Abstract
Gaucher disease (GD) is the most common inherited lysosomal storage disorder. It is a multi-system disease secondary a deficient activity of glucocerebrosidase-β-acid by variants in the GBA gene. The wide variability in the severity of clinical manifestations causes it to be diagnosed at any age. Only about 30% of patients are identified to have it in their childhood. Leaving aside the most serious forms of the diseases that are observed in the first weeks of life, most of the manifestations focus on the increase of visceral size, cytopenias, growth retardation, and bone pain crisis. The introduction of enzymatic replacement therapy (ERT) 28 years ago was a revolution and a change in the treatment landscape of GD, the eradication of splenectomy, and the reduction of bone complications when ERT begins in childhood has been definitive for the control of the disease and improvement of the quality of life. The treatment is effective in most patients without neurological involvement and with few adverse effects; however, the need for an intravenous administration every 2 weeks, indefinitely, not having the property to cure the disease has motivated the search for more alternatives that are comfortable, effective, and definitive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gaucher disease (GD)(OMIM#230800) is the most common inherited lysosomal storage disorder. It is a recessively autosomal disease arising from variants in the glucocerebrosidase-β-acid gene (GBA) located in chromosome 1 in the q21 region [1]. The consequence is a partial or total lack of lysosomal glucocerebrosidase-β-acid (GBA) enzymatic activity. GBA is a hydrolase responsible for the degradation of the glycosphingolipid complex; the deficiency leads to an accumulation of complex molecules of glucosylceramide and glucosylsphingosine inside the lysosomes in macrophage cells, mainly in the liver, spleen, and bone marrow [2, 3]. In a subset of patients, the central nervous system is damaged.
According to neurological involvement, there are several subtypes described. Type 1 GD or non-neuronopathic GD is the most common form of presentation in western countries. Type 3 GD is the second most frequent and their clinical course is characterized by the heterogeneity in the neurological manifestations like ataxia, saccadic eye movements, seizures or myoclonic epilepsy, and some type 3 subtypes without neurological features have heart valves infiltration or kyphosis and other characteristics known as Norbottnian-like [4, 5]. Type 2 GD or acute neuronopathic form is the most aggressive presentation related to severe neurological impairment early in life (newborns to 1 year old) with a short lifespan, usually around 2 years of age [6]. At present, the tendency is to consider that the types in lysosomal disorders are a continuum of manifestations in GD from the more severe, the type 2 form, followed by the intermediate disease of type 3 to milder or non-symptomatic phenotypes of some type 1 patients [7, 8]. These multi-system manifestations are based on the grade of residual GBA enzymatic activity and the association of some mutations with a high risk of neurologic involvement [9, 10].
The variability in the severity of clinical manifestations causes, that despite being a hereditary disease, it to be diagnosed at any age [11]; around 49% of the cases (reported from the international GD registry, ICCG) are diagnosed before 10 years of age [11]; in Spain, according data from the Spanish Registry of Gaucher Disease (SpRGD) coordinated by the “Fundacion Española para el Estudio y Terapeutica de la Enfermedad de Gaucher” (FEETEG), a total of 416 GD patients have been reported (www.feeteg.org) and about the 30.2% of patients are diagnosed below 18 years [12]. In children, the wide variability ranges from the tremendously affected newborns with severe deterioration or with irreparable damage to the skin until mild cases with scarce increase in visceral volume, mild cytopenias, or growth retardation [6]. This last non-specific profile of the GD contributes to the delay in the diagnosis in childhood if the patient without family history or the physician does not consider the possibility of an accumulation disease due to its low frequency out of Ashkenazi Jews origin. Despite the fact that symptoms and signs of the disease appear during childhood in the majority of GD patients, diagnosis is often delayed for many years, even until adulthood. Delayed recognition of the disease leads to late treatment, which in turn translates into a higher probability of irreversible consequences [12, 13].
Since the introduction of enzymatic replacement therapy (ERT) in early 1990s, the natural history of disease has been modified. Prior to ERT, only spleen removal and red blood cell package transfusions to improve hematologic parameters were available [14]. The accumulation of glucocerebroside in viscera and bone marrow can be clarified in the majority of patients with ERT after some months on therapy. Nevertheless, the aberrant lysosomal function and the permanent stimulation of the monocyte-macrophage are not fully controlled by the therapy with persistence of inflammatory complex and variable manifestations like fatigue, neuronopathic symptoms in GD3, pulmonary complications and some bone crisis and reduction of skeletal mass. Type 2 GD has not any kind of effective treatment because the neurological damage is present in prenatal life [15].
Current Therapy for GD
Types of Enzyme Replacement Therapy
The development of the enzyme replacement treatment was due to Roscoe Brady’s research work in the NIH laboratories USA, obtaining the glucocerebrosidase enzyme from human placentas, which after manage various purification procedures to expose the mannose terminations of the molecule that facilitates its incorporation into the macrophage mannose receptor pathway [16]. This enzyme was subsequently produced by Genzyme Corporation under the name Ceredase® (alglucerase) and it was applied to the first child patient. Treatment with this protein was approved in the USA and in the UK (1991 and 1994, respectively, for long-term therapy of patients with confirmed diagnosis of type 1 GD who presented manifestations of the disease). However, the use of Ceredase® was limited by the availability of placentas and the possibility, although small, of transmitting infectious agents or interfering with certain hormones.
Subsequently, the recombinant form of the enzyme, imiglucerase (Cerezyme®), was produced by genetic recombinant engineering in Chinese hamster ovary (CHO) cells, developed by Genzyme Corporation. Cerezyme® was approved in the USA in November 1994 and in Europe in 1998. The experience of more than 25 years in the use of ERT is already available [14, 17].Two other recombinant enzymes have subsequently been developed in different cell lines (velaglucerase alfa [VPRIV], Shire Human Genetic Therapies, Lexington, MA, USA, obtained in culture of human fibroblasts [18], and taliglucerase alfa [ELELYSO], Pfizer Labs, New York, NY, USA) obtained in carrot vegetable cells; both enzymes are widely used, although taliglucerase alfa has not been approved so far in Europe [19].
In 2014, an imiglucerase biosimilar, Abcertin® (ISU302, ISU Abxis, Seoul, Korea), was also developed in CHO cells and its efficacy and safety have been proven in a limited number of patients compared with imiglucerase [20].
The ERT is recommended for symptomatic children and adult GD1or GD3 patients [21]. The early application of therapy in children may prevent the development of irreversible complications as osteonecrosis and bone infarcts. The treatment in pediatric patients also has demonstrated to improve growth and bone mineralization. ERT is administered intravenously for at least 1 h and periodically every 2 weeks at doses between 15 and 60 units/kg [12, 22]. The response occurs mostly from 6 months after the start of therapy, producing a reduction in liver and splenic volume, an increase in hemoglobin concentration and platelet counts, evidence in the disappearance of bone painful, recovery from osteopenia, and prevention of osteonecrosis. The reduction in bone marrow infiltration [23, 24] and improvement of the quality of life require more time on therapy to analyze the effectiveness [25]. The stability or minimally active disease generally is acquired after 4–5 years on therapy according to the definition of di Rocco et al. [26].
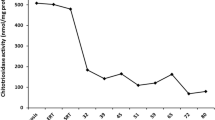
The plasma biomarkers related to the disease (chitotriosidase, CCL18/PARC, glucosylsphingosine (LysoGb1 a deacylated form of glucosylceramide. Lyso-Gb1 was proved to be a highly sensitive and specific biomarker for diagnosis and monitoring of adults and children patients with GD) are progressively decreasing reaching therapeutic objectives according to established criteria [27].
ERT is generally well tolerated and hypersensitivity or anaphylaxis reactions are rare [28]. Nevertheless, ERT can induce weight gain and insulin resistance [29]. Despite the efficacy of ERT, it has limitations in the resolution of bone complications, the control of lung involvement, or in the prevention of neurological complications [17] since it does not cross the blood brain barrier and therefore has no activity in neurological lesions in types GD2 and GD3 and does not prevent the occurrence of cholelithiasis, Parkinson’s disease, or neoplasms.
Since the 1990s, the introduction of ERT for types 1 and 3, the goals of therapy according to the consensus established through the European Group of Gaucher Disease (EWGGD) [24] in the assessment of the response, based in the therapeutic objectives defined by G Pastores in 2004, are detailed in the Table 1.
In relation to visceral manifestations, splenectomy should be avoided because it is the greatest risk factor for developing bone complications.
With regard to bone disease, the objectives are set in the longer term due to the characteristics of the tissue and difficulty in accessing the enzyme, eliminating chronic bone pain in 1 to 2 years of treatment.
• Reduce bone marrow infiltration, measured by the bone marrow burden (BMB) score.
• Recovery bone mineral density in adults at 2 years of treatment with T score lower than − 2.5 baseline.
• Normalize growth at 2 years.
The efficacy of treatment on visceral and hematological involvement has been demonstrated in various clinical studies [30, 31]. The results of the first clinical studies in adults and children with alglucerase and imiglucerase are detailed in Table 2. The evaluation of the efficacy in bone disease has been carried out in several observational studies, such as that performed in 33 patients (only one patient was less than 22 years old) not previously treated and with skeletal manifestations such as osteopenia, history of bone crisis, or other documented bone pathology, who received imiglucerase 60 U/kg/2 weeks, for 4 years. Substantial improvements in bone pain were observed already 3 months after the start of the ERT (p < 0.001 compared with the initial value) and continued progressively throughout the study, with 39% of patients showing pain at 48 months versus 73% at the beginning of the study. Only 2 patients produced new bone crises. There was a constant improvement in bone mineral density in the spine and femoral neck, measured by DEXA, the average Z score in the column increased from − 0.72 ± 1302 at the beginning to almost normal levels (− 0.09 ± 1503) a 48 months (p = 0.042) and for the femoral neck from − 0.59 ± 1352 to − 0.17 ± 1206 (p = 0.035) already in month 36 [32].
Alglucerase/Imiglucerase
Taliglucerase Alfa
Taliglucerase alfa is the first plant cell–expressed recombinant therapeutic protein approved for use in humans and is approved for the treatment of patients with GD in multiples countries. Glycosylation of glucocerebrosidase occurs inside the plant cell without requiring the addition of other components in the production process [33]. It is indicated for treatment of adults with GD1 in the USA, Israel, Australia, Canada, Chile, Brazil, and other countries, and is approved for treatment of pediatric patients in the USA, Australia, and Canada, and for hematologic manifestations in pediatric patients with GD3 in Canada.
Pivotal clinical trials have been conducted in adult and pediatric GD patients who were ERT naïve and who had been switched from imiglucerase to taliglucerase alfa and are detailed in Table 3 [34,35,36,37,38]. Recently, a review of all results from the phase 3 clinical studies of taliglucerase alfa in adults and children with GD has been published [39].
Taliglucerase alfa has demonstrated long-term efficacy in patients treated in the first line with the plant enzyme and stability in patients switched from imiglucerase. The objectives of visceral and hematological response were achieved as well as reduction in biomarkers in both adults and prospective pediatric studies. The tolerance is good and the adverse effects are of mild or moderate degree and transient in a similar percentage to those observed with imiglucerase [39]. In three studies in adults with bone marrow infiltration, the results of taliglucerase alfa on bone disease have been assessed by quantitative magnetic resonance, measuring the fat fraction with respect to water in the lumbar spine (QCSI), the response evaluated in 8 patients with GD1 treated in the first line with taliglucerase alfa, compared with 15 untreated patients and with a 1-year follow-up, a significant clearance in bone marrow was detected with respect to the initial moment (p = 0.012) and comparatively with untreated patients (p = 0.005), the assessment at 4 and 5 years of follow-up in patients treated with taliglucerase alfa showed an increase in lumbar spine fat fraction or stability since the onset of treatment [40, 41].
The study was carried out in 11 pediatric patients treated with taliglucerase in two branches at doses of 30 or 60 U/kg after 1 year of treatment. The analysis of bone mineral density by DEXA showed a mean decrease (± SE) of the Z score in the lumbar spine of − 0.20 (± 0.20; n = 6) and of the femoral neck of − 0.30 (± 0.28; n = 5) in the group receiving taliglucerase alfa at a dose of 30 U/kg while the group receiving 60 U/kg, the average Z score (± SE) increased in the lumbar spine of 0.27 (± 0.05; n = 4) and in the femoral neck 0.20 (± 0.421; n = 4) [36].
Velaglucerase Alfa
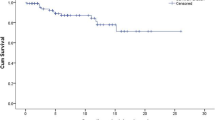
Velaglucerase alfa was assessed in adult and pediatric patients in three clinical trials that were followed by a single extension study (Table 4). The first pivotal study with velaglucerase alfa was named TKT-025 with 12 adult GD1 patients completed the phase I/II clinical trial [42]. Later in the extension study completing 7 years of follow-up, the evaluation of bone marrow infiltration using the BMB score showed a reduction of infiltration in lumbar vertebra and/or femoral. TKT032 trial was an assessment of two doses of velaglucerase alfa in 25 GD1patients [43]. TKT034 was a phase II/III trial assessing velaglucerase alfa in GD1 patients previously treated with imiglucerase [44]. HGT-GCB-039 was a non-inferiority study comparing velaglucerase alfa with imiglucerase [45].
In 8 of 12 adult patients who completed the phase I/II clinical trials with velaglucerase alfa and later in the extension study completing 7 years of follow-up, the assessment of the medullary infiltration using the BMB score showed a reduction of infiltration at least at 2 points at the level of the lumbar spine and/or femurs. Bone mineral density was evaluated as an exploratory variable in phase I/II studies with velaglucerase alfa, observing a constant improvement over time. In 57 patients evaluated in the follow-up phase after clinical trials conducted with velaglucerase alfa (TKT032, HGT-GCB-039) and who continued to receive velaglucerase alfa in the extension study, after 2 years of follow-up, a mean increase was observed in the bone mineral density measured by the lumbar spine and femur neck Z score of 0.62 SD and 0.12 SD respectively. In this study, the mean change in the Z score in the femoral neck was smaller than in the lumbar spine, although without statistical significance, in this study, more than 64% of patients initially had a normal bone mineral density in the femoral neck [46, 48, 49].
HGT-GCB-044 was an extension study of the preceding trials TKT032, HGT-GCB-039, and TKT034; the primary objective was to evaluate the long-term safety of velaglucerase alfa [46]. The analysis reported the safety and efficacy of velaglucerase alfa in pediatric population of the extension study (25% of the study population), including assessments conducted specifically in children to evaluate bone disease.
HGT-GCB-058 clinical trial was conducted with velaglucerase alfa in GD1 patients in the USA to evaluate the safety treatment protocol during a global supply shortage of imiglucerase [47].
The current recommendations for starting ERT on children [50] are based in the presence of hemorrhages, growth tendency, bone pain, visceral enlargement, hematological alterations, and the presence of genotypes such as L444P, D409H, or other severe genotypes associated with diagnosis of a GD3 or more aggressive GD1. The recommendation is to start ERT as soon as possible. For asymptomatic patients diagnosed below 20 years old, it is recommended to start ERT if they have a family member or sibling who requires therapy. It is important to keep in mind that the prevalence of genotypes varies among the different populations; in Ashkenazi Jews, N370S is the most frequent mutation [51] and more than the 40% of those pediatric patients are homozygous for N370S mutation, with predominant mild phenotype. In contrast, other population as Asiatic countries [52, 53] or Egypt with higher presence of consanguineous parents, the prevalence of severe genetic variants is corresponding with more aggressive phenotypes.
To eradicate the spleen removal is mandatory; the prevalence of splenectomized pediatric patients before ERT was approximately 20% (data from the ICGG Registry) [14]. The severity of bone manifestations is highly significant (bone crisis, ischemic bone events, and bone pain) in splenectomized patients compared with patients with intact spleen (P < .0001) [14]. Fortunately, in the era of ERT, the decreasing prevalence of ischemic bone events is significant in pediatric patients with intact spleens after ERT started.
In this way, the Spanish experience in the follow-up of patients diagnosed in the pediatric age and who have received ERT since the first years of life shows, in comparison with the cohort of patients diagnosed in childhood but who had not received ERT until adulthood or have not received it, the risk of developing bone complications in this group was 1.7 times higher (p = 0.025) compared with patients treated from the first years of life [12]. Bone alterations were present in 37.9% of cases, with a clear predominance in the subset of patients diagnosed before 1994 without possibilities to receive ERT.
Inhibition of substrate therapy
The introduction of another approach to therapy of Gaucher’s disease has the objective to reduce the formation of glycosphingolipids by inhibition of the enzyme glucosylceramide synthase, in order that residual enzyme activity can metabolize the lysosomal substrate. At present, two substrate inhibitors are authorized. This form of therapy represents an oral alternative by acting and by decreasing the synthesis of glucosylceramide, less accumulated amount is produced in the lysosomal compartment of macrophages. In 2002, miglustat (Zavesca®, Actelion Pharmaceuticals, Allschwil, Switzerland) [54] a small molecule of iminosugar, was approved in Europe as second-line oral treatment for type GD1 adult patients who do not want or cannot receive ERT. Due to the small size of the molecule, it is speculated that access to bone tissue will be easier [55].
The clinical trials carried out with miglustat were reported as efficacy data of drug at a dose of 100 mg t.i.d. at the level of bone disease; they come from the pooled analysis of the data collected prospectively from 72 patients who participated in 3 multinational clinical trials open for 2 years [56] (Table 5). The problem with this therapy is the high incidence of gastrointestinal disturbances that cause early discontinuation [57].
The other substrate reduction therapy Eliglustat (Cerdelga®, Sanofi Genzyme, Cambridge, MA, USA) is a potent and selective inhibitor of glucosylceramide synthase and has been approved in Europe in 2015 as a first-line treatment for adults with GD1, whether fast, intermediate, or slow metabolizers (categories that apply to more than 90% of genotyped) for CYP2D6 variants.
The four clinical studies with more than 400 adults GD patients exposed to eliglustat have shown similar efficacy in the hematological and visceral response when comparing the results against imiglucerase. The majority of adverse effects detected in clinical studies with eliglustat have been mild, occasional, and transient in general, not related to the drug and that did not motivate the withdrawal of treatment [58,59,60,61,62].
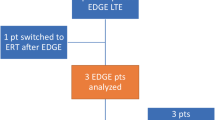
At present, a clinical multicenter trial with eliglustat in pediatric patients under 18 years old with GD1 and GD3 is conducted and sponsored by Sanofi Genzyme, to evaluate the pharmacokinetics, safety, and efficacy of the drug in children. Clinical trials in children are necessary to produce high-class evidence of the efficacy of new medications in the pediatric population. Most currently used GD treatments are injectable. These treatments are by nature associated with increased patient burden in terms of time, inconvenience, and discomfort when compared with oral treatments.
As an oral alternative for GD treatment, eliglustat may reduce the burden and stress on children and their caregivers (Table 6).
Future Therapy for GD
Pharmacological Chaperone Therapy
Pharmacological chaperone therapy is a new approach for GD. The chaperone treatments have been applied to various lysosomal storage disorders. The rationale is that small molecules of pharmacological chaperones selectively bind to misfolded enzyme and contribute to the correctly folding of the defective protein involved in the pathophysiology of the disease and the enzyme to recover its functional activity. Defective and misfolded proteins are degraded by cellular machinery in the endoplasmic reticulum. The chaperones due to their small size can cross the blood-brain barrier so they would meet all the characteristics to be a targeted therapy in the GD and in this line, research is still being investigated [63, 64].
By screening more than 1000 compounds by thermal denaturation assays, ambroxol a drug with a long history to use as mucolytic, expectorant was identified as a potential chaperone that significantly increased the enzymatic activity of acid betaglucosidase and reduced the substrate accumulation in cell lines with mutations associated with GD1 and GD2/3 [65]. Research conducted with ambroxol in cell cultures and normal mice to assess efficacy and toxicity has shown a significant increase in the activity of acid betaglucosidase in the spleen, heart, and cerebellum of mice and absence of toxicity at increasing doses. Therefore, ambroxol could be a useful therapeutic chaperone in the treatment of GD patients with neurological involvement [66].
A pilot study has been conducted in five GD3 patients receiving high doses oral of ambroxol in combination with ERT. No adverse effects have been observed and a decrease in glucosylsphingosine levels in cerebrospinal fluid is detected with improvement of some neurological symptoms. At present, further randomized controlled studies are required to answer questions such as ambroxol shows efficacy on visceral and hematologic manifestations, it has protective effects against neurological complications, and bone disease, it is a good substrate inhibitor, and it has synergistic effects plus ERT [67, 68].
Gene Therapy
The latest advances in genome editing technology based on genetic engineering have made it possible to improve the correction of gene variants and currently the strategy of gene therapy can offer advantages that allow the cure of some diseases with a single intervention. The objective of gene therapy in monogenic diseases is to ensure that the corrected gene variant expresses the protein in an enough and lasting way [69].
Since 2016, the regulatory agencies EMA and FDA have already approved 6 products to apply, 2 in CAR-T for B-line lymphomas, and 4 for monogenic diseases: β-thalasemia, a type of blindness, spinal muscular atrophy and a primary immunodeficiency.
Two types of gene therapy have also been implemented that are also applicable to lysosomal disorders. Ex vivo gene therapy with better lentiviral vector designs and efficient large-scale production that ensures robust grafting of genetically corrected stem cells.
In vivo gene therapy with adeno-associated virus vectors, in which vectors are directly injected with the correct genetic sequence that is incorporated into the target cells, depends on tissue-specific targeting or local delivery and/or target cell–specific gene expression. This procedure requires suitable management of the immune response induced by the vector.
In short, these approaches offer unquestionable possibilities for lysosomal disorders that lack treatment or that do not achieve the desired objectives with current therapies. These therapies are developing now in an industrial way with the union between biotechnology and pharmaceutical companies and several clinical trials are launched in various entities including Gaucher disease to develop and standardize procedures, monitoring the results and management of adverse effects [69, 70].
The experiments performed in a murine disease model of type GD1, using gammaretroviral vectors harboring strong viral promoters to drive glucosidase β-acid gene expression as a proof-of-concept, have demonstrated a reversal of symptoms secondary the increase in the activity of glucosidase β-acid induced the regression of splenomegaly, reduced bone marrow infiltration, and normalized hematological parameters [71].
The first-in-human study evaluates the safety and efficacy of ex vivo lentiviral-mediated GBA gene therapy investigational gene therapy candidate, AVR-RD-02 in GD1 patients a phase 1/2 study in GD1 patients is conducted with the goal of enabling sustained expression of endogenous, functional enzyme. The design of the study has been presented in the 13th European Working Group on Gaucher Disease Congress (EWGGD 2019), Clermont-Ferrand, France, 4–6 July 2019 [72].
Conclusions
Gaucher’s disease continues to face ongoing challenges in every day medical practice. First of all, the early diagnosis that requires constant insistence on the dissemination of knowledge so that doctors who find a case for the first time can easily identify it and make a complete and correct assessment to define the patient’s situation and start treatment as soon as possible to avoid complications.
The appearance of ERT in the 1990s was a revolution and a change in the therapeutic landscape of GD, the eradication of splenectomy, and the reduction of bone complications when ERT begins in childhood have been definitive for the control of the disease and improvement of the quality of life.
However, challenges remain to be achieved and those patients who do not respond well to treatment and those who develop neurological complications do not have a better alternative solution at present. We hope that the new strategies of gene therapy will be able to correct these deficiencies and we will be closer to achieving a definitive cure for the disease.
References
Devine EA, Smith M, Arredondo-Vega FX, Shafit-Zagardo B, Desnick RJ. Regional assignment of the structural gene for human acid beta- glucosidase to q42 leads to qter on chromosome 1. Cytogenet Cell Genet. 1982;33:340–4.
Beutler E, Gelbart T. Glucocerebrosidase (Gaucher disease). Hum Mutat. 1996;8(3):207–13.
Brady RO, Kanfer JN, Shapiro D. Metabolism of glucocerebrosides. II. Evidence of an enzymatic deficiency in Gaucher’s disease. Biochem Biophys Res Commun. 1965;18:221–5.
Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83(1–2):6–15.
Pastores GM, Hugues DA. Gaucher Disease. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews®. Seatle: University of Washington; 1993-2019. 2000 Jul 27 [updated 2018 Jun 21].http://www.ncbi.nlm.nih.gov/books/NBK1269/.
Goker-Alpan O, Schiffmann R, Park JK, Stubblefield BK, Tayebi N, Sidransky E. Phenotypic continuum in neuronopathic Gaucher disease: an intermediate phenotype between type 2 and type 3. J Pediatr. 2003;143:273–6.
Mistry PK, Belmatoug N, vom Dahl S, Giugliani R. Understanding the natