Abstract
Macrophages are the main source of cytokines in atherosclerotic plaques. Modified low-density lipoproteins may stimulate macrophages to produce large quantities of proinflammatory cytokines that promote atherosclerosis. Berberine is the main component of the traditional Chinese medicine umbellatine, which has a widespread effect and was used to treat many diseases clinically. Our previous study found that berberine could increase adipophilin expression in macrophages, which is a target gene of PPARγ. PPARγ agonist could decrease proinflammatory cytokines in macrophage. In this study, we investigated the effects and the mechanism of action of berberine on the expression and secretion of TNFα, MCP-1, and IL-6 in vitro to identify new pharmacological actions of berberine. The results of RT-PCR and ELISA shows that berberine may inhibit the expression and secretion of the tumor necrosis factor α (TNFα), monocyte chemoattractant protein 1 (MCP-1), and interleukin-6 (IL-6) in macrophages stimulated by acetylated low-density lipoprotein (AcLDL), whereas the peroxisome proliferator-activated receptor γ (PPARγ) inhibitor GW9662 could attenuate this effect of berberine. This study demonstrates that berberine may inhibit the expression and production of TNF-α, MCP-1, and IL-6 in AcLDL-stimulated macrophages. This effect might be partially mediated through PPARγ activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a multifactorial disease and numerous risk factors associated with its manifestation have been identified [1]. Increased levels of plasma cholesterol, in particular of low-density lipoprotein (LDL), are recognized as a major cardiovascular risk factor, similar as hypertension, diabetes mellitus and smoking. Inflammation plays a critical role in the mechanism of atherosclerosis throughout every stage of the disease [2]. Proinflammatory cytokines are mainly secreted from macrophages in atherosclerotic plaques [3, 4]. Plasma LDL can cross the endothelium and accumulate in the intima, where it can undergo oxidative modification and acquire several potentially proatherogenic biological properties. These include stimulating vascular endothelial cells and macrophages to produce large number of proinflammatory cytokines [5, 6].
Berberine is the main component of the traditional Chinese medicine umbellatine, which has a widespread effect and were used to treat many diseases clinically. Recently, it was found that berberine has cholesterol-lowering effect [7], inhibits rat vascular-smooth-muscle-cell proliferation and migration [8], and has anti-tumor effect [9, 10]. It has also been shown that berberine can inhibit acetaldehyde-induced interleukin-1b and tumor necrosis factor-α production in HepG2 cells [11]. Tumor necrosis factor α (TNFα) [12], interleukin (IL)-6 [13, 14], and monocyte chemoattractant protein 1 (MCP-1) have important roles in the development and acceleration of atherosclerosis [15, 16].
Our previous studies observed berberines’ effect on glucose in HepG2 cell line and found that berberine was able to exert a glucose-lowering effect in hepatocytes [17]. Also in our previous study, we found that adipophilin expression was higher in aorta tissue of monkey with diabetes than aorta tissue of monkey without diabetes. It was found that berberine could increase adipophilin expression in macrophages in the process of finding the factors of affect adipophilin expression [18]. As we known, adipophilin is a PPAR-γ target gene [19], PPARγ agonist could decrease proinflammatory cytokines in macrophage. To determine the novel pharmacological action of berberine, we investigated the effects and the mechanism of action of berberine on the AcLDL-stimulated levels of TNFα, IL-6, and MCP-1 expression in macrophages cultured in vitro. We found that berberine could inhibit AcLDL-induced proinflammatory cytokine expression and secretion in THP-1 macrophages.
Material and methods
Drugs and reagents
Berberine hydrochloride was produced by the Tianjin Chinese medicine factory and supervised by National Institute for the Control of Pharmaceutical and Biological Products, and that was a gift from Dr. Zhou LB, Institute of Endocrine and Metabolism of Shanghai, China. GW9662 and rosiglitazone were purchased from Sigma Company. Fetal calf serum (FCS), RPMI1640 culture medium, and DMSO were purchased from Gibco Company. The THP-1 cell line was obtained from the American Type Culture Collection (ATCC). Total RNA extract kit purchased from QIAGEN Company. The ELISA kits to detect TNF-α, MCP-1, and IL-6 were purchased from BIOSOURSE Company. FastStart DNA Master SYBR Green I kit was purchased from Roche Company. Primers were synthesized by the Shanghai Shengong Company.
