Abstract
Classical congenital adrenal hyperplasia (CAH) is characterized by the defects in cortisol and aldosterone secretion, and accompanied with adrenal hyperandrogenism. It is likely that the impaired adrenocortical function and intermittent treatment-related hypercortisolism may predispose patients to the development of metabolic syndrome in adulthood. Our aim was to assess the impact of hyperandrogenism on metabolic profiles in CAH women without glucocorticosteroid treatment. We evaluated the clinical characteristics and metabolic profiles in 30 untreated Chinese female adults with simple virilizing congenital adrenal hyperplasia (SV–CAH). Mutation analysis was performed by sequencing the entire 21-hydroxylase gene (CYP21A2). As compared with the controls, CAH patients had higher BMI (BMI, 21.5 ± 2.1 vs. 20.0 ± 1.8 kg/m2, P < 0.05), higher 2 h post-load plasma glucose levels (6. 35 ± 1.74 vs. 5. 35 ± 1.17 mmol/l, P < 0.05), higher serum triglycerides (TG) (1.12 ± 0.64 vs. 0.63 ± 0.15 mmol/l, P < 0.01), and lower high-density lipoprotein cholesterol (HDL-c) (1.30 ± 0.39 vs. 1.67 ± 0.29 mmol/l, P < 0.01). Moreover, CAH patients had higher fasting insulin and homeostasis model assessment of insulin resistance (HOMA-IR) (1.81 ± 0.99 vs. 1.24 ± 0.50, P < 0.05), while ΔIns30/ΔGlu30 showed no statistically significant difference in two groups. In addition, a marked reduction of serum adiponectin levels were observed in CAH patients (7.0 ± 3.3 vs. 13.2 ± 4.8 μg/ml, P < 0.001), however, serum CRP levels were not different between patients and the controls. Further regression analysis showed that higher serum testosterone concentrations were associated with metabolic disorder indexes and reduction of serum adiponectin. Our study demonstrates that untreated CAH patients are prone to have metabolic disorders in association with elevated serum testosterone levels and reduced insulin insensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
21-Hydroxylase deficiency (21-OHD) is one of the most common causes of genital ambiguity, accounting for over 90% congenital adrenal hyperplasia (CAH). Defects in the CYP21A2 gene, which codes for the steroid 21-hydroxylase, lead to various degrees of impaired cortisol and aldosterone synthesis, and the excess of androgen [1]. Based on the extent of enzyme impairment, classic 21-OHD is subdivided into salt wasting (SW) with deficiency in both mineralocorticosteroids and glucocorticosteroids, and simple virilizing (SV) form with major cortisol deficiency. Androgen excess in both classes results in virilization of external genitals, precocious pubarche, hirsutism, menstrual disturbances, and infertility in females. Usually, glucocorticoids are administrated to suppress the elevated adrenocortical secretion of androgen steroid precursors in classic CAH patients from childhood [2].
Recent studies suggest that androgen excess plays a role in pathogenesis of obesity and insulin resistance in women [3, 4]. The close relationship between androgen excess and hyperinsulinemia and insulin insensitivity has been shown in nonclassic CAH [5, 6]. Indeed, a progressive increase in fat mass during childhood has been reported in CAH patients [7]. However, most of the studies have addressed the consequences of glucocorticoid replacement on lipid metabolism and glucose control. In this study, we investigated a special population with SV–CAH, which has never been treated by gulcocorticoid and other androgen lowering medicine before, in order to recognize the effect of long-term excess androgen on metabolic disorders and the risk factors for cardiovascular diseases.
