Abstract
Deteriorated bone microarchitecture is a major health concern affecting millions worldwide, amounting to high mortality along with psychological, social, and economic burden. Hypoxia has been known to affect bone mineral metabolism in various in vitro and in vivo experiments in an inconclusive manner and only a few studies are available on natives or travelers of high altitude, pointing towards the deterioration of bone health. HIF proteins, fundamental to hypoxia signaling have also been shown to affect bone remodeling by mediating osteoblastogenesis and osteoclastogenesis but the underlying mechanism of this process is not clear. Most studies have been reported in men but only few in female, while it has been already established that estrogen plays a major role in protecting skeletal health and recent reports identify estrogen as a major player in determining bone quality in men as well. The tough terrain and lack of transport in these areas require optimal bone quality to be maintained for continuous locomotion and load-bearing capacity. The Wnt pathway is involved in load-induced bone formation and sclerostin; the inhibitor of this pathway has been reported to be regulated by both estrogen and HIF proteins. However, the hypobaric hypoxia-operated molecular mechanism regulating the bone quality and microarchitecture in both male and female is still not fully elucidated. Therefore, in this review, available literature on the bone health status under sustained hypoxic exposure focusing on the significance and crosstalk of HIF proteins, Wnt pathway, and estrogen are compiled and discussed to open new aspects of high-altitude bone health research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis, characterized by low bone mass and bone deterioration, is a major escalating health concern with about 200 million people being affected worldwide [1]. The local microenvironment is an important determinant of skeletal cell functions. A small change in inspired or delivered oxygen (O2) influences skeletal cell homeostasis, i.e., the balance between bone formation and bone resorption [2]. Pathological and environmental hypoxia has been reported to influence bone health [3,4,5,6]. Approximately 140 million people live at elevations equal to or above 2500 m defined as high altitude (HA) while another 40 million people are visiting HA every year for recreational and occupational purposes [7, 8]. A range of physiological adjustments are required for survival at HA due to extreme environmental conditions like cold, dehydration, solar radiation, malnutrition, and hypobaric hypoxia [9]. With the increase in altitude, there is a decrease in barometric pressure resulting in fewer O2 molecules in the inspired air and subsequently organ tissues, causing hypoxia [10]. Fortunately, acclimatization allows partial compensation for lowered O2 levels and the body responds to hypobaric hypoxia via. molecular, cellular, and systemic adaptations across cardiovascular, respiratory, musculoskeletal, hormonal, reproductive, and metabolic organ systems [11,12,13,14,15,16,17]. At the molecular level, adaptation to hypoxia is driven majorly by the hypoxia-inducible factor (HIF) family of transcription factors [18]. Skeletal cells also respond to hypoxia via hypoxia signaling pathway consisting of HIF proteins and reports have highlighted the importance of HIF proteins in regulating several steps during growth, development, and repair in all skeletal cell types viz. chondrocytes, osteoblasts, osteocytes, and osteoclasts [19].
Hypoxia has also been reported to alter various pathways like vascular endothelial growth factor (VEGF) bone morphogenetic proteins (BMP) and Wnt pathway involved in the maintenance of bone mineral homeostasis [20, 21]. Bone health parameters like vitamin D (Vit D), calcium, phosphorus, alkaline phosphatases (ALP), and hormones like sex steroid hormones, intact parathyroid hormone (iPTH), calcitonin, etc. are also affected by hypoxic exposure [22,23,24]. The great Himalayas are a major geographical feature of the Indian sub-continent and a large population inhabits these altitudes. Almost no study has reported the epidemiology of osteoporosis among natives of these altitudes, but two studies have reported osteoporosis and a significant percentage of osteopenia after exposure to the HA environment [22, 24]. Bone microarchitecture and bone mineral density (BMD) have a profound effect on bone strength and load-carrying capacity, [25] required for continuous movement on tough terrains of HA [26]. Literature exploring the field of bone health at HA is scanty and most of the available studies have been reported in males. Females constitute nearly half the demography of any geographical area and also a large number of women are visiting these altitudes nowadays for professional and recreational purposes. Participation of women in the Indian army, particularly posted at HA has also increased over the last decade. We are committed to studying the physiology of Indian soldiers posted at HA and stress like hypobaric hypoxia has been reported to deteriorate bone health at these altitudes. We have, therefore, reviewed available literature describing the role of sustained hypoxia in regulating bone health in relation to Wnt pathway and estrogen at HA.
Bone Mineral Metabolism and Incidences of Osteoporosis in India
Bone is often misunderstood as merely an organ that supports the body structurally, but it has many other functions like protecting our internal organs from injury, enabling us to move, serving as a reservoir of minerals like phosphorus and calcium, and providing a suitable environment for hematopoiesis [27,28,29]. Most bones grow in length through endochondral ossification where primary spongiosa is created by bone-eating osteoclasts which mediate resorption of calcified cartilage, finally replaced by bone-forming osteoblasts which then deposit the bone matrix [30, 31]. Despite its static and rigid impression, bone is continuously remodeled where old bone tissue is digested by osteoclasts, and new bone tissue is laid down by osteoblasts in a tightly coordinated manner to ensure same rate of resorption and formation, thus maintaining skeletal integrity [27]. Bone remodeling allows for the adaptation of the skeleton to meet changing mechanical needs and homeostasis of minerals like phosphorus and calcium [28]. Bone remodeling is regulated by systemic regulators, including hormones (e.g., parathyroid, calcitonin, growth, and sex hormones), glucocorticoids, prostaglandins, and local regulators including many cytokines and growth factors [27, 32,33,34,35,36,37]. Slight alterations in the optimal values of the above mentioned regulators may disturb the bone homeostasis leading to sub-optimal quality. WHO has stratified the following definitions of 4 categories of bone health based on standard deviations (SDs) below the mean peak bone mass of young healthy adults as determined by DEXA, with BMD at the femoral neck as the reference site: Normal: a BMD value of 1 SD below, Osteopenia: a BMD value between 1 and 2.5 SD below, Osteoporosis: a BMD value > 2.5 SD below, and Severe (established) Osteoporosis: a BMD value > 2.5 SD below with one or more fragility fractures [38, 39].
The prevalence of osteoporosis is becoming a major public health issue due to the universal increase in life expectancy; in particular, more rapidly in the developing countries of Asia, South America, and Africa [38]. In India, there are around 50 million individuals with osteoporosis or osteopenia [40, 41]. The Indian Council for Medical Research (ICMR) in its attempt to generate an India-specific database showed that Indians have lower BMD than their North American counterparts [42]. Several factors like a predominant vegetarian diet containing high amounts of phytates, oxalates, and fiber, low consumption of milk and milk products, negligible intake of calcium and Vit D supplements, reduced sunlight exposure, lack of physical activity, increased glucocorticoid consumption, genetic differences, and poor level of awareness contribute to osteoporosis in India [43, 44]. A study reported a high prevalence of osteopenia (52%) and osteoporosis (29%) among Indian women aged 30–60 years of low‑income groups which may be attributed to inadequate nutrition [45]. Another study observed that osteoporosis at the spine and hip when computed using the Hologic database was present in 42.7% and 11.4% of subjects respectively but only in 27.7% and 8.3% of subjects using the ICMR database [46]. The decrease in bone mass and microarchitectural deterioration of bone tissue also result in an increased risk of fracture and therefore almost 40% of women and 20% of men with osteoporosis have been reported to have at least one osteoporotic fracture during their lifespan [47, 48]. Reports suggest that more than 4.5 million women in India above the age of 60 years have fractured spine and approximately 0.25 million people sustain a hip fracture every year due to osteoporosis [49]. The annual incidence rate of hip fractures above the age of 55 years has been reported to be 163 and 121 per 100,000 people per year for women and men, respectively [44]. Initially, it was thought that women are more predisposed to osteoporosis, but reports have indicated it to be a major health concern among males as well [50,51,52]. A significant percentage of men in various studies showed compromised bone quality [44, 53, 54]. A study even reported earlier age of fracture in men as compared to women in north India [54].
Bone Health Status at HA
To understand the effect of hypobaric hypoxia on bone health, it is important to look at the bone health status of natives of HA, living in these regions for generations and therefore, may represent long-term effect of hypobaric hypoxia exposure on skeletal health. High-altitude natives (HANs) have been reported to have short stature, fracture-prone thin metacarpals, and enlarged medullary cavity in long bones [55]. An enlarged medullary cavity could be an adaptation for increased erythropoiesis in order to compensate for low O2 [55]. A study showed that Vit D was very low, and bone-related diseases were significantly higher in Tibet [56]. Another study which compared ALP activity in dwellers of moderately HA at 1495 m and low altitude of 250 m in Meghalaya, India, reported increased ALP activity among HANs which may be due to the elevated rate of bone turnover at these altitudes [57].
Few short-term hypobaric hypoxia exposure studies have also been reported where the bone health of the participants was evaluated. When BMD was assessed in 24 members of the Himalayan expedition party who stayed at 3700 m for 60 days and 5400 m for 37 days, a decrease in BMD of the radius was observed which recovered partially in 12 months post expedition [3]. The Indian armed force composed of healthy males who stayed at 3450 m for about 4 months demonstrated a decrease in bone health measured by speed of sound (SOS) values and T scores of the radius, metatarsal, and phalanx, with a higher percentage of subjects having osteopenia and osteoporosis compared to their counterparts at sea level [22]. Another study by the same authors at extreme HA of 5400–6700 m reported similar results and 62% of these subjects had osteopenia while 2.8% had osteoporosis after de-induction [24]. Likewise, a group of five healthy, active male adults, who participated in an expedition of 24 weeks at 2500 m in Antarctica, showed a decrease in BMD-spine [58]. Another group that studied bone metabolism in middle-aged and older mountaineers and compared them with those who walk regularly and those who do not exercise regularly showed no significant difference in osteo sono-assessment index of the right calcaneus as an indicator of bone strength among the three groups [59]. However, it is equally interesting that the study on Ladakhi soldiers, native to altitudes greater than 3500 m showed decreased bone mineral content after a stay of 44 days at sea level [60]. In summary, several authors have shown bone health to be deteriorating at HA.
Changes in Bone Remodeling Factors at HA
Residency at HA changes different hormonal, biochemical, and morphological indicators of bone remodeling. A significant decrease in plasma ionized calcium and phosphate levels with fluctuating PTH concentration was reported on exposure to HA of 4424 m for 5 days [61]. Similarly decreased serum calcium, calcitonin, 25-Vit D, C-PTH with increased serum and urinary phosphorus along with urinary calcium levels was reported post-Himalayan expedition at HA of 3700 m for 60 days and extreme altitude of 5400 m for 37 days [3]. Bone formation and resorption markers were measured in healthy individuals after a stay at different high to extreme altitudes [22, 24] and it was observed that 40 weeks stay at an extreme altitude of 5400–6700 m led to decreased bone health associated with decreased bone formation markers viz. ALP, bone-specific alkaline phosphatase (BAP), and calcitonin indicating decreased bone formation [24]. Calcium levels were maintained at extreme altitudes [24]. At HA, BAP, urinary deoxypyridinole to creatine ratio (DPD/Cr), C-terminal collagen propeptide (CICP), collagen type 1 cross-linked N-telopeptide (NTX) were reported to be lower than sea level reflecting a lower bone turnover but ALP and calcium levels increased [22]. Serum 25-Vit D showed significant reduction at both extreme and HA [22, 24]. 2-week expedition at 3200–3616 m caused a significant decrease in 25-Vit D levels with a significant increase osteoprotegerin (OPG) levels but soluble receptor activator of NF-κB ligand (sRANKL) levels showed no changes after the expedition [62]. A significant decrease in the 25-Vit D concentration in the alpinist climbers after their return from the mountaineering expedition with no changes in serum PTH and calcium was also reported [63]. iPTH is a regulator of calcium metabolism and aids the production of osteoclast which speeds up bone resorption, releasing calcium and other minerals into circulation [64, 65]. Plasma osteopontin (OPN) levels were reduced after exposure at 3500 m for 1 day in healthy adults [66]. One group reported that 10 to 20 years of mountaineering lead to non-significant changes in BAP, procollagen type 1 amino-terminal propeptide (P1NP) and bone formation/resorption ratio in male and female mountaineers with significantly higher tartrate-resistant acid phosphastase-5b (TRACP-5b) levels [59]. Table 1 summarizes studies done on travelers of HA to understand the effect of hypobaric hypoxia on bone metabolism. Studies report significant changes in biochemical and endocrinal factors related to bone mineral metabolism. However, it is difficult to conclude the effect of HA on bone health based on these factors and more studies are required in the field to come to conclusion.
Effect of Hypoxia on Bone Remodeling Parameters In Vitro and In Vivo
To understand the effect of hypobaric hypoxia on bone mineral homeostasis we looked into the existing literature on the gene and protein expression of various markers of bone remodeling under the effect of sustained chronic hypoxia during osteogenic differentiation. Runt-related transcription factor 2 (RUNX2) is a transcription factor that regulates the expression of osteogenic differentiation-related genes like ALP, type I collagen (COL1A1), OPN, and osteocalcin (OCN) [67, 68]. While most studies showed a decrease in RUNX2 gene and protein expression when exposed to 1–2% hypoxia for 5–21 days [69,70,71,72,73,74,75,76,77,78,79], results are not conclusive as expression was maintained in few studies and even increased after 2–5 days of exposure to chronic hypoxia at 1.5–5% O2 [72, 80,81,82]. Protocols involving 1–2% O2 for 5–21 days reported a decrease in ALP gene expression [73, 74, 76], while 2–5% O2 for 3–14 days showed increased expression [67, 72, 80, 83]. ALP protein expression was also found to be increased when less severe hypoxia of > 2% O2 for 2–21 days was applied [84,85,86,87] while ALP activity was decreased after severe hypoxic exposure of 1–2% O2 for 2–28 days [68,69,70, 73, 75,76,77,78, 88]. Col1A1 also showed contradictory results as the expression of the Col1A1 gene was unaltered at 2 and 5% O2; it decreased after exposure to 2% O2 for 7, 14, or 21 days, and increased with 2% O2 for 12 days and 3% O2 for 3 days with a decrease after 9 days, indicating a decreased osteogenic potential [67, 71,72,73, 76, 83, 89, 90]. A lower gene expression of OPN was observed following chronic hypoxic exposure of 1–2% O2 for 21 days, but expression was maintained at 5% [70, 74, 78, 91,92,93]. Lower expression of the OCN gene was reported when severe hypoxic exposure of 5–28 days were applied while four studies showed increased expression with a moderate dose of > 2% O2 for a short exposure time of 3–12 days [67, 72, 74, 76, 80, 83, 91,92,93,94]. Further, when calcium depositions were investigated, no change in calcium deposition was found after exposure to 1–5% O2 for 14–21 days, while a severe dose of < 2% O2 resulted in decreased calcium deposits [67, 70, 74, 76, 88, 91, 93, 95,96,97]. Table 2 summarizes various in vitro studies included in reviewing the effect of sustained hypoxia on various bone parameters. Based on the above discussions, it may be concluded that chronic hypoxic exposure of 1–2% O2 with extended exposure time resulted in bone resorption. The results of in vitro studies on osteogenic differentiation are difficult to conclude as there are differences in observations which may have resulted due to differences in the amount and duration of hypoxia used in the experiments. Also, the different cell lines used may add to variations observed.
The response of cultured cell lines to normobaric hypoxia may not give full insight into the physiological response to hypoxia especially hypobaric hypoxia because at the systemic level, the body tries to compensate for low O2 availability by physiological adjustments like increased erythropoiesis, heart rate, and ventilation eventually reaching sufficient O2 at the tissue level. Therefore, we looked into various in vivo studies to evaluate the effect of systemic hypoxia on bone health in mice and rat models. Table 3 summarizes various animal studies on rat or mouse models included in this review. Healthy rats exposed to simulated hypoxia at 6000 m for 3 weeks, showed a decrement in the bone volume to tissue volume ratio (BV/TV), tubercular number (Tb.N), and BMD-total [98]. In another study where rats were exposed to hypobaric hypoxia equivalent to 4100 m for varied periods of 2, 4, 6, or 8 months, a decrease in femur length, femur dry weight, cortical area, load fracture, bone stiffness, and second moment of inertia of cortical bone (CSMI) were observed [99]. Similar results of reduced bone mineral content, bone strength, and elasticity were observed in other studies of stimulated hypobaric hypoxia exposure at 2000–5500 m [100,101,102,103,104]. Alterations in genetic and protein expression of various biomarkers were also reported in rats and mice after hypobaric hypoxia exposure. Rats exposed to 4000 m for 3 weeks showed a decrease in RUNX2 and OCN protein levels while no change in levels of ALP was observed which may be due to exposure to the lesser altitude as other parameters of bone strength like BMD, bone volume (BV), BV/TV, trabecular thickness (Tb.Th), and Tb.N did not alter the experiment [105]. In another study where mice were exposed to 19,000 m for 4 days, no change was observed in BV/TV, but increased numbers of osteoclasts along with decreased numbers of osteoblasts and colony-forming unit (CFU) were reported [106]. Overall major in vivo studies are pointing towards deteriorated bone quality when animals were exposed to stimulated hypobaric hypoxia.
Oxygen Sensing in Bone
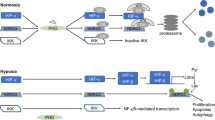
Bone has a highly hypoxic microenvironment with partial pressure of oxygen (pO2) ranging from < 1% in highly hypoxic regions to 6% in sinusoidal cavities [107]. Hypoxia in bone is likely a result of successive drops in velocity and oxygenation in sinusoidal blood and enormous O2 consumption by hematopoietic cells [108,109,110]. Prolyl hydroxylase (PHD) enzymes (PHD1, PHD2, and PHD3) are the primary cellular oxygen sensors [111]. Along with HIF proteins, PHD enzymes are fundamental for any cell to adapt and survive in hypoxic situation. In well-oxygenated tissues, proline residues of HIF-α are hydroxylated by PHD which provides a binding site for von Hippel–Lindau tumor-suppressor protein (VHL), initiating proteolytic degradation of HIF-α. When O2 levels are limited, PHD-dependent hydroxylation is reduced allowing HIF-α to dimerize with HIF-β and binds to hypoxia response elements (HRE) in the nucleus [112, 113]. HIF signaling influences cell and tissue functions at multiple levels to reverse the decreased O2 tension by stimulating angiogenesis and manipulating cellular metabolism [114]. HIF signaling induces transcription of various genes of bone remodeling such as VEGF production in osteogenic cells attracts blood vessels for the supply of oxygen, nutrient, osteogenic factors, and possibly osteoprogenitors, and thereby stimulating bone formation [114]. HIF-signaling also drives glycolysis in osteoprogenitor cells and controls bone homeostasis by regulating EPO, RUNX2, OPN, OCN, ALP, and sclerostin (SOST) secretion in bone cells [114].
Bone Remodeling Under Hypoxia Condition
Bone remodeling maintains the skeletal integrity by maintaining a balance between bone formation and bone resorption [19]. Osteoblasts are bone-forming cells and the process of maturation of mesenchymal stem cells into mature osteoblasts is known as osteoblastogenesis [115]. Osteocytes are formed when osteoblasts get buried in the bone matrix and differentiate to develop a dendritic process that extends into the bone marrow and bone surface for the transfer of nutrients and gases as well as communication [116]. Osteoclasts are bone-degrading skeletal cells and formation of mature osteoclasts from monocytes is known as osteoclastogenesis [117]. At puberty when the body is trying to reach its peak bone mass there is an increase in bone formation improving bone mass and quality while there is an increase in bone resorption in old age deteriorating bone [118, 119]. The smallest changes in the bone microenvironment such as hypoxia can disturb this homeostasis between osteoblastogenesis and osteoclastogenesis which is a dynamic process known as bone remodeling [2].
Hypoxia and Bone Formation
Hypoxia has been reported to limit bone formation by delaying osteoblast growth differentiation and bone mineralization [120,121,122]. During an in vitro experiment, the formation of mineralized bone nodules decreased by 11-fold following exposure to 2% O2 while it almost stopped at 0.2% O2. This effect was partly due to decreased osteoblast proliferation [123]. Hypoxia is also known to reduce ALP activity, and OPN, OCN, and RUNX2 expression in osteoblast cell lines [124, 125]. In ovariectomized rats, short-term hypoxic exposure was shown to suppress osteoblastogenesis [126] which may be linked to reduced RUNX2 expression, which in turn reduced the differentiation of mesenchymal cells to immature osteoblasts [127,128,129,130]. Limited expression and activity of ALP as well as collagen in hypoxic environments resulted in the inhibition of osteoblast proliferation. Also, the collagen produced had a lower amount of hydroxyl residue leading to reduced matrix mineralization [123]. Inhibition of osteoblast function by hypoxia could also be the result of reduced activity of proline hydrolases and/or lysyl oxidase which are O2-dependent enzymes [123, 131] and required for post-translational modification of collagen [123, 132]. Hypoxia has also been reported to increased SRY box 9 (SOX9) levels, a negative regulator of osteoblastogenesis through decrease in RUNX2 levels [71, 80]. Hypoxia-mediated apoptosis due to mitochondrial dysfunction and mitophagy may be another reason of bone loss upon exposure [133]. The osteogenic differentiation and expression of VEGF reached its peak after 3 days of hypoxia implying that the duration of hypoxia is an important factor for the differentiation of precursor cells [134].