Cell culture
Human monocytic THP-1 cells were maintained in RPMI 1640 medium with 10% FCS. Three days before treatment, cells were seeded in 6-well culture dishes at a density of 2 × 106 cells/well. Differentiation of THP-1 monocytes to macrophages occurred in the presence of 160 nM of phorbol 12-myristate 13-acetate (PMA) for 72 h. The cells were found adhered to the bottom of culture dishes and presented hetermorphism.
Cell treatment
Berberine was freshly prepared in normal saline. GW9662 and rosiglitazone were dissolved in DMSO in a 1000× concentration. The THP-1 derived macrophages were washed three times with Hanks’ solution and cultured in RPMI1640 medium with 10% FCS. Cells were treated for 48 h with 50 μg/ml AcLDL and various amounts of berberine (5 μmol/l, 10 μmol/l, normal saline, or AcLDL alone as control 1 and control 2, respectively). Some cells were harvest at 24 h extract total RNA for measured the expression levels of TNF-α, IL-6, and MCP-1. The supernatants were collected after treating 24 and 48 h to measure the content of proinflammatory cytokines.
MTT assay
Cell viability was monitored by MTT colorimetric assay. Cells were treated with berberine or rosiglitazone for 24 h. One-tenth volume of 5 mg/ml MTT was then added to the culture medium. After 4-h incubation at 37°C, equal cell culture medium volume of 0.04 N HCl in isopropanol was added to dissolve the MTT formazan, and the absorbance value was measured using an ELISA plate reader.
Real-time RT-PCR
The total cell RNA was extracted with Trizol (QIAGEN Company). 1 μg RNA was used for reverse transcription. The expression of TNF-α, MCP-1, and IL-6 in macrophages was detected by method of real-time-PCR. β-actin was used as an internal standard and various concentrations of plasmid inserted with target PCR products were used to generate a standard curve. The LightCycler® 2.0 System with the matched LightCycler® FastStart DNA Master SYBR Green I kit was used for real-time PCR.
Enzyme-linked immunosorbent assay (ELISA)
Levels of the cytokines IL-6, MCP-1, and TNF-α were measured by ELISA using the human TNF-α, MCP-1, and TNF-α sets (BIOSOURCE). Macrophages were treated with various amounts of berberine (5 μmol/l, 10 μmol/l, or normal saline as control) in the presence of 50 μg/ml AcLDL for 24 h. Supernatants were then added to wells, which were coated with monoclonal antibody against IL-6, MCP-1, and TNF-α. After three washes with washing buffer (0.05% Tween-20 in phosphate-buffered saline, PBS), peroxidase-conjugated avidin, biotinylated antibodies against IL-6, MCP-1, and TNF-α, and chromogenic substrates were added to each well. The absorbance was read at 450 nm in an ELISA plate reader.
Statistical analysis
Data were presented as mean ± standard error (SE). Analysis of variance (ANOVA) was used for comparisons multiple means. A value of P < 0.05 was considered statistically significant.
Results
The cytotoxicity of berberine and rosiglitazone to macrophages
The results of MTT assay showed that berberine in concentration of 5 or 10 μmol/l and rosiglitazone in concentration of 10 nmol/l did not show significant cytotoxicity. As shown in Fig. 1, the relative viability of berberine- or rosiglitazone-treated macrophages were all greater than 88%.
The cytotoxicity of berberine and rosiglitazone to macrophages. Macrophages were treated with various concentrations of berberine (5 or 10 μmol/l) or rosiglitazone (10 nmol/l) in the presence of 50 μg/ml AcLDL for 48 h. Relative viability was measured using MTT assay. Values are mean ± SE of three independent experiments. Samples were measured in triplicate (con1, normal saline; BBR 5 μmol, 50 μg/ml AcLDL, and 5 μmol/l berberine; BBR 10 μmol, 50 μg/ml AcLDL, and 10 μmol/l berberine; Rosi 10 nmol, 50 μg/ml AcLDL and 10 nmol rosiglitazone)
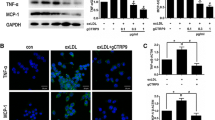
Berberine could inhibit the expression and secretion of TNF-α, IL-6, and MCP-1 in macrophages stimulated by AcLDL
AcLDL may stimulate macrophages to produce proinflammatory cytokines, the expression of which was correlated with the development of artherosclerosis. To determine the effect of berberine on the expression and secretion of cytokines in macrophages stimulated by AcLDL, we treated the macrophages with various amount of berberine (5 μmol/l, 10 μmol/l, or normal saline as control) in the presence of 50 μg/ml AcLDL for 48 h and collected the supernatant after treating 24 and 48 h. Some cells were harvest at 24 h extract total RNA for measured the expression levels of TNF-α, IL-6, and MCP-1 with real-time RT-PCR. The levels of TNF-α, IL-6, and MCP-1 in the supernatant were detected by ELISA analysis.
As shown in Fig. 2a, macrophages that were not treated with AcLDL (con1) expressed little TNF-α, IL-6, and MCP-1, but the levels of TNF-α, IL-6, and MCP-1 increased significantly after treatment with AcLDL (increased almost 2-fold). We found that berberine could inhibit the expression of TNF-α, IL-6, and MCP-1 in macrophages stimulated by AcLDL in a dose-dependent manner. After treatment with 5 μmol/l or 10 μmol/l berberine, the levels of TNF-αmRNA expression decreased by 28% and 50%, respectively, the levels of MCP-1 mRNA expression decreased by 37% and 57%, respectively, and the levels of IL-6 mRNA expression decreased by 16% and 44%, respectively, compared with controls.
The effects of berberine on the TNF-α, IL-6 and MCP-1 expression and, secretion in AcLDL-stimulated macrophages. a Macrophages cultured in 6-well culture dishes were treated various amounts of berberine (5 μmol/l, 10 μmol/l) in the presence of 50 μg/ml AcLDL for 24 h, and the expression levels of TNF-a, IL-6 and MCP-1 were measured by real-time RT-PCR. The levels of TNF-a, IL-6, and MCP-1 mRNA are compared with those of β-actin mRNA. b and c Macrophages were treated with various amounts of berberine (5 μmol/l, 10 μmol/l, or normal saline as control) in the presence of 50 μg/ml AcLDL for 48 h. The cells supernatant were collected at 24 b and 48 h c after treating. The levels of TNF-a, IL-6, and MCP-1 in the cells supernatant were detected with ELISA. Values are mean ± SE of three independent experiments and each sample were detected three times. *P < 0.05, **P < 0.01 compared with con2. (con1, normal saline; con2, 50 μg/ml AcLDL; BBR 5 μmol, 50 μg/ml AcLDL and 5 μmol/l berberine; BBR 10 μmol, 50 μg/ml AcLDL and 10 μmol/l berberine)
As shown in Fig. 2b, c, the levels of TNF-α, IL-6, and MCP-1 increased significantly after treatment with AcLDL whereas berberine could inhibit the expression of TNF-α, IL-6, and MCP-1 in macrophages stimulated by AcLDL in a dose-dependent manner. After treatment with 5 μmol/l or 10 μmol/l berberine, the levels of TNF-α in the supernatant decreased by 43.9% and 62.8%, respectively, after 24 h, and decreased by 47.8% and 65.2%, respectively, after 48 h compared with controls. After treatment with 5 μmol/l or 10 μmol/l berberine, the levels of MCP-1 in the supernatant were reduced by 33.6% and 63.2%, respectively, after 24 h, and were reduced by 42.3% and 68.4%, respectively, after 48 h, compared with controls. After treatment with 5 μmol/l or 10 μmol/l berberine, the levels of IL-6 in the supernatant reduced by 57.6% and 67.5%, respectively, after 24 h, and by 57.1% and 70.9%, respectively, after 48 h, compared with controls.
PPARγ inhibitor GW9662 could attenuate the effect of berberine on the expression of TNFα, MCP-1, and IL-6 mRNA in macrophages stimulated by AcLDL
It was found in our previous study that berberine could increase adipophilin, a target gene of PPAR-γ expression in macrophages. To investigate whether berberine affects the expression levels of TNFα, MCP-1, and IL-6 mRNA via activating PPAR-γ pathway, we pretreated the macrophages with GW9662 and than co-treated cells with berberine and AcLDL or rosiglitazone and AcLDL. Rosiglitazone is an agonist of a PPAR-γ and was used as a positive control in this study.