Results
Clinical characteristics
All the CAH patients (n = 30) were newly diagnosed and did not receive any treatment before. 90% (27/30) of patients presented with primary amenorrhea and the other 10% (3/30) patients also had irregular menses. The signs of hyperandrogenism were observed, including hirsutism (100%, 30/30), coarse skin (90%, 27/30), acne (100%, 30/30), laryngeal prominence (80%, 24/30), and undeveloped breasts (100%, 30/30), clitoris enlargement (100%, 30/30) and labioscrotal fusion (complete, 7%, 2/30; incomplete, 13%, 4/30). Nine patients received a feminizing genitoplasty due to ambiguous genitalia at age of 2 until 10 years. Hyper-pigmentation or darkening of the skin was found in all the patients. Most patients had accelerated growth from age 4–8 years, but ceased gradually from age 11–12 years. The bone age for all the patients indicated complete epiphyseal fusion and their mean final height (154.5 ± 5.8 cm) was lower than that of the female controls. The computerized tomography (CT) scan showed bilateral adrenal hyperplasia with different size of nodules (maximum diameter 23 mm) in all patients. Six patients had polycystic ovaries and other 24 patients had immature uterus and ovaries by ultrasonography. As compared with the controls, the patients had significantly lower plasma cortisol, and higher plasma ACTH, 17-hydroxyprogesterone (17-OHP), and testosterone levels (Table 1). FSH, LH, estradiol, and aldosterone levels were basically normal in the patients.
Sequencing analysis demonstrated that the I172N substitution at exon 4 was the most common mutation (38%, 23/60 alleles) in CAH patients (Supplemental Table 1). The IVS2-13A/C-G substitution was the next common mutation (31.7%). Compound heterozygous mutations were identified in 20 patients, accounting for 66.7%, while the genotype of compound heterozygous I172N and IVS2-13A/C-G mutation represented 30% of the patients.
Metabolic characteristics
None of the patients was overweight or obese, while BMI was greater in CAH patients as compared with the controls (Table 2). The patients had significant lower plasma adiponectin concentrations than that of the controls (Fig. 1a). The percentage of metabolic syndrome diagnosed in the patients was about 3.3%, and approximately 50% of the patients presented more than one components of metabolic syndrome.
Insulin sensitivity and pancreatic beta cell function
Oral glucose tolerance test (OGTT) showed that fasting plasma glucose (FPG) levels were not significantly different between the CAH patients and controls, while fasting insulin concentrations and 2 h post-load plasma glucose levels were markedly elevated in CAH patients as compared with the controls (Fig. 2; Table 2). As compared with the controls, the area under curve (AUC) of insulin levels was significantly higher in CAH patients, but the AUC of glucose levels in OGTT showed no statistically significant difference (Table 2). The HOMA-IR was higher in CAH patients, while HOMA-β showed no statistically significant difference between two groups. Serum insulin levels after oral glucose load had a rapid increase in CAH patients at 30 min, and were constantly higher until 3 h. The insulin sensitivity index (ISI) was lower in CAH patients, while ΔIns30/ΔGlu30 showed no statistically significant difference between the patients and controls.
Associations of serum testosterone with other variables
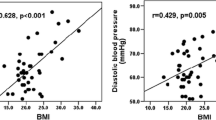
In the patients, plasma testosterones levels were inversely correlated with serum adiponectin (Fig. 1b), HDL and ISI, while positively correlated with fasting insulin level, TG, systolic blood pressure, HOMA-IR and ΔIns30/ΔGlu30 (Table 3). After adjustment for BMI, plasma testosterone levels were positively correlated with serum TG (r = 0.41, P < 0.01), and negative associated with HDL-c (r = −0.32, P < 0.05). Plasma 17-OHP levels was also positively correlated with serum TG (r = 0.40, P < 0.05) and inversely correlated with serum HDL-c (r = −0.42, P < 0.01). By multiple regression analysis, after adjustment for FPG, BMI, age, serum total cholesterol (TC), TG, HDL-c and low-density lipoprotein (LDL), systolic blood pressure, diastolic blood pressure, and plasma adiponectin, plasma testosterone was significantly correlated with HOMA-IR (P < 0.01); moreover, after adjustment for FPG, BMI, age, serum TC, TG, HDL-c and LDL, systolic blood pressure, and diastolic blood pressure, plasma adiponectin was significantly correlated with plasma testosterone (P < 0.05). The upper 75th percentile of the HOMA-IR distribution is defined as insulin resistance. Logistic regression analysis showed that the odds ratio of plasma testosterone levels for insulin resistance was 2.02 (95% CI, 1.09–3.73) after adjustment for fasting glucose, age, TC, TG, HDL-c, LDL, systolic blood pressure, and diastolic blood pressure (Table 4). The odds ratio was 1.41 (95% CI 1.11–1.78) after further adjustment for adiponectin and BMI.
Discussion
In this study, we recruited 30 young women with newly diagnosed SV–CAH. This special group might have long-term hyperandrogenism due to lack of treatment. Majority of those patients were from remote rural area and did not seek medical attention until they had no menarche. Even all the patients had predominant abnormality of external genitalia; only 30% (9/30) were aware of it and received feminizing genitoplastic surgery. Since no further treatment was given to lower the excess androgen, virilizing symptoms such as hirsutism, coarse skin and acne, were not improved and clitoris enlargement were recurrent in six of those with plastic surgery.
Our findings revealed the clear differences in some metabolic risk factors between this special population and the control. The untreated female patients with SV form of CAH had higher body weight, higher blood pressures, lower insulin sensitivity and more metabolic disorders, including higher serum TG, and lower HDL-c. As there were no impacts from exogenous glucocorticoids, the differences were most likely due to the long-term exposure to excess androgen in such a young patient group.
Further investigation of underlying factors revealed that plasma testosterone was positively associated with the body weight, dyslipidemia, systolic hypertension, and other metabolic disorders. Actually, androgen had an important impact on glucose homeostasis, lipid metabolism and body fat distribution; therefore androgen excess might play a role in the development of metabolic syndrome in women. Some studies showed that elevated plasma androgen levels were associated with insulin resistance, metabolic disorders, and even coronary artery diseases in obese women [8–10], but not in men [11]. Our findings may have direct implications in body weight accumulation in these CAH patients. Because androgen receptors have been identified in adipocytes [12], it is plausible that elevated plasma androgen levels play an important role in regional fat distribution, particularly visceral fat accumulation [13–15]. Furthermore, some other studies showed that testosterone promoted lipolysis in adipose tissues through upregulating lipoprotein lipase and hormone-sensitive lipase activities [16, 17]. Thus, hyperandrogenism could be a major factor involved in metabolic disorders in these untreated CAH patients.
Lines of evidence suggested that elevated free testosterone was associated with higher fasting insulin and post-load insulin; and considered to be a major predictor of metabolic syndrome in PCOS patients [10, 18, 19]. Several studies also reported hyperinsulinemia and insulin insensitivity in women with non-classical CAH or classic CAH [6, 20–22], but few were free from the influences of glucocorticoids replacement. Our results showed that the patients had significantly higher insulin levels and lower insulin sensitivity, which would be the further evidence for hyperandrogenism as a predictor of metabolic disorders.
We also demonstrated that the serum adiponectin levels were significantly reduced in SV–CAH patients and negatively correlated with plasma testosterone levels. It was reported that serum adiponectin levels were down-regulated by androgen [23, 24], which could be a risk for insulin resistance and atherosclerosis [25]. We further observed that the association of testosterone with HOMA-IR was markedly reduced after adjustment for serum adiponectin levels. It implied that elevated testosterone levels resulted in insulin resistance partially through down-regulation of adiponectin in patients with SV–CAH. Therefore, we speculate that exposure to high testosterone levels might contribute to reduction of serum adiponectin levels, and that in turn may impair insulin sensitivity in SV–CAH patients.
In conclusion, the newly diagnosed patients with SV–CAH have significantly lower adiponectin concentrations, higher insulin levels, and other metabolic indexes, compared with the normal control group. These differences might reflect the impacts from the long-term hyperandrogenism due to 21-OHD. Furthermore, all the metabolic disorder indexes may expose the patients to the increased risk of metabolic syndrome and cardiovascular disease.
Materials and methods
Subjects
Thirty unrelated Chinese female patients (age, 21.0 ± 4.3 years) with SV form of 21-OHD were investigated. All the patients were newly diagnosed as CAH in the Clinic of Department of Endocrinology and Metabolism, Rui-Jin Hospital, Shanghai and had never received glucocorticoid treatment and other lowering androgen agents before this study. The diagnosis for all the patients was established according to the clinical manifestations, basal hormone levels, and imaging evidence. The SV form is defined as variable degree of ambiguous external genitalia and without SW evidence. A mutation analysis of the CYP21A2 gene by direct sequencing was performed in all patients as reported previously [26].