HIF-1α overexpression significantly stimulated osteoblast cell viability, which was suppressed by silencing HIF-1α and led to reduced expression of osteogenic markers, including RUNX2, ALP, and OCN [135]. Mice overexpressing HIF‐1α showed increased vascularity and bone formation, whereas mice lacking HIF‐1α had reduced repair efficiency [136]. The improved bone quality in ovariectomized mice after PHD inhibition could be linked to increased number of osteoblasts in mice overexpressing HIF‐1α compared to controls [137, 138]. HIF‐1α-driven bone formation may act via upregulation of glycolytic activity as VHL deletion in osteoblasts boosted cellular glycolysis [139, 140]. Hypoxic exposure may therefore increase HIF‐1α-induced VEGF transcription, improving local vascularization which in turn might increase nutrient availability for increased cellular glycolysis and activation of target genes in progenitor cells finally enhancing bone formation through osteogenic–angiogenic coupling [20, 136, 138,139,140,141,142]. The osteoanabolic response of HIF‐1α stimulation could also be the result of osteogenic‐angiogenesis through VEGF, aiding in reaching sufficient O2 levels. VEGF may not be able to yield the same anabolic response in the absence of O2 in in vitro systems [123]. HIF‐2α which was initially thought to have no impact on osteoblast has also been implicated in osteoblastogenesis [142,143,144,145]. Reduced HIF‐2α expression promoted osteoblast differentiation leading to increased bone mass [143, 144]. HIF-2 α promoted SOX9 through a reduction in the expressions of RUNX2 and SP7 which are osteoblast differentiation mediators [144, 146]. In hypoxic environment with increased HIF (both 1α and 2α) stabilization, there is an increase in TWIST expression leading to reduction in RUNX2 and OCN expressions resulting in decreased osteoblast mineralization and bone mass [127,128,129,130, 143, 147]. Major studies have reported hypoxia to be inhibiting osteoblast number and function in in vitro systems however, HIF-1α has been reported to increase osteoblastogenesis through osteoanabolic response of VEGF in in vivo models. At the same time, two new studies have reported HIF-2α impairing osteoblastogenesis.
Hypoxia and Bone Resorption
Hypoxia was shown to be a major stimulator of osteoclast formation and bone resorption as a fourfold increase in osteoclast number and 21-fold increase in its activity was reported after exposure to 2% O2 [123, 148,149,150]. A reduction in O2 level to 2% increased the mean area of osteoclasts nearly 8-folds and exhibited a 13-fold increase in resorption lacunae in bone slices [151]. In another study, 2% O2 exposure resulted in tenfold increase in resorption pit formation [149]. However, some studies also reported inhibition of osteoclast number and activity upon exposure to severe hypoxia which they attributed to extensive cell death following constant hypoxic exposure as reoxygenation after 2–3 days promoted osteoclastogenesis but a recent report also reports reduced osteoclast formation and function without affecting cell viability upon constant exposure of 1% O2 [150, 152]. Although hypoxia has been reported to increase osteoclast number and activity, hypoxia was also shown to suppress OPG, preventing RANK‐induced osteoclast formation and function [126, 142,143,144, 152,153,154,155,156]. Osteoclasts require high amount of ATP for their bone-resorbing function, and therefore, excessive accumulation of mitochondrial ROS during low oxygen tension for longer periods may promote apoptosis and limit bone resorption [157]. A significant decrease in bone calcium content with increased osteoclast differentiation after exposure to low oxygen levels has been reported in rainbow trouts which is consistent with the report of a fivefold increase in osteoclast-mediated calcium release after exposure to 2% O2 in a 3-day organ culture of mouse calvarial bones [148, 158].
Knowles and Athanasou, for the first time, showed that HIF‐1α siRNA nullified the hypoxic increase in bone resorption [150]. HIF-1α not only enhanced osteoclast-related RUNX2 and ALP gene as well as protein expression but also the number of TRAP-positive osteoclasts [159]. Shirakura et al. demonstrated that HIF‐1α knockdown increased OPG expression under hypoxic conditions [156]. Heterozygous deletion of PHD2 increased expression of proresorptive genes in bone marrow cells, resulting in 3.7‐fold higher resorption as compared to wild type [153]. Similarly, in vivo depletion of PHD2 led to increased bone resorption, reducing BV/TV and Tb.N, increased Tb.Sp suggesting the role of PHD2 and HIF‐1α in hypoxic-induced bone resorption [153]. Stimulation of proresorptive genes and glycolytic activity seems to be the mechanism by which HIF‐1α affects osteoclast function as knockdown of HIF-1α suppressed hypoxia-mediated glycolysis and acid secretion for bone resorption [160,161,162]. It is well established that HIF‐1α induces an increase in osteoclast activity but its role in osteoclastogenesis needs more understanding. Digoxin, an inhibitor of HIF-1α, has been reported to attenuate osteoclastogenesis through downregulation of RANK/RANKL signaling [163]. Several reports have similarly noted increased osteoclast differentiation following HIF‐1α stimulation [6, 164, 165], while a study noted that HIF1-α inactivation through siRNA accelerated osteoclast cell fusion and HIF‐1α induction moderately inhibited its differentiation [153]. In few reports, HIF‐1α induction caused a small decrease in osteoclast numbers, which authors reasoned to the longer reoxygenation time compared to other studies [148,149,150,151, 153]. Homozygous knockdown of PHD3 in animal models increased osteoclast formation, which was associated with increased expression of the differentiation marker NFATC1, which however, did not change the final number of osteoclasts formed but increased the final resorption activity [153, 166].
Initially, it was thought HIF‐2α had no role in osteoclastic resorption as silencing of HIF‐2α in monocyte‐derived osteoclasts had no effect on the hypoxia-induced resorption [150, 167, 168]. However, a recent study reported that HIF‐2α was capable of stimulating and accelerating osteoclastogenesis in M-CSF- and RANKL-treated osteoclasts [143]. The overexpression of HIF‐2α resulted in increased osteoclast formation with large cytoplasmic compartments, suggesting its role in osteoclast maturation, similar to HIF‐1α [143, 153]. Similarly, inhibition of HIF‐2α led to reduced number of nuclei and expression of osteoclast‐related genes finally reducing osteoclast differentiation. TRAF6 which is an adapter of the RANK gene may have role in HIF‐2α mediated osteoclastogenesis [143]. TRAF6 activates NFATC1 and promotes osteoclastogenesis [143, 169, 170]. HIF‐2α overexpression further enhanced TRAF6 expression in RANKL-mediated osteoclast differentiation. Inhibition of HIF‐2α lead to inhibition of TRAF6 and induced increase in osteoclast formation and differentiation [143]. Therefore, HIF‐2α may increase bone resorption by increased expression of osteoclast activity genes like TRAP and NFATC1 mediated through increased osteoclast number associated with HIF‐2α stabilization [143]. It is difficult to conclude the role of hypoxia on osteoclast as contradictory reports have emerged in various studies. Amount and duration of hypoxia may be playing role in determining whether hypoxia stimulates osteoclast growth and function or promote apoptosis through cell death. However major studies report hypoxia through HIF (1α and 2α) to stimulate osteoclast number and function.
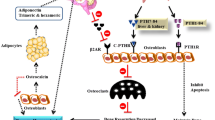
Crosstalk Between Osteoclasts and Osteoblasts
Osteoclastogenic response to hypoxia may be mediated through both independent cell action as well as osteoblast–osteoclast crosstalk as osteoclast requires RANKL signals from osteoblasts for their proliferation, differentiation, and activity [166, 171,172,173]. Adenosine triphosphate (ATP) released from osteoblast in response to low oxygen tension was shown to inhibit bone formation while it stimulated bone resorption via the P2Y1 receptor and therefore could affect the balance between formation and resorption of bone through local purinergic signaling [174, 175]. In presence of osteoblasts, the PHD inhibitor, FG-4592’s ability to inhibit osteoclastogenesis was improved but bone resorption activity was reduced [166]. HIF‐2α was also reported to be an important mediator of osteoblast-osteoclast crosstalk as osteoclast‐specific loss of HIF‐2α solely affected osteoclasts, whereas osteoblast‐specific loss of HIF‐2α affected both osteoblasts and osteoclasts [143]. HIF‐2α seems to bind to RANKL promoter directly to increase osteoclast differentiation [143, 176] while the HIF target gene, OPG and its intermediary, interleukin 33, may be responsible for osteoblast-mediated inhibition of osteoclastogenesis [142, 144, 154, 155]. Though there are evidences of hypoxia‐induced increase in osteoclast number to be the result of osteoblast–osteoclast crosstalk [126, 156, 173] but hypoxia in absence of osteoblast was also able to demonstrate increased differentiation of osteoclasts. Therefore, exploring downstream signaling might improve our understanding of this crosstalk [148, 149].
Hypoxia and Osteocytes
Osteocytes contribute significantly to bone formation through β-catenin which is required for the osteoanabolic effect of mechanical loading and constitutively expressing β-catenin in osteocytes has been reported to increase bone mass in rat models [177, 178]. The disruption of the lacuno-canalicular network formed by osteocytes, necessary for nutrient and gaseous exchange, resulted in localized hypoxia in bone [179, 180]. Hypoxia caused by mechanical unloading has been reported to induce osteocytic apoptosis [181, 182].
One percent O2 induced hypoxia after 16 h and up to 72 h. It significantly reduced osteocyte number at 8 and 48 h, induced cell death at 8, 24, and 48 h and induced apoptosis at 16, 24, and 48 h [183]. Acute oxygen deprivation at 2% O2 for 4–12 h resulted in 2.1- to 3.7-fold upregulation of HIF-1a protein expression in MLO-Y4 osteocyte-like cells compared to cells cultured in normoxia [184]. Osteocyte cells exposed to hypoxia simulated by 100 μmol/L CoCl2 or 2% O2 stably expressed HIF-1α proteins and upregulated the expression of RANKL at both gene and protein levels [185]. Also, knockdown of HIF-1α in osteocyte cell lines downregulated the expression of RANKL [185]. Osteocyte-like cells exposed to hypoxia augmented secretion of chemotactic factors [186] and GDF15 to promote osteoclastogenesis [180] and influenced osteoblast-to-osteocyte transition [73]. Hypoxic osteocytes have been reported to increase their expression of OPN, a potent osteoclast chemotaxant, which increases the migration and attachment of osteoclasts [187].
Hypoxia with increased HIF-1α expression was reported in mice osteocytes following mechanical unloading and disuse [184, 187, 188] and mice lacking HIF-1 in osteocytes reported increased bone formation when subjected to tibia loading as a result of increased osteoblast activity [188]. Conditional knockout of PHD2 in osteocytes resulted in high bone mass phenotype and blunted osteoporotic bone loss induced by estrogen deficiency or mechanical unloading by enhancing bone modeling in young and adult mice while reducing bone remodeling in aged mice [189]. Mice conditionally lacking Von Hippel–Lindau (VHL) protein in osteocytes (10-kb Dmp1-Cre) exhibited a high-bone mass phenotype [190]. Osteocytic HIF-1α not contributing to skeletal development has also been reported in one study where Dmp1-Cre; Hif1af/f mice were phenotypically indistinguishable from Cre-negative control mice, with insignificant differences in trabecular bone volume fraction, cortical area fraction, or bone formation rate suggesting that osteocytes utilize an alternate HIF-α isoform, or HIF-independent signaling, to influence the skeleton [190]. Reduced expression of HIF-1α has also been reported to be involved in osteocyte-mediated osteoclastogenesis [191]. Osteocytes play a role in communication among cells in bone and reports indicate hypoxia to inhibit osteocyte growth and differentions and promote osteoclast-mediated resorption but osteocytic HIF-1α seems to increase bone mass through osteo-angiogenesis coupling in osteoblasts.
Role of Wnt Pathway in Bone Mineral Metabolism
A variety of signaling pathways like Hedgehog (Hh), Wnt, BMP, and Notch pathway have been shown to function at specific and/or multiple stages in the differentiation cascade of osteoblasts and osteoclasts [20, 192, 193]. HA inhabitants and soldiers posted at these altitudes are exposed to a greater extent of external loads because of tough terrain and scanty transportation facilities [26]. Induction of Wnt signaling leads to load-induced bone formation in response to external loads [194,195,196]. Wnt family proteins are abundantly expressed in skeletal tissue and regulate endochondral ossification through both canonical and non-canonical pathways [197]. In the canonical Wnt pathway, Wnt proteins bind to Frizzled receptors and the Lrp5/6, which leads to the stabilization of beta-catenin (β-catenin) and its translocation to the nucleus to activate the transcription of target genes [198,199,200]. Regulation of Wnt signaling occurs via secreted decoy receptors or antagonists like sclerostin and DKK that bind to Lrp4-6 to prevent Wnt-Lrp interaction and signal transduction [196, 201]. Loss-of-function mutations in Lrp5 cause low bone mass condition: osteoporosis pseudoglioma (OPPG) and missense mutations lead to high bone mass phenotype [202, 203]. Activating mutations in Lrp4-6 promoted high bone mass phenotypes while complimentary phenotypes emerged from the deletion of Lrp4/5/6 [177, 178, 188, 204,205,206,207,208,209,210]. Wnt/β-catenin signaling plays a crucial role in the osteogenic differentiation and maturation of osteoprogenitor cells by promoting RUNX2 expression [211, 212]. It has also been demonstrated that the activation of Wnt signaling pathway inhibits bone resorption by increasing the expression of OPG from osteoblasts and decreasing the secretion of RANKL, thus altering OPG and RANKL ratio opposing osteoclast formation [213]. In non-canonical pathway, G protein-coupled phosphatidylinositol and PKCD activation are involved in the Wnt-mediated osteoblast differentiation [214] while Wnt5a in the non-canonical pathway induces in vitro osteoblastogenesis and Wnt-5α+/− mice show bone loss presumably because of less osteoblast to adipocyte number ratio [215].
Interaction of Wnt Pathway with HIF
Several in vivo studies in rats and mice have reported a decrease in bone strength parameters viz. maximum load, ultimate load, structural stiffness, load at fracture, load at yielding, and elastic modulus after exposure to hypobaric hypoxia [98,99,100,101,102, 104]. In vivo and in vitro evidences reveal functional crosstalks between Wnt and HIF signaling. HIF-1α was reported to inhibit osteoblast proliferation by inhibiting Wnt pathway through downregulation of β-catenin, cyclin D1, and c-Myc [207]. Active β-catenin in osteocytes is essential for osteoanabolic effect of mechanical loading and increases both cortical and trabecular bone mass [216, 217]. Conditional knockout studies using Bglap-Cre, HIF-1αKO mice displayed increased load-induced bone formation through β-catenin and Bglap-Cre; VHLcKO mice showed attenuated osteocyte differentiation where both Osterix, the osteoblast-specific transcription factor, and HIF-1α cooperatively reduced Wnt signaling [79, 218]. Contradictory reports have also indicated that hypoxia or hypoxia mimetics increased Wnt signaling in bone [219, 220]. Two recent reports demonstrated a relationship between hypoxia and Wnt/β-catenin signaling in osteocytes: VHL and PHD2 deletion in osteocytes decreased sclerostin and increased canonical Wnt signaling leading to increased bone mass [36, 221]. Activation of HIF signaling in osteocytes epigenetically repressed sclerostin expression resulting in high bone mass phenotype caused by increased bone formation and decreased bone resorption [21, 207, 210]. Hypoxia was reported to decrease sclerostin levels and increase Wnt signaling while a contradictory report of increased sclerostin levels by 62% when compared to the normoxia group has also been reported in cell culture studies [208, 222].
Role of Estrogen in Bone Mineral Metabolism
Reports have associated exposure to HA with disturbance in steroidogenesis which was more prominent during early exposure [223]. Higher levels of augmented nitric oxide (eNOS) which activated estrogen in both young and middle-aged Ladakhi women was reported and similarly increased levels of circulating estrone and 17β-estradiol as a result of greater aromatase activity were reported during HA residency [224,225,226]. The same study also reported increased mRNA levels of estrogen receptors at HA [226]. It is well established that estrogen protected women from bone loss and post-menopausal women undergo more bone resorption than formation leading to bone loss in old age [227]. An interventional study had reported that estrogen accounted for more than 70% of the effect of sex steroid hormone on bone resorption in men and studies since then have reported that bioavailable estrogen which maintains cortical bone constituting nearly 80% of bone mass, is more closely associated with bone density than bioavailable testosterone in both male and female [52, 228,229,230,231,232]. Estrogen has been reported to decrease the number, activity, and apoptosis of osteoclasts leading to decreased bone resorption while increase osteoblast proliferation, differentiation, and activity which is therefore reduced estrogen levels at old age leads to loss of bone formation [233,234,235,236]. Though contradictory reports have also been reported, estrogen has also been reported to disrupt absorption of parathyroid hormone (PTH), which extracts calcium from bone to maintain systemic calcium levels and therefore promotes bone formation [237]. In the animal model, ovariectomy lead to decreased bone strength, distorted bone microarchitecture, and increased markers of bone turnover, serum ALP, serum OCN, urinary calcium, urinary phosphorus, and urinary Cr [238]. Authors of the same study noted increased expression of HIF-1α and NF-κB and decreased expression of VEGF and β-catenin after ovariectomy [238].
Interaction of Estrogen with Wnt Pathway and HIF Pathway
Estrogen has been reported to increase osteoblast-mediated production of transforming growth factor-β (TGF-β), resulting in increased Wnt production by osteoclast [239]. Mice with Lrp5 mutations were resistant to sclerostin and had no or reduced bone loss following ovariectomy [240]. Human studies have provided considerable evidences of estrogen regulating the production of sclerostin [241]. Treatment of postmenopausal women with estrogen, or raloxifene, an estrogen receptor modulator, reduced circulating sclerostin levels [242,243,244,245], and correspondingly, bone sclerostin mRNA levels were also lower in the estrogen-treated women [242]. These studies are consistent with the observation that mice with osteocyte-specific deletion of estrogen receptor α had increased bone sclerostin mRNA levels [246]. Inhibition of sclerostin using a humanized monoclonal antibody, romosozumab, resulted in an increase in bone formation and reduction in bone resorption, qualitatively similar to results of estrogen treatment in postmenopausal women [241, 247]. These studies indicate a potential role for sclerostin, the inhibitor of the Wnt pathway in mediating estrogen-deficiency-mediated bone loss but the underlying mechanism of this effect needs further investigations.
Studies have also revealed crosstalk between estrogen signaling pathway and HIF-α regulation in bone remodeling [6, 248, 249]. 17β-estradiol was reported to increase HIF-1α and VEGF protein levels and partially stimulate MSC proliferation and HIF-1α was shown to be required for osteoclast activation in postmenopausal osteoporosis [6, 250]. Bone density, bone vessels, and bone formation were reported to be reduced in HIF-1α conditional knockout (KO) ovariectomized mice when compared to wild-type ovariectomized mice [248]. The expression of HIF-1α and VEGF decreased in ovariectomized mice but not in VHL KO ovariectomized mice while activation of the HIF-α pathway increased angiogenesis as well as osteogenesis thereby conferring protection from ovariectomy-induced bone loss [249]. These reports are consistent with the study by Yen et al., who demonstrated that diosgenin, having estrogenic effects, induced HIF-1 activation and angiogenesis through the SRC kinase, P38 MAPK, and AKT signaling pathways in osteoblasts [251]. These reports therefore point towards the role of decreased stimulation of HIF-1α and angiogenesis contributing to bone loss during estrogen deficiency. Though far stretched, reports by various authors give an impression of crosstalk among HIF signaling, Wnt pathway, and estrogen signaling which may be regulating bone mineral homeostasis in HA environments.
Summary and Conclusion
This review aims to provide available data on the role of hypoxia in bone health in relation to the Wnt pathway and estrogen at HA. Studies indicate a proportional relationship between altitude and bone health which deteriorates with increasing altitude. BMD and bone strength parameters have been reported to be decreased by various groups in both human and animal models respectively. Biochemical and endocrinal indicators of bone health also indicate decreased bone turnover at HA as compared to sea level. Duration of stay or exposure is also an important parameter along with altitude and therefore oxygen concentration, while assessing the role of hypobaric hypoxia on bone health. Extended stay as well as higher altitudes proportionally increased the deterioration of bone quality in human and animal studies. Crosstalks among the mediators of the hypoxia-sensing pathway, Wnt pathway, and estrogen have been reported in various studies which need validation in relation to bone health and mineral metabolism at HA. In conclusion, it is evident that there is only a handful of reports on the effects of hypobaric hypoxia on bone health. The tough terrain is a major challenge to conduct studies at HA. Also, there are many factors such as cold, dehydration, and high UV radiation operating simultaneously at these altitudes making it difficult to isolate the effect of hypobaric hypoxia on bone health from human studies. Major instruments used for measuring BMD, define osteopenia and osteoporosis based mainly on T score values prepared from a reference database of the western population which may not be reflecting the true bone health status of the Indian population due to geographical and ethnic differences. Therefore, detailed studies are required to prepare a database for the Indian population.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Vijayakumar R, Büsselberg D. Osteoporosis: an under-recognized public health problem. J Local Glob Heal Sci. 2016;2:1–13.
Dirckx N, Van Hul M, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res Part C - Embryo Today Rev. 2013;99:170–91.
Tanaka H, Minowa K, Satoh T, Koike T. Bone atrophy at high altitude. J Bone Miner Metab. 1992;10:31–6.
Terzi R, Yılmaz Z. Bone mineral density and changes in bone metabolism in patients with obstructive sleep apnea syndrome. J Bone Miner Metab. 2016;34:475–81.
Ma J, Li D, Zhang Z, Li Y, Wang Y, Cao Z. Correlating oxidative stress-related factors with bone metabolic markers in older adult male patients exhibiting degenerative osteoporosis in the high-altitude hypoxic area of China: study protocol for a non-randomized controlled trial. Clin Trials Degener Dis. 2017;2:53–8.