As shown in Fig. 3a, we found that the effect of rosiglitazone on the expression of TNFα, MCP-1, and IL-6 mRNA in AcLDL-stimulated macrophages was almost completely abolished by GW9662, whereas the effect of berberine was partially repressed by GW9662. The TNFα mRNA level was reduced by 50% after treatment with 10 μmol/l berberine for 24 h, whereas the TNFα mRNA expression level was reduced by only 18% in macrophages pretreated with 1 μmol/l GW9662. The TNFα mRNA expression level was reduced by 46% after treatment with 10 nmol/l rosiglitazone for 24 h, whereas the TNFα mRNA expression level was reduced by only 2% in macrophages pretreated with 1 μmol/l GW9662.
PPARγ inhibitor GW9662 reduces the effects of berberine on TNFα, MCP-1, and IL-6 mRNA expression levels. The macrophages were treated with 10 μmol/l berberine or 10 μmol/l rosiglitazone for 24 h in the presence of 50 μmol/l AcLDL, normal saline alone as control 1, 50 μmol/l AcLDL alone as control 2. The other two wells contained cells that had been pretreated with 1 μmol/l GW9662 for 30 min and then treated with 10 μmol/l berberine or 10 nmol/l rosiglitazone for 24 h. Cells were harvest to extract total RNA and the supernatant of cells were collected after treating 24. a TNFα, MCP-1, and IL-6 mRNA expression levels were measured by real-time RT-PCR and are shown relative to those of β-actin mRNA and b The levels of TNF-α, IL-6, and MCP-1 in the supernatant were detected by ELISA analysis. Values are mean ± SE of three independent experiments. Samples were measured in triplicate. *P < 0.05, **P < 0.01, compared with control 2
The MCP-1 mRNA expression level was reduced by 63.2% after treatment with 10 μmol/l berberine for 24 h, whereas the MCP-1 mRNA expression level was reduced by only 22.5% in macrophages pretreated with 1 μmol/l GW9662. The MCP-1 mRNA expression level was reduced by 65% after treatment with 10 nmol/l rosiglitazone for 24 h, whereas the MCP-1 mRNA expression level was reduced by only 2.5% in macrophages pretreated with 1 μmol/l GW9662.
The IL-6 mRNA expression level was reduced by 46.7% after treatment with 10 μmol/l berberine for 24 h, whereas the IL-6 mRNA expression level was reduced by only 22.2% in macrophages pretreated with 1 μmol/l GW9662. The IL-6 mRNA expression was reduced by 44.4% after treatment with 10 nmol/l rosiglitazone for 24 h, whereas the IL-6 mRNA expression was reduced by only 2.2% in macrophages pretreated with 1 μmol/l GW9662.
Also as shown in Fig. 3b, the levels of TNF-α, MCP-1, and IL-6 in the supernatant were significantly decreased after treated with berberine or rosiglitazone for 48 h. While pretreated with GW9662, the levels of TNF-α, MCP-1, and IL-6 in the supernatant were decreased after treated with berberine, but the extent of decrease were less than that of not pretreated with GW9662. As a positive control, pretreated with GW9662, the levels of TNF-α, MCP-1 and IL-6 in the supernatant were not decreased significantly after treated with rosiglitazone.
Discussion
The macrophages in arterial wall are mainly derived from circulation monocytes, which are play important role in the process of artherosclerosis. AcLDL is similar to oxidative LDL (OXLDL), which can induce macrophages to produce large amounts of proinflammatory cytokines [5, 6]. PMA can induce transformation of human monocytes to macrophages in vitro, which have many characteristics similar to the macrophages isolated from peritoneal in vivo [20]. Therefore, in the present study, we used the macrophages induced from THP-1 cell line to investigate the effects of berberine and AcLDL on production of proinflammatory cytokines.
Previously, studies have showed that berberine is an alkaloid and posses antiatherogenic effects in vitro and in vivo [7, 8], but this mechanism is remain incompletely understand. It was found in our previous study that berberine could increase adipophilin, a target gene of PPAR-γ expression in macrophages. In this study, we found that berberine could inhibit the expression and secretion of TNF-α, MCP-1, and IL-6 in AcLDL-stimulated macrophages. Inflammation of vessel wall due to dyslipidemia is important in the development of atherosclerosis, and TNFα, MCP-1, and IL-6 have important roles in this inflammatory process [5, 6]. TNFα is a proinflammatory cytokine that is produced by macrophages, lymphoid and vascular smooth muscle cells in the vessel wall and by monocytes and peripheral tissue macrophages in the blood [2, 3]. TNFα could facilitate vascular-smooth-muscle-cell proliferation and hasten macrophages death in vessel wall, stimulate synthesis of matrix proteases by vascular smooth muscle cells and macrophages in plaque. All these effects could facilitate development of atheromatous plaques to an advanced stage [21–25]. Brane et al. has shown that inhibition of TNF-α reduces atherosclerosis in apolipoprotein-E-knockout mice [12].
MCP-1 and its receptor CCR2 are key mediators in vascular inflammation, and is one of the most potent chemotactic agents to monocytes. MCP-1 has been shown to play a pivotal role in spontaneous atherosclerosis and post-angioplasty restenosis. Recently, it has been shown that blocking of the MCP-1/CCR2 pathway results in reduced atherosclerosis and restenosis by inhibition of monocyte adhesion to the vascular wall and reduces macrophage content in atherosclerotic lesion [26–28].
IL-6 is a major proinflammatory cytokine that is central to the inflammatory response, regulating the hepatic synthesis of acute-phase proteins such as fibrinogen, C-reactive protein, and albumin. IL-6 mRNA is present in atherosclerotic arteries at levels 10- to 40-fold higher than in non-atherosclerotic vessels and increased levels of IL-6 are associated with increased cardiovascular risk. Functional variations in the IL-6 gene may modify cardiovascular risk by affecting serum IL-6 levels and in some cases changing the structure of the IL-6 protein, which indicates that IL-6 has a role in the pathogenesis of atherosclerosis [13, 14, 29–31]. These effects of TNF-α, MCP-1, and IL-6 connect dyslipidemia and atherosclerosis, but berberine might block this link by inhibition of TNF-α, MCP-1, and IL-6 production in macrophages to prevent the atherosclerosis.
In present study, we have found that the effect of berberine on the expression and secretion of TNF-α, MCP-1, and IL-6 could be partially blocked by PPAR-γinhibitor. Recently reports showed that berberine could inhibit acetaldehyde-induced NF-κB activity through inhibition of IκB phosphorylation and degradation, resulting in the suppression of IL-1b and TNF-α production in acetaldehyde induced HepG2 cells [11]. It could not clear-out a causal correlation between the berberine’s effect of inhibiting NF-κB activity and suppression of IL-1b and TNF-α production in acetaldehyde-induced HepG2 cells in that study. We presumed that beberine implement its effect of inhibiting TNF-α, MCP-1, and IL-6 expression and secretion via multitarget and various pathway.
In addition, it was notable that functional response elements of nuclear factors AP1 and NFκB are found in the promoter region of the TNF-α gene [32]. PPAR-γ may reduce the level of TNF-α, MCP-1, and IL-6 expression in macrophages by inhibition of the activity of nuclear factors AP1 and NFκB [33–35]. Bong et al. found that berberbine can reduce the expression and secretion of TNF-α in 3T3-L1 cells [36]. In addition, berberine may also reduce plasma LDL levels [7], inhibit vascular-smooth-muscle-cell proliferation [8], improve insulin sensitivity and inhibit fat synthesis [36]. These effects are similar to those of PPARγ agonists and NFκB inhibitors. Based on the results, it is tempting to speculate that the effects of berberine in macrophages may be through activating the PPARγ pathway. Interestingly, the fact that GW9662 (PPARγ inhibitor) could attenuate the inhibitory effect of berberine on the expression levels of TNF-α, MCP-1, and IL-6 in AcLDL-stimulated macrophages in the present study, supporting that berberbine’s anti-inflammatory effects might, at least in part, be mediated through activation of PPARγ.
Conclusion
Berberine can inhibit the expression and production of TNF-α, MCP-1, and IL-6 in AcLDL-stimulated macrophages. These effects might be partially mediated through activation of the PPARγ pathway.
References
R. Ross, Am. Heart J. 138(5 Pt 2), S419–S420 (1999)
G.K. Hansson, N. Engl. J. Med. 352, 1685–1695 (2005)
P. Libby, Nature 420, 868–874 (2002)
A. Tedgui, Z. Mallat, Physiol. Rev. 86, 515–581 (2006)
D. Steinberg, J. Biol. Chem. 272, 20963–20966 (1997)
D. Steinberg, S. Parthasarathy, T.E. Carew et al., N. Engl. J. Med. 320, 915–924 (1989)
W. Kong, J. Wei, P. Abidi et al., Nat. Med. 10, 1344–1351 (2004)
S. Lee, H.J. Lim, H.Y. Park et al., Atherosclerosis 186, 29–37 (2006)
C.L. Kuo, C.W. Chi, T.Y. Liu, Cancer Lett. 203, 127–137 (2004)
K. Fukuda, Y. Hibiya, M. Mutoh et al., J. Ethnopharmacol. 66, 227–233 (1999)
C.Y. Hsiang, S.L. Wu, S.E. Cheng et al., J. Biomed. Sci. 12, 791–801 (2005)
L. Branen, L. Hovgaard, M. Nitulescu et al., Arterioscler. Thromb. Vasc. Biol. 24, 2137–2142 (2004)
Y. Seino, U. Ikeda, M. Ikeda et al., Cytokine 6, 87–91 (1994)
P.M. Ridker, N. Rifai, M.J. Stampfer et al., Circulation 101, 1767–1772 (2000)
S. Inoue, K. Egashira, W. Ni et al., Circulation 106, 2700–2706 (2002)
W. Ni, K. Egashira, S. Kitamoto et al., Circulation 103, 2096–2101 (2001)
J. Yin, R. Hu, M. Chen et al., Metabolism 51, 1439–1443 (2002)
Feng-Ling Chen, Zhi-hong Yang, Xuan-Chun Wang et al., J. Xinxiang Med. Coll. 24, 544–547 (2007)
I. Bildirici, C.R. Roh, W.T. Schaiff et al., J. Clin. Endocrinol. Metab. 88, 6056–6062 (2003)
G. Larigauderie, M.A. Bouhlel, C. Furman et al., Arterioscler. Thromb. Vasc. Biol. 24, 504–510 (2004)
Y.J. Geng, P. Libby, Arterioscler. Thromb. Vasc. Biol. 22, 1370–1380 (2002)
I. Tabas, Cell Death Differ. 11(Suppl 1), S12–S16 (2004)
Z.S. Galis, M. Muszynski, G.K. Sukhova et al., Circ. Res. 75, 181–189 (1994)
Z.S. Galis, M. Muszynski, G.K. Sukhova et al., Ann. N. Y. Acad. Sci. 748, 501–507 (1995)
L.S.M. Boestena, A.S.M. Zadelaar, A. van Nieuwkoop et al., Cardiovas. Res. 6, 179–185 (2005)
E.J. Leonard, A. Skeel, T. Yoshimura, Adv. Exp. Med. Biol. 305, 57–64 (1991)
K. HoonHan, R.K. Tangirala, S.R. Green et al., Arterioscler. Thromb. Vasc. Biol. 18, 1983–1991 (1998)
E. Mori, K. Komori, T. Yamaoka et al., Circulation 105, 2905–2910 (2002)
M. Romano, M. Sironi, C. Toniatti et al., Immunity 6, 315–325 (1997)
S. Morimoto, T. Nabata, E. Koh et al., J. Cardiovasc. Pharmacol. 17(Suppl. 2), S117–S118 (1991)
D.U. Lee, Y.J. Kang, M.K. Park et al., Life Sci. 73, 1401–1412 (2003)
S. Takashiba, L. Shapira, S. Amar et al., Gene 131, 307–308 (1993)
V. Pasceri, H.D. Wu, J.T. Willerson et al., Circulation 101, 235–238 (2000)
C. Jiang, A.T. Ting, B. Seed, Nature 391, 82–86 (1998)
N. Marx, G. Sukhova, C. Murphy et al., Am. J. Pathol. 153, 17–23 (1998)
B.H. Choi, I.S. Ahn, Y.H. Kim et al., Exp. Mol. Med. 38, 599–605 (2006)
Acknowledgments
This study has been funded by grants to RMH from the Key Project of National Science Foundation of China (30230380), the National Nature Science Foundation of China (39900072, 30670999, 30711120573), the High Tech Program (2002BA711A05 and 2001AA221201), the National Key Basic Research and Development Program (2002CB713703), the Shanghai Commission for Science and Technology (01JC14026), and by grant to Chen FL from Shanghai Commission for Science and Technology (07ZR14071) and from the National Nature Science Foundation of China (30870954). The authors also would like to thank Tang JF of Shanghai Institute of Endocrinology for ELISA technique in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, F.L., Yang, Z.H., Liu, Y. et al. Berberine inhibits the expression of TNFα, MCP-1, and IL-6 in AcLDL-stimulated macrophages through PPARγ pathway. Endocr 33, 331–337 (2008). https://doi.org/10.1007/s12020-008-9089-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-008-9089-3