheng, W.W. Chan, B. Butler, A. Barreto Jr., R.G. Smith, Evidence for a role of protein kinase-C in His-d-Trp-Ala-Trp-d-Phe-Lys-NH2-induced growth hormone release from rat primary pituitary cells. Endocrinology 129(6), 3337–3342 (1991)
T. Takei, K. Takano, J. Yasufuku-Takano, T. Fujita, N. Yamashita, Enhancement of Ca2+ currents by GHRH and its relation to PKA and [Ca2+]i in human GH-secreting adenoma cells. Am. J. Physiol. 271(5 Pt 1), E801–E807 (1996)
E.F. Adams, T. Lei, M. Buchfelder, C.Y. Bowers, R. Fahlbusch, Protein kinase C-dependent growth hormone releasing peptides stimulate cyclic adenosine 3′,5′-monophosphate production by human pituitary somatotropinomas expressing gsp oncogenes: evidence for crosstalk between transduction pathways. Mol. Endocrinol. 10(4), 432–438 (1996)
D.J. Hartt, T. Ogiwara, A.K. Ho, C.L. Chik, Cyclic GMP stimulates growth hormone release in rat anterior pituitary cells. Biochem. Biophys. Res. Commun. 214(3), 918–926 (1995)
Y. Shimekake, S. Ohta, K. Nagata, C-type natriuretic peptide stimulates secretion of growth hormone from rat-pituitary-derived GH3 cells via a cyclic GMP-mediated pathway. Eur. J. Biochem. 222(2), 645–650 (1994)
T.S. Kostic, S.A. Andric, S.S. Stojilkovic, Spontaneous and receptor-controlled soluble guanylyl cyclase activity in anterior pituitary cells. Mol. Endocrinol. 15(6), 1010–1022 (2001)
A.D. Uretsky, J.P. Chang, Evidence that nitric oxide is involved in the regulation of growth hormone secretion in goldfish. Gen. Comp. Endocrinol. 118(3), 461–470 (2000)
D. Sachs, F.Q. Cunha, S.H. Ferreira, Peripheral analgesic blockade of hypernociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc. Natl. Acad. Sci. USA 101(10), 3680–3685 (2004)
J. Han, N. Kim, E. Kim, W.K. Ho, Y.E. Earm, Modulation of ATP-sensitive potassium channels by cGMP-dependent protein kinase in rabbit ventricular myocytes. J. Biol. Chem. 276(25), 22140–22147 (2001)
F. Rodriguez-Pacheco, R.M. Luque, S. Garcia-Navarro, F. Gracia-Navarro, J.P. Castano, M.M. Malagon, Ghrelin induces growth hormone (GH) secretion via nitric oxide (NO)/cGMP signaling. Ann. NY Acad. Sci. 1040, 452–453 (2005)
F. Rodriguez-Pacheco, R.M. Luque, M. Tena-Sempere, M.M. Malagon, J.P. Castano, Ghrelin induces growth hormone secretion via a nitric oxide/cGMP signaling pathway. J. Neuroendocrinol. 20(3), 406–412 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, X., Zhu, Y., Zhao, Y. et al. Ghrelin reduces voltage-gated calcium currents in GH3 cells via cyclic GMP pathways. Endocrine 40, 228–236 (2011). https://doi.org/10.1007/s12020-011-9520-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-011-9520-z