Miyauchi Y, Sato Y, Kobayashi T, Yoshida S, Mori T, Kanagawa H, et al. HIF1α is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci U S A. 2013;110:16568–73.
San T, Polat S, Cingi C, Eskiizmir G, Oghan F, Cakir B. Effects of high altitude on sleep and respiratory system and theirs adaptations. Sci World J. 2013;1–7.
Singh GK. High altitude dermatology. Indian J Dermatol. 2017;62:59–65.
Bouverot P. Adaptation to altitude-hypoxia in vertebrates. In: Heinrich B, Johansen K, Langer H, Neuweile G, Randall DJ, editors. New York: Springer-Verlag Berlin; 1985. 1st ed, pp. 1–18
Brown JPR, Grocott MPW. Humans at altitude: physiology and pathophysiology. Contin Educ Anaesthesia Crit Care Pain. 2013;13:17–22.
Beretta E, Lanfranconi F, Grasso GS, Bartesaghi M, Alemayehu HK, Pratali L, et al. Air blood barrier phenotype correlates with alveolo-capillary O2 equilibration in hypobaric hypoxia. Respir Physiol Neurobiol. 2017;246:53–8.
Hinkelbein J, Jansen S, Iovino I, Kruse S, Meyer M, Cirillo F, et al. Thirty minutes of hypobaric hypoxia provokes alterations of immune response, haemostasis, and metabolism proteins in human serum. Int J Mol Sci. 2017;18:1–9.
Kylhammar D, Rådegran G. The principal pathways involved in the in vivo modulation of hypoxic pulmonary vasoconstriction, pulmonary arterial remodelling and pulmonary hypertension. Acta Physiol. 2017;219:728–56.
Leali PT, Muresu F, Melis A, Ruggiu A, Zachos A, Doria C. Skeletal fragility definition. Clin Cases Miner Bone Metab. 2011;8:11–3.
Verratti V, Ietta F, Paulesu L, Romagnoli R, Ceccarelli I, Doria C, et al. Physiological effects of high-altitude trekking on gonadal, thyroid hormones and macrophage migration inhibitory factor (MIF) responses in young lowlander women. Physiol Rep. 2017;5:1–9.
Verratti V, Falone S, Fanò G, Paoli A, Reggiani C, Tenaglia R, et al. Effects of hypoxia on nocturnal erection quality: a case report from the Manaslu Expedition. J Sex Med. 2011;8:2386–90.
West JB. High-altitude medicine. Am J Respir Crit Care Med. 2012;186:1229–37.
Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–73.
Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J Cell Sci. 2011;124:991–8.
An SY, Heo JS. Low oxygen tension modulates the osteogenic differentiation of mouse embryonic stem cells. Tissue Cell. 2018;52:9–16.
Yellowley CE, Genetos DC. Hypoxia signaling in the skeleton: implications for bone health. Curr Osteoporos Rep. 2019;17:26–35.
Basu M, Malhotra AS, Pal K, Kumar R, Bajaj R, Verma SK, et al. Alterations in different indices of skeletal health after prolonged residency at high altitude. High Alt Med Biol. 2014;15:170–5.
Bhattarai HK, Shrestha S, Rokka K, Shakya R. Vitamin D, calcium, parathyroid hormone, and sex steroids in bone health and effects of aging. J Osteoporos. 2020;1–10.
Basu M, Malhotra AS, Pal K, Chatterjee T, Ghosh D, Haldar K, et al. Determination of bone mass using multisite quantitative ultrasound and biochemical markers of bone turnover during residency at extreme altitude: a longitudinal study. High Alt Med Biol. 2013;14:150–4.
Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47:11–20.
Chatterjee T, Bhattacharyya D, Pramanik A, Pal M, Majumdar DD, Majumdar DD. Soldiers’ load carriage performance in high mountains: a physiological study. Mil Med Res. 2017;4:1–9.
Hadjidakis DJ, Androulakis II. Bone remodeling. Ann NY Acad Sci. 2006;396:385–96.
Grabowski P. Physiology of bone. Endocr Dev. 2009;28:32–48.
Florencio-silva R, Da Sasso GRS, Sasso-cerri E, Simões MJ, Cerri PS, Rodrigues G. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;1–17.
Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6.
Gunson D, Gropp KE, Varela A. Bone and joints. Fundam Toxicol Pathol Third Ed Elsevier Inc. 2018.
Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol. 2004;180:247–55.
Kim CH, Takai E, Zhou H, Von Stechow D, Müller R, Dempster DW, et al. Trabecular bone response to mechanical and parathyroid hormone stimulation: the role of mechanical microenvironment. J Bone Miner Res. 2003;18:2116–25.
Chapuy MC, Arlot ME, Duboef F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327:1637–42.
Weinstein RS, Jilka RL, Michael Parfitt A, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts end osteocytes by glucocorticoids potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82.
Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, et al. Wnt/β-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor α. J Biol Chem. 2007;282:20715–27.
Stegen S, Carmeliet G. The skeletal vascular system – breathing life into bone tissue. Bone. 2018;115:50–8.
Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int. 1999;10:259–64.
Kanis J, Melton L, Christiansen C, Johnston C, Khaltaev N. The diagnosis of osteoporosis. J Korean Med Assoc. 1994;9:1137–41.
Kaushal N, Vohora D, Jalali RK, Jha S. Prevalence of osteoporosis and osteopenia in an apparently healthy Indian population - a cross-sectional retrospective study. Osteoporos Sarcopenia. 2018;4:53–60.
Mithal A, Kaur P. Osteoporosis in Asia : a call to action. Curr Osteoporos Rep. 2012;10:245–7.
Vaidya R, Shah R. Bone mineral density and reference standards for Indian women. J Midlife Health. 2010;1:55.
Malhotra N, Mithal A. Osteoporosis in Indians. Indian journl Med Res. 2015;263–8.
Mithal A, Bansal B, Kyer SC, Ebeling P. The Asia-Pacific Regional Audit-Epidemiology, costs, and burden of osteoporosis in India 2013: a report of International Osteoporosis Foundation. Indian J Endocrinol Metab. 2014;18:449–54.
Shatrugna V, Kulkarni B, Kumar PA, Balakrishna N, Rani US. Bone status of Indian women from a low-income group and its relationship to the nutritional status. Osteoporos Sarcopenia. 2005;16:1827–35.
Shetty S, Kapoor N, Naik D, Asha HS, Thomas N, Paul TV. The impact of the Hologic vs the ICMR database in diagnosis of osteoporosis among south Indian subjects. Clin Endocrinol. 2014;81:519–22.
Kraenzlin M. Biochemical markers of bone turnover and osteoporosis management. BoneKEy. 2007;4:191–203.
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57.
Mehta N, Garg B, Malhotra R. Management of fragility fractures in India. Best Pract Res Clin Rheumatol. 2019;33:301–9.
Adler RA. Osteoporosis in men: insights for the clinician. Ther Adv Musculoskelet Dis. 2011;3:191–200.
Orwoll E, Ebeling P. Osteoporosis in men. The new Engl J Med. 2008;358:1474–82.
Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–64.
Kotwal N, Upreti V, Nachankar A, Hari Kumar KVS. A prospective, observational study of osteoporosis in men. Indian J Endocrinol Metab. 2018;22:62–6.
Dhibar D, Gogate Y, Aggarwal S, Garg S, Bhansali A, Bhadada S. Predictors and outcome of fragility hip fracture: a prospective study from North India. Indian J Endocrinol Metab. 2019;23:282–8.
Mazess RB, Mather W. Bone mineral content of North Alaskan Eskimos. Am J Clin Nutr. 1974;27:916–25.
Gelsor N, Ma L, Duan J, Wangmu T, Gelsor N. Human vitamin D deficiency in Tibet. Food Nutr Sci. 2017;8:1127–36.
Ranhotra HS, Sharma R. Moderately high altitude habitation modulates lipid profile and alkaline phosphatases activity in aged Khasis of Meghalaya. Indian J Clin Biochem. 2010;1:51–6.
O’Brien KA, Pollock RD, Stroud M, Lambert RJ, Kumar A, Atkinson RA, et al. Human physiological and metabolic responses to an attempted winter crossing of Antarctica: the effects of prolonged hypobaric hypoxia. Physiol Rep. 2018;6:1361301–13.
Nakamaru S, Sakuraba K, Fujita S. Characteristics of bone metabolism in middle-aged and older mountaineers. Juntendo Med J. 2018;64:278–85.
Bharadwaj H, Jain SC, Nayar HS. Body composition of high altitude natives on descent to the plains: a densitometric, hydrometric, and anthropometric evaluation. Eur J Appl Physiol Occup Physiol. 1981;47:65–72.
Khan DA, Aslam M, Khan ZU. Changes in plasma electrolytes during acclimatisation at high altitude. J Pak Med Assoc. 1996;46:128–31.
Śliwicka E, Cisoń T, Kasprzak Z, Nowak A, Pilaczyńska-Szcześniak Ł. Serum irisin and myostatin levels after 2 weeks of high-altitude climbing. PLoS One. 2017;12: e0181259.
Kasprzak Z, Sliwicka E, Hennig K, Pilaczyńska-Szczeniak A, Huta-Osiecka A, Nowak A, Vitamin D. iron metabolism, and diet in alpinists during a 2-week high-altitude climb. High Alt Med Biol. 2015;16:230–5.
Bingham PJ, Brazell IA, Owen M. The effect of parathyroid extract on cellular activity and plasma calcium levels in vivo. J Endocrinol. 1969;45:387–400.
Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15:60–5.
Tang XG, Wen J, Zhang XS, Jiang DC. Association between decreased osteopontin and acute mountain sickness upon rapid ascent to 3500 m among young Chinese men. J Travel Med. 2018;25:1–6.
Ciapetti G, Granchi D, Fotia C, Savarino L, Dallari D, Del Piccolo N, et al. Effects of hypoxia on osteogenic differentiation of mesenchymal stromal cells used as a cell therapy for avascular necrosis of the femoral head. Cytotherapy. 2016;18:1087–99.
Zhang P, Ha N, Dai Q, Zhou S, Yu C, Jiang L. Hypoxia suppresses osteogenesis of bone mesenchymal stem cells via the extracellular signal-regulated 1/2 and p38-mitogen activated protein kinase signaling pathways. Mol Med Rep. 2017;16:5515–22.
Xu Y, Malladi P, Chiou M, Bekerman E, Giaccia AJ, Longaker MT. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981–93.
Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y, et al. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol. 2013;94:33–9.
Zhao AG, Shah K, Freitag J, Cromer B, Sumer H. Differentiation potential of early- and late-passage adipose-derived mesenchymal stem cells cultured under hypoxia and normoxia. Stem Cells Int. 2020;1–11.
Xu Q, Liu Z, Guo L, Liu R, Li R, Chu X, et al. Hypoxia mediates runt-related transcription factor 2 expression via induction of vascular endothelial growth factor in periodontal ligament stem cells. Mol Cells. 2019;42:763–72.
Zahm A, Bucaro M, Srinivas V, Shapiro I, Adams C. Oxygen tension regulates preosteocyte maturation and mineralization. Bone. 2008;43:25–31.
Ding H, Chen S, Yin JH, Xie XT, Zhu ZH, Gao YS, et al. Continuous hypoxia regulates the osteogenic potential of mesenchymal stem cells in a time-dependent manner. Mol Med Rep. 2014;10:2184–90.
Merceron C, Vinatier C, Portron S, Masson M, Amiaud J, Guigand L, et al. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol. 2010;298:355–64.
Huang YC, Zhu HM, Cai JQ, Huang YZ, Xu J, Zhou Y, et al. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2012;113:1407–15.
Ma HP, Ma XN, Ge BF, Zhen P, Zhou J, Gao YH, et al. Icariin attenuates hypoxia-induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro. Cell Prolif. 2014;47:527–39.
Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl Med. 2014;3:206–17.
Lee JS, Park JC, Kim TW, Jung BJ, Lee Y, Shim EK, et al. Human bone marrow stem cells cultured under hypoxic conditions present altered characteristics and enhanced in vivo tissue regeneration. Bone. 2015;78:34–45.
Balogh E, Tóth A, Méhes G, Trencsényi G, Paragh G, Jeney V. Hypoxia Triggers osteochondrogenic differentiation of vascular smooth muscle cells in an HIF-1 (hypoxia-inducible factor 1)-dependent and reactive oxygen species-dependent manner. Arterioscler Thromb Vasc Biol. 2019;39:1088–99.
Buravkova LB, Ezdakova MI, Andrianova IV, Golikova EA, Andreeva ER. Differential expression of bipotent commitment-related genes in multipotent mesenchymal stromal cells at different O2 levels. Dokl Biochem Biophys. 2020;491:67–9.
Lambertini E, Penolazzi L, Angelozzi M, Bergamin LS, Manferdini C, Dalla Sega FV. Hypoxia preconditioning of human MSCs: a direct evidence of HIF-1α and collagen type XV correlation. Cell Physiol Biochem. 2018;51:2237–49.
Gu Q, Gu Y, Shi Q, Yang H. Hypoxia promotes osteogenesis of human placental-derived mesenchymal stem cells. Tohoku J Exp Med. 2016;239:287–96.
Gao YS, Ding H, Xie XT, Zhang CQ. Osteogenic induction protects rat bone marrow-derived mesenchymal stem cells against hypoxia-induced apoptosis in vitro. J Surg Res. 2013;184:873–9.
Inagaki Y, Akahane M, Shimizu T, Inoue K, Egawa T, Kira T, et al. Modifying oxygen tension affects bone marrow stromal cell osteogenesis for regenerative medicine. World J Stem Cells. 2017;9:98–106.
Wei L, Zhang B, Zhang J, Tan Q, Zhang Y, Fan Y, et al. Application of a grading system in the treatment of frontal lobe contusion in high-altitude regions. World Neurosurg. 2018;116:e975–82.
Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882–8.
Pattappa G, Thorpe SD, Jegard NC, Heywood HK, De Bruijn JD, Lee DA. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng - Part C Methods. 2013;19:68–79.
Salamanna F, Cepollaro S, Contartese D, Giavaresi G, Brodano GB, Griffoni C, et al. Biological rationale for the use of vertebral whole bone marrow in spinal surgery. Spine (Phila Pa 1976). 2018;43:1401–10.
Hung SP, Ho JH, Shih YRV, Lo T, Lee OK. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res. 2011;30:260–6.
Lee WYW, Lui PPY, Rui YF. Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Eng - Part A. 2012;18:484–98.
Hsu SH, Chen CT, Wei YH. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2013;31:2779–88.
Park IH, Kim KH, Choi HK, Shim JS, Whang SY, Hahn SJ, et al. Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp Mol Med. 2013;45:44–11.
Wang YY, Li J, Wang YY, Lei L, Jiang C, An S, et al. Effects of hypoxia on osteogenic differentiation of rat bone marrow mesenchymal stem cells. Mol Cell Biochem. 2012;362:25–33.
Binder BYK, Saguna JE, Leacha JK. Reduced serum and hypoxic culture conditions enhance the osteogenic potential of human mesenchymal stem cells. Stem Cell Rev. 2015;11:387–93.
Cicione C, Muiños-López E, Hermida-Gómez T, Fuentes-Boquete I, Díaz-Prado S, Blanco FJ. Effects of severe hypoxia on bone marrow mesenchymal stem cells differentiation potential. Stem Cells Int. 2013;1–11.
Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One. 2011;6:e23965.
Wang W, Yun Z, Peng H-Z, Yan S-J, Zhang H-T, Qiu X-C, et al. The hypobaric hypoxia environment impairs bone strength and quality in rats. Int J Clin Exp Med. 2017;10:9397–406.
Bozzini C, Picasso EO, Champin GM, Alippi RM, Bozzini CE. Structural and material mechanical quality of femoral shafts in rats exposed to simulated high altitude from infancy to adulthood. High Alt Med Biol. 2016;17:50–3.
Lezon C, Bozzini CEC, Agûero Romero A, Pinto P, Champin G, Alippi RM, et al. Effect of chronic undernutrition on body mass and mechanical bone quality under normoxic and altitude hypoxic conditions. Br J Nutr. 2016;115:1687–95.
Bozzini C, Champin GM, Alippi RM, Bozzini CE. Static biomechanics in bone from growing rats exposed chronically to simulated high altitudes. High Alt Med Biol. 2013;14:367–74.
Bozzini C, Olivera MI, Huygens P, Alippi RM, Bozzini CE. Long-term exposure to hypobaric hypoxia in rat affects femur cross-sectional geometry and bone tissue material properties. Ann Anat. 2009;191:212–7.
Del Pilar MM, Bozzini C, Olivera MI, Dmytrenko G, Conti MI. Aluminum bone toxicity in immature rats exposed to simulated high altitude. J Bone Miner Metab. 2011;29:526–34.
Conti MI, Terrizzi AR, Lee CM, Mandalunis PM, Bozzini C, Piñeiro AE, et al. Effects of lead exposure on growth and bone biology in growing rats exposed to simulated high altitude. Bull Environ Contam Toxicol. 2012;88:1033–7.
Yin BH, Chen HC, Zhang W, Li TZ, Gao QM, Liu JW. Effects of hypoxia environment on osteonecrosis of the femoral head in Sprague-Dawley rats. J Bone Miner Metab. 2020;38:780–93.
Durand M, Collombet J-M, Frasca S, Sarilar V, Lataillade J-J, Le Bousse-Kerdilès M-C, et al. Separate and combined effects of hypobaric hypoxia and hindlimb suspension on skeletal homeostasis and hematopoiesis in mice. Hypoxia. 2019;7:41–52.
Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–61.
Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II Modified Kroghian Models Biophys J. 2001;81:685–96.
Winkler IG, Barbier V, Wadley R, Zannettino ACW, Williams S, Lévesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: Serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116:375–85.
Rankin EB, Giaccia AJ, Schipani E. A central role for hypoxic signaling in cartilage, bone, and hematopoiesis. Curr Osteoporos Rep. 2011;9:46–52.
Semenza GL. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3.
Prabhakar NR, Semenza GL. Oxygen sensing and homeostasis. Physiology. 2015;30:340–8.
Pugh CW, Ratcliffe PJ. New horizons in hypoxia signaling pathways. Exp Cell Res. 2017;356:116–21.
Stegen S, Carmeliet G. Hypoxia, hypoxia-inducible transcription factors and oxygen-sensing prolyl hydroxylase s in bone development and homeostasis. Curr Opin Nephrol Hypertens. 2019;328–35.
Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–48.
Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007;1116:281–90.
Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–35.
Rizzoli R, Bonjour JP. Determinants of peak bone mass and mechanisms of bone loss. Osteoporos Int. 1999;9:17–23.
Mosekilde L. Age-related changes in bone mass, structure, and strength - Effects of loading. Z Rheumatol. 2000;59:1–9.
Yang M, Liu H, Wang Y, Wu G, Qiu S, Liu C, et al. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect Tissue Res. 2019;60:583–96.
Chen Q, Wu S, Lu T, Chen J, Xu Z, Chen J. The effect of sulforaphane on the activity and mineralization of osteoblasts under oxidative stress. Pharmacology. 2019;104:147–56.
Yoon DK, Park JS, Rho GJ, Lee HJ, Sung IY, Son JH, et al. The involvement of histone methylation in osteoblastic differentiation of human periosteum-derived cells cultured in vitro under hypoxic conditions. Cell Biochem Funct. 2017;35:441–52.
Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res. 2006;312:1693–702.
Hollborn M, Brück R, Kuhrt H, Wiedemann P, Bringmann A. Osmotic and hypoxic induction of osteopontin in retinal pigment epithelial cells: involvement of purinergic receptor signaling. Mol Vis. 2020;26:188–203.
Wang XM, Liu H, Li JY, Wei JX, Li X, Zhang YL, et al. Rosamultin attenuates acute hypobaric hypoxia-induced bone injuries by regulation of sclerostin and its downstream signals. High Alt Med Biol. 2020;0:273–86.
Wang G, Wang J, Sun D, Xin J, Wang L, Huang D, et al. Short-term hypoxia accelerates bone loss in ovariectomized rats by suppressing osteoblastogenesis but enhancing osteoclastogenesis. Med Sci Monit. 2016;22:2961–71.
Komor T. Regulation of osteoblast and odontoblast differentiation by RUNX2. J Oral Biosci. 2010;52:22–5.
Ontiveros C, Irwin R, Wiseman RW, McCabe LR. Hypoxia suppresses runx2 independent of modeled microgravity. J Cell Physiol. 2004;200:169–76.
Park JH, Park BH, Kim HK, Park TS, Baek HS. Hypoxia decreases Runx2/Cbfa1 expression in human osteoblast-like cells. Mol Cell Endocrinol. 2002;192:197–203.
Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007–16.
Arnett TR. Acidosis, hypoxia and bone. Arch Biochem Biophys. 2010;503:103–9.
Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22:15–24.
Yang CN, Kok SH, Wang HW, Chang JZC, Lai EHH, Shun CT, et al. Simvastatin alleviates bone resorption in apical periodontitis possibly by inhibition of mitophagy-related osteoblast apoptosis. Int Endod J. 2019;52:676–88.
Yu X, Wan Q, Ye X, Cheng Y, Pathak JL, Li Z. Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling. Cell Mol Biol Lett. 2019;24:1–17.
Xu G. HIF-1-mediated expression of Foxo1 serves an important role in the proliferation and apoptosis of osteoblasts derived from children’s iliac cancellous bone. Mol Med Rep. 2018;17:6621–31.
Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, et al. Role of HIF-1α in skeletal development. Ann N Y Acad Sci. 2010;1192:322–6.
Liu X, Tu Y, Zhang L, Qi J, Ma T, Deng L. Prolyl hydroxylase inhibitors protect from the bone loss in ovariectomy rats by increasing bone vascularity. Cell Biochem Biophys. 2014;69:141–9.
Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–26.
Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, et al. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc Natl Acad Sci U S A. 2014;111:8673–8.
Dirckx N, Tower RJ, Mercken EM, Vangoitsenhoven R, Moreau-Triby C, Breugelmans T, et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J Clin Invest. 2018;128:1087–105.
Niu X, Chen Y, Qi L, Liang G, Wang Y, Zhang L, et al. Hypoxia regulates angeogenic-osteogenic coupling process via up-regulating IL-6 and IL-8 in human osteoblastic cells through hypoxia-inducible factor-1α pathway. Cytokine. 2018;113:117–27.
Wu C, Rankin EB, Castellini L, Fernandez-Alcudia J, Lagory EL, Andersen R, et al. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 2015;29:817–31.
Lee SY, Park KH, Yu HG, Kook E, Song WH, Lee G, et al. Controlling hypoxia-inducible factor-2α is critical for maintaining bone homeostasis in mice. Bone Res. 2019;7:1–13.
Merceron C, Ranganathan K, Wang E, Tata Z, Makkapati S, Khan MP, et al. Hypoxia-inducible factor 2α is a negative regulator of osteoblastogenesis and bone mass accrual. Bone Res. 2019;7:1–13.
Shomento SH, Wan C, Cao X, Faugere MC, Bouxsein ML, Clemens TL, et al. Hypoxia-inducible factors 1α and 2α exert both distinct and overlapping functions in long bone development. J Cell Biochem. 2010;109:196–204.
Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, et al. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci U S A. 2006;103:19004–9.
Liu Y, Huang X, Yu H, Yang J, Li Y, Yuan X, et al. HIF-1α-TWIST pathway restrains cyclic mechanical stretch-induced osteogenic differentiation of bone marrow mesenchymal stem cells. Connect Tissue Res. 2019;60:544–54.
Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8.
Utting JC, Flanagan AM, Brandao-Burch A, Orriss IR, Arnett TR. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem Funct. 2010;28:374–80.
Knowles HJ, Athanasou NA. Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis. J Pathol. 2009;218:256–64.
Muzylak M, Price JS, Horton MA. Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcif Tissue Int. 2006;79:301–9.
Ma Z, Yu R, Zhao J, Sun L, Jian L, Li C, et al. Constant hypoxia inhibits osteoclast differentiation and bone resorption by regulating phosphorylation of JNK and IκBα. Inflamm Res. 2019;68:157–66.
Hulley PA, Bishop T, Vernet A, Schneider JE, Edwards JR, Athanasou NA, et al. Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J Pathol. 2017;242:322–33.
Kang H, Yang K, Xiao L, Guo L, Guo C, Yan Y, et al. Osteoblast hypoxia-inducible factor-1α pathway activation restrains osteoclastogenesis via the interleukin-33-microRNA-34a-Notch1 pathway. Front Immunol. 2017;8:1–15.
Shao J, Zhang Y, Yang T, Qi J, Zhang L, Deng L. HIF-1α disturbs osteoblasts and osteoclasts coupling in bone remodeling by up-regulating OPG expression. Vitr Cell Dev Biol - Anim. 2015;51:808–14.
Shirakura M, Tanimoto KK, Eguchi H, Miyauchi M, Nakamura H, Hiyama K, et al. Activation of the hypoxia-inducible factor-1 in overloaded temporomandibular joint, and induction of osteoclastogenesis. Biochem Biophys Res Commun. 2010;393:800–5.
Arnett TR, Orriss IR. Metabolic properties of the osteoclast. Bone. 2018;115:25–30.
Hou ZS, Wen HS, Li JF, He F, Li Y, Qi X. Environmental hypoxia causes growth retardation, osteoclast differentiation and calcium dyshomeostasis in juvenile rainbow trout (Oncorhynchus mykiss). Sci Total Environ. 2020;705: 135272.
Tian Y, Shao Q, Tang Y, Li X, Qi X, Jiang R, et al. HIF-1α regulates osteoclast activation and mediates osteogenesis during mandibular bone repair via CT-1. Oral Dis. 2020;1–26.
Tang Y, Zhu J, Huang D, Hu X, Cai Y, Song X, et al. Mandibular osteotomy-induced hypoxia enhances osteoclast activation and acid secretion by increasing glycolysis. J Cell Physiol. 2019;234:11165–75.
Knowles H. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia. 2015;3:73–82.
Indo Y, Takeshita S, Ishii KA, Hoshii T, Aburatani H, Hirao A, et al. Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res. 2013;28:2392–9.
Igari K, Kelly MJ, Yamanouchi D. Digoxin attenuates receptor activation of NF-κB ligand-induced osteoclastogenesis in macrophages. J Vasc Res. 2019;56:55–64.
Bozec A, Bakiri L, Hoebertz A, Eferl R, Schilling AF, Komnenovic V, et al. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature. 2008;454:221–5.
Leger AJ, Altobelli A, Mosquea LM, Belanger AJ, Song A, Cheng SH, et al. Inhibition of osteoclastogenesis by prolyl hydroxylase inhibitor dimethyloxallyl glycine. J Bone Miner Metab. 2010;28:510–9.
Hulley PA, Papadimitriou-Olivgeri I, Knowles HJ. Osteoblast-osteoclast co-culture amplifies inhibitory effects of FG-4592 on osteoclast formation and reduces bone resorption activity. bioRxiv. 2019;1–18.
Knowles HJ. Hypoxia, hypoxia-inducible factor ( HIF ) and bone homeostasis : focus on osteoclast-mediated bone resorption. Trends Cell Mol Biol. 2015;10:91–104.
Knowles HJ, Cleton-Jansen A-M, Korsching E, Athanasou NA. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J. 2010;24:4648–59.
Gohda J, Akiyama T, Koga T, Takayanagi H, Tanaka S, Inoue JI. RANK-mediated amplification of TRAF6 signaling to NFATc1 induction during osteoclastogenesis. EMBO J. 2005;24:790–9.
Kanemoto S, Kobayashi Y, Yamashita T, Miyamoto T, Cui M, Asada R, et al. Luman is involved in osteoclastogenesis through the regulation of DC-STAMP expression, stability and localization. J Cell Sci. 2015;128:4353–65.
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–57.
Hannah SS, McFadden S, McNeilly A, McClean C. “Take My Bone Away?” Hypoxia and bone: a narrative review. J Cell Physiol. 2021;1–20.
Dandajena TC, Ihnat MA, Disch B, Thorpe J, Currier GF. Hypoxia triggers a HIF-mediated differentiation of peripheral blood mononuclear cells into osteoclasts. Orthod Craniofacial Res. 2012;15:1–9.
Morrison MS, Turin L, King BF, Burnstock G, Arnett TR. ATP is a potent stimulator of the activation and formation of rodent osteoclasts. J Physiol. 1998;511:495–500.
Orriss IR, Knight GE, Utting JC, Taylor SEB, Burnstock G, Arnett TR. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol. 2009;220:155–62.
Ryu JH, Chae CS, Kwak JS, Oh H, Shin Y, Huh YH, et al. Hypoxia-inducible factor-2α is an essential catabolic regulator of inflammatory rheumatoid arthritis. PLoS Biol. 2014;12:e1001881.
Javaheri B, Stern A, Lara N, Dalla M, Khao H, Liu Y, et al. Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2014;29:705–15.
Tu X, Delgado-calle J, Condon KW, Maycas M, Zhang H, Carlesso N. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. PNAS. 2015;478–86.
Dodd JS, Raleigh JA, Gross TS. Osteocyte hypoxia: a novel mechanotransduction pathway. Am J Physiol - Cell Physiol. 1999;598–602.
Hinoi E, Ochi H, Takarada T, Nakatani E, Iezaki T, Nakajima H, et al. Positive regulation of osteoclastic differentiation by growth differentiation factor 15 upregulated in osteocytic cells under hypoxia. J Bone Miner Res. 2012;27:938–49.
Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol - Cell Physiol. 2005;289:633–43.
Wang H, Ji B, Liu XS, Van Oers RFM, Guo XE, Huang Y, et al. Osteocyte-viability-based simulations of trabecular bone loss and recovery in disuse and reloading. Biomech Model Mechanobiol. 2014;13:153–66.
Montesi M, Jähn K, Bonewald L, Stea S, Bordini B, Beraudi A. Hypoxia mediates osteocyte ORP150 expression and cell death in vitro. Mol Med Rep. 2016;14:4248–54.
Gross TS, Akeno N, Clemens TL, Komarova S, Srinivasan S, Weimer DA, et al. Physiological and genomic consequences of intermittent hypoxia: selected contribution: osteocytes upregulate HIF-1α in response to acute disuse and oxygen deprivation. J Appl Physiol. 2001;90:2514–9.
Zhu J, Tang Y, Wu Q, Ji YC, Feng ZF, Kang FW. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J Cell Physiol. 2019;1–11.
Raheja LF, Genetos DC, Yellowley CE. Hypoxic osteocytes recruit human MSCs through an OPN/CD44-mediated pathway. Biochem Biophys Res Commun. 2008;366:1061–6.
Gross T, King K, Rabaia N, Pathare P, Srinivasan S. Upregulation of osteopontin by osteocytes deprived of mechanical loading or oxygen. J Bone Miner Res. 2005;20:250–6.
Riddle RC, Leslie JM, Gross TS, Clemens TL. Hypoxia-inducible factor-1α protein negatively regulates load-induced bone formation. J Biol Chem. 2011;286:44449–56.
Stegen S, Stockmans I, Moermans K, Thienpont B, Maxwell PH, Carmeliet P, et al. Osteocytic oxygen sensing controls bone mass through epigenetic regulation of sclerostin. Nat Commun. 2018;9:1–15.
Loots GG, Robling AG, Chang JC, Murugesh DK, Bajwa J, Carlisle C, et al. Vhl deficiency in osteocytes produces high bone mass and hematopoietic defects. Bone. 2018;116:307–14.
Song X, Tang Y, Zhu J, Tian Y, Song Z, Hu X, et al. HIF-1α induces hypoxic apoptosis of MLO-Y4 osteocytes via JNK/caspase-3 pathway and the apoptotic-osteocyte-mediated osteoclastogenesis in vitro. Tissue Cell. 2020;67: 101402.
Hojo H, Ohba S, Chung U Il. Signaling pathways regulating the specification and differentiation of the osteoblast lineage. Regen Ther. 2014;1–6.
Kim JH, Kim N. Signaling pathways in osteoclast differentiation. Chonnam Med J. 2016;52:12–7.
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–7.
Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, et al. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem. 2003;278:24113–7.
Nakanishi R, Shimizu M, Mori M, Akiyama H, Okudaira S, Otsuki B. Secreted frizzled-related protein 4 is a negative regulator of peak. J Bone Miner Res. 2006;21:1713–21.
Andrade AC, Nilsson O, Barnes KM, Baron J. Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone. 2007;40:1361–9.
Mao J, Wang J, Liu B, Pan W, Farr GH, Flynn C, et al. Binds to axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–9.
Pinson KI, Brennan J, Monkley S, Avery BJ. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–8.
Laine CM, Joeng KS, Campeau PM, Tarkkonen K, Grover M, Lu JT, et al. WNT1 Mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368:1809–16.
Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, et al. The regulation of bone metabolism and disorders by wnt signaling. Int J Mol Sci. 2019;20:5525.
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-roman S, Reginato AM, et al. LDL Receptor-Related Protein 5 ( LRP5) Affects bone accrual and eye development. Cell. 2001;107:513–23.
Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Bénichou O, Scopelliti D, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003;72:763–71.
Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15:928–35.
Balemans W, Ebeling M, Patel N, Van HE, Olson P, Dioszegi M, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein ( SOST ). Hum Mol Genet. 2001;10:537–44.
Bodine PVN, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–37.
Chen D, Li Y, Zhou Z, Xing Y, Zhong Y, Zou X, et al. Synergistic inhibition of Wnt pathway by HIF-1 a and osteoblast-specific transcription factor Osterix (Osx) in osteoblasts. PLoS One. 2012;7:e52948.
Genetos DC, Toupadakis CA, Raheja LF, Wong A, Savvas E, Fyhrie DP, et al. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem. 2010;110:457–67.
Peng J, Lai ZG, Fang ZL, Xing S, Hui K, Hao C, et al. Dimethyloxalylglycine prevents bone loss in ovariectomized C57BL/6J mice through enhanced angiogenesis and osteogenesis. PLoS One. 2014;9:e112744.
Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, et al. Prolyl hydroxylase-1 negatively regulates IkB kinase-B, giving insight into hypoxia-induced NFkB activity. PNAS. 2006;103:18154–9.
Cai T, Sun D, Duan Y, Wen P, Dai C, Yang J, et al. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res. 2016;345:206–17.
Moorer MC, Riddle RC. Regulation of osteoblast metabolism by Wnt signaling. Endocrinol Metab. 2018;33:318–30.
Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, Kariya Y. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195–9.
Tu X, Joeng KS, Nakayama KI, Nakayama K, Carroll TJ, Mcmahon AP, et al. Noncanonical Wnt signaling through G protein-linked PKCδ activation promotes bone formation. Dev Cell. 2007;12:113–27.
Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat Cell Biol. 2007;9:1273–85.
Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, et al. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–64.
Prisby RD, Dominguez JM, Muller-Delp J, Allen MR, Delp MD. Aging and estrogen status: a possible endothelium-dependent vascular coupling mechanism in bone remodeling. PLoS One. 2012;7:e48564.
Chang J, Wang Z, Tang E, Fan Z, Mccauley L, Guan K, et al. Inhibition of osteoblast functions by IKK/NF-κB in osteoporosis. 2009;15:682–9.
Melotte V, Qu X, Ongenaert M, Criekinge W, Bruïne AP, Baldwin HS, et al. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010;24:4153–66.
Xing W, Pourteymoor S, Mohan S. Ascorbic acid regulates osterix expression in osteoblasts by activation of prolyl hydroxylase and ubiquitination-mediated proteosomal degradation pathway. Physiol Genomics. 2011;43:749–57.
Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol. 2012;8:358–66.
Fujiwara M, Kubota T, Wang W, Ohata Y, Miura K, Kitaoka T, et al. Successful induction of sclerostin in human-derived fibroblasts by 4 transcription factors and its regulation by parathyroid hormone, hypoxia, and prostaglandin E2. Bone. 2016;85:91–8.
Parraguez VH, Mamani S, Cofré E, Castellaro G, Urquieta B, De los Reyes M, et al. Disturbances in maternal steroidogenesis and appearance of intrauterine growth retardation at high-altitude environments are established from early pregnancy. Effects of treatment with antioxidant vitamins. PLoS One. 2015;10:e0140902.
Pooja, Ghosh D, Bhargava K, Sethy NK. Post-translational modifications of eNOS augment nitric oxide availability and facilitates hypoxia adaptation in Ladakhi women. Nitric Oxide - Biol Chem. 2018;78:103–12.
Friedl KE, Plymate SR, Bernhard WN, Mohr LC. Elevation of plasma estradiol in healthy men during a mountaineering expedition. Cancer Res. 1988;20:239–42.
Pooja, Sharma M, Singh K, Himashree G, Bhaumik G, Kumar B, et al. Estrogen receptor (ESR1 and ESR2)-mediated activation of eNOS–NO–cGMP pathway facilitates high altitude acclimatization. Nitric Oxide - Biol Chem. 2020;102:1–20.
Lufkin EG, Wahner HW, O’Fallon WM, Hodgson SF, Kotowicz MA, Lane AW, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med. 1992;117:1–9.
Falahati-Nini A, Riggs BL, Atkinson EJ, Michael O’fallon W, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560.
Finkelstein JS, Lee H, Leder B, Burnett-Bowie S-A, Goldstein D, Hahn C, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Inves. 2016;126:1114–25.
Idan A, Griffiths KA, Harwood DT, Seibel MJ, Turner L, Conway AJ, et al. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: A randomized, placebo-controlled trial. Ann Intern Med. 2010;153:621–32.
Smith MR, Malkowicz SB, Brawer MK, Hancock ML, Morton RA, Steiner MS. Toremifene decreases vertebral fractures in men younger than 80 years receiving androgen deprivation therapy for prostate cancer. J Urol. 2011;186:2239–44.
Uebelhart B, Herrmann F, Pavo I, Draper MW, Rizzoli R. Raloxifene treatment is associated with increased serum estradiol and decreased bone remodeling in healthy middle-aged men with low sex hormone levels. J Bone Miner Res. 2004;19:1518–24.
Venken K, Callewaert F, Boonen S. Sex hormones, their receptors and bone health. Osteoporos Int. 2008;19:1517–25.
Hughes DE, Dai A, Tiffee JC, Li HH, Munoy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-. Nat Med. 1996;2:1132–5.
Tobias JH, Compston JE. Does Estrogen stimulate osteoblast function in postmenopausal women? Bone. 1999;24:121–4.
Robinson JA, Harris SA, Riggs BL, Spelsberg TC. Estrogen regulation of human osteoblastic cell proliferation and differentiation. Endocrinology. 1997;138:2919–27.
Wu S, Du X. Body mass index may positively correlate with bone mineral density of lumbar vertebra and femoral neck in postmenopausal females. Med Sci Monit. 2016;22:145–51.
Fayed HA, Barakat BM, Elshaer SS, Abdel-Naim AB, Menze ET. Antiosteoporotic activities of isoquercitrin in ovariectomized rats: role of inhibiting hypoxia inducible factor-1 alpha. Eur J Pharmacol. 2019;865: 172785.
Khosla S. Odanacatib: location and timing are everything. J Bone Miner Res. 2012;27:509–23.
Niziolek PJ, Bullock W, Warman ML, Robling AG. Missense mutations in LRP5 associated with high bone mass protect the mouse skeleton from disuse-and ovariectomy-induced osteopenia. PLoS One. 2015;10:e0140775.
Drake MT, Khosla S. Hormonal and systemic regulation of sclerostin. Bone. 2017;96:8–17.
Fujita K, Roforth MM, Demaray S, McGregor U, Kirmani S, McCready LK, et al. Effects of estrogen on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in postmenopausal women. J Clin Endocrinol Metab. 2014;99:81–8.
Farr JN, Roforth MM, Fujita K, Nicks KM, Cunningham JM, Atkinson EJ, et al. Effects of age and estrogen on skeletal gene expression in humans as assessed by RNA sequencing. PLoS One. 2015;10:e0138347.
Mödder UI, Roforth MM, Hoey K, McCready LKP, Eterson JM, Monroe DG, et al. Effects of estrogen on osteoprogenitor cells and cytokines/bone-regulatory factors in postmenopausal women. Bone. 2011;23:1–7.
Chung YE, Lee SH, Lee SY, Kim SY, Kim HH, Mirza FS, et al. Long-term treatment with raloxifene, but not bisphosphonates, reduces circulating sclerostin levels in postmenopausal women. Osteoporos Int. 2012;23:1235–43.
Kondoh S, Inoue K, Igarashi K, Sugizaki H, Shirode-Fukuda Y, Inoue E, et al. Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice. Bone. 2014;60:68–77.
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–43.
Liu XD, Deng LF, Wang J, Qi J, Zhou Q, Wang JS, et al. The regulation of hypoxia inducible factor-1alpha on osteoblast function in postmenopausal osteoporosis. Zhonghua Wai Ke Za Zhi. 2007;45:1274–8.
Zhao Q, Shen X, Zhang W, Zhu G, Qi J, Deng L. Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone. 2012;50:763–70.
Yun SP, Lee MY, Ryu JM, Song CH, Han HJ. Role of HIF-1α and VEGF in human mesenchymal stem cell proliferation by 17β-estradiol: involvement of PKC, PI3K/Akt, and MAPKs. Am J Physiol - Cell Physiol. 2009;296:317–27.
Men LY, Jen LS, Chung LC, Kuang WT, Ching YY, Wei FC, et al. Diosgenin induces hypoxia-inducible factor-1 activation and angiogenesis through estrogen receptor-related phosphatidylinositol 3-kinase/Akt and p38 mitogen-activated protein kinase pathways in osteoblasts. Mol Pharmacol. 2005;68:1061–73.
Tsang WP, Shu Y, Kwok PL, Zhang F, Lee KKH, Tang MK, et al. CD146 + human umbilical cord perivascular cells maintain stemness under hypoxia and as a cell source for skeletal regeneration. PLoS One. 2013;8:1–13.
Sengupta S, Park SH, Patel A, Carn J, Lee K, Kaplan DL. Hypoxia and amino acid supplementation synergistically promote the osteogenesis of human mesenchymal stem cells on silk protein scaffolds. Tissue Eng - Part A. 2010;16:3623–34.
Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:1–12.
Yao Y, Deng Q, Sun C, Song W, Liu H, Zhou Y. A genome-wide analysis of the gene expression profiles and alternative splicing events during the hypoxia-regulated osteogenic differentiation of human cartilage endplate-derived stem cells. Mol Med Rep. 2017;16:1991–2001.
Burian E, Probst F, Palla B, Riedel C, Saller MM, Cornelsen M, et al. Effect of hypoxia on the proliferation of porcine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells in 2- and 3-dimensional culture. J Cranio-Maxillofacial Surg. 2017;45:414–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Babu, L.K., Ghosh, D. Looking at Mountains: Role of Sustained Hypoxia in Regulating Bone Mineral Homeostasis in Relation to Wnt Pathway and Estrogen. Clinic Rev Bone Miner Metab 20, 18–36 (2022). https://doi.org/10.1007/s12018-022-09283-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-022-09283-4