Thirty control subjects (age, 22.5 ± 3.1 years) were recruited from postgraduate students of Shanghai Jiao Tong University, Shanghai. The study protocol was approved by the Institutional Review Board of the Rui-Jin Hospital and the informed consent was obtained from each participant.
Clinical and biochemical measurements
Body mass index (BMI) was calculated as body weight in kilogram divided by body height squared in meters. BMI of 25.0–29.9 kg/m2 was defined as overweight, whereas 30 kg/m2 or greater as obese. Blood pressure was measured at the right arm with a mercury meter three times after at least 5 min rest in a seated position. These three readings were averaged for analysis. The signs of hyperandrogenism were recorded by an endocrinologist. Hirsutism was evaluated according to the modified Ferriman–Gallwey scoring method [27].
Blood samples were drawn in the morning after an overnight fast for the biochemical and hormone measurements. Serum TC, TG, HDL-c, and LDL cholesterol were measured using the SYNCHRONLX Systems (Beckman Coulter Inc., Fullerton, CA). Serum insulin, cortisol, and testosterone were measured by fluoroimmunoassay (AutoDelfia Wallac Inc, Turku, Finland). Urine aldosterone, serum 17-OHP, and dehydroepiandrostenedione sulfate (DHEAS) were measured by radioimmunoassay (Abbott Laboratories Inc, Illinois, USA), Serum C-reactive protein (CRP) and adiponectin levels were measured by ELISA (Linco Laboratories Inc, Missouri, USA). The HOMA-IR was calculated as fasting insulin (μIU/ml) × fasting glucose (mmol/l)/22.5, and HOMA-β was calculated as 20 × fasting insulin (μIU/ml)/{fasting glucose (mmol/l) − 3.5}. Most patients and age-matched control subjects received OGTT and insulin release test (IRT). The ΔIns30/ΔGlu30 was calculated as = (Ins30 − Ins0)/(Glu30 − Glu0), and ISI represented 1/{fasting glucose (mmol/l) × fasting insulin (μIU/ml)}.
According to the definition recommended by the Asia Pacific Working Party on NAFLD 2006 [28], metabolic syndrome is defined as any three or more of the following: (1) Central obesity: waist circumference >90 cm (male), >80 cm (female), and/or BMI >25 kg/m2 in both sexes. (2) Hypertriglyceridaemia: TG ≥1.7 mmol/l. (3) Low HDL-c: HDL-c <1.03 mmol/l (male) and <1.29 mmol/l (female). (4) Elevated blood pressure: blood pressure ≥130/85 mmHg. (5) Elevated fasting glucose: FPG ≥5.6 mmol/l or previously diagnosed type 2 diabetes.
Statistical analysis
Results are presented as mean ± SD if not otherwise stated. Measurements with a skewed distribution were normalized by logarithmic transformation. Comparisons of means and proportions were performed with the standard normal z-test and χ2 tests, respectively. To allow for covariates and confounders, we performed analysis of multiple linear regression and logistic regression. The definition of the upper normal limit used for defining normal range of insulin resistance is set at the 75th upper percentile of the HOMA-IR distribution. Statistical significance was defined as P < 0.05.
References
P.C. White, M.I. New, Genetic basis of endocrine disease 2: congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 74(1), 6–11 (1992)
D.P. Merke, S.R. Bornstein, Congenital adrenal hyperplasia. Lancet 365(9477), 2125–2136 (2005)
E. Diamanti-Kandarakis, C. Christakou, H. Kandarakis, Polycystic ovarian syndrome: the commonest cause of hyperandrogenemia in women as a risk factor for metabolic syndrome. Minerva Endocrinol. 32(1), 35–47 (2007)
A. Corbould, Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J. Endocrinol. 192(3), 585–594 (2007)
P.W. Speiser et al., Insulin insensitivity in adrenal hyperplasia due to nonclassical steroid 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 75(6), 1421–1424 (1992)
F. Saygili, A. Oge, C. Yilmaz, Hyperinsulinemia and insulin insensitivity in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency: the relationship between serum leptin levels and chronic hyperinsulinemia. Horm. Res. 63(6), 270–274 (2005)
R.E. Cornean, P.C. Hindmarsh, C.G. Brook, Obesity in 21-hydroxylase deficient patients. Arch. Dis. Child. 78(3), 261–263 (1998)
L. Poretsky et al., The insulin-related ovarian regulatory system in health and disease. Endocr. Rev. 20(4), 535–582 (1999)
F.C. Wu, A. von Eckardstein, Androgens and coronary artery disease. Endocr. Rev. 24(2), 183–217 (2003)
S.H. Golden et al., Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the atherosclerosis risk in communities study. Am. J. Epidemiol. 160(6), 540–548 (2004)
D. Simon et al., Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: The Telecom Study. J. Clin. Endocrinol. Metab. 82(2), 682–685 (1997)
M.N. Dieudonne et al., Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am. J. Physiol. 274(6 Pt 1), C1645–C1652 (1998)
I. Janssen et al., Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity 18(3), 604–610 (2010)
D.J. Evans, J.H. Barth, C.W. Burke, Body fat topography in women with androgen excess. Int. J. Obes. 12(2), 157–162 (1988)
J.C. Seidell et al., Androgenicity in relation to body fat distribution and metabolism in 38-year-old women—the European Fat Distribution Study. J. Clin. Epidemiol. 43(1), 21–34 (1990)
R. Pasquali, Obesity and androgens: facts and perspectives. Fertil. Steril. 85(5), 1319–1340 (2006)
J.S. Mayes, G.H. Watson, Direct effects of sex steroid hormones on adipose tissues and obesity. Obes. Rev. 5(4), 197–216 (2004)
J.A. Marcondes et al., Metabolic syndrome in women with polycystic ovary syndrome: prevalence, characteristics and predictors. Arq. Bras. Endocrinol. Metabol. 51(6), 972–979 (2007)
J.C. Marshall, Obesity in adolescent girls: is excess androgen the real bad actor? J. Clin. Endocrinol. Metab. 91(2), 393–395 (2006)
F.J. Paula et al., Androgen-related effects on peripheral glucose metabolism in women with congenital adrenal hyperplasia. Horm. Metab. Res. 26(11), 552–556 (1994)
E. Charmandari et al., Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: potential clinical implications. J. Clin. Endocrinol. Metab. 87(5), 2114–2120 (2002)
L. Green-Golan et al., Patients with classic congenital adrenal hyperplasia have decreased epinephrine reserve and defective glycemic control during prolonged moderate-intensity exercise. J. Clin. Endocrinol. Metab. 92(8), 3019–3024 (2007)
M. Berra et al., Testosterone decreases adiponectin levels in female to male transsexuals. Asian J. Androl. 8(6), 725–729 (2006)
S.T. Page et al., Testosterone administration suppresses adiponectin levels in men. J. Androl. 26(1), 85–92 (2005)
H. Nishizawa et al., Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 51(9), 2734–2741 (2002)
H.J. Zhang et al., Variations in the promoter of CYP21A2 gene identified in a Chinese patient with simple virilizing form of 21-hydroxylase deficiency. Clin. Endocrinol. 70(2), 201–207 (2009)
D. Ferriman, J.D. Gallwey, Clinical assessment of body hair growth in women. J. Clin. Endocrinol. Metab. 21, 1440–1447 (1961)
J.G. Fan et al., What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J. Gastroenterol. Hepatol. 22(6), 794–800 (2007)
Acknowledgments
We are grateful to the consideration and cooperation of all the participants of the study. This study has been supported with the grants from Natural Science Foundation of China (No. 30725037 and 30973912) and Shanghai Committee of Science and Technology (No. 09DJ1400402 and 09XD1403400).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hui-Jie Zhang and Jun Yang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, HJ., Yang, J., Zhang, MN. et al. Metabolic disorders in newly diagnosed young adult female patients with simple virilizing 21-hydroxylase deficiency. Endocr 38, 260–265 (2010). https://doi.org/10.1007/s12020-010-9382-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-010-9382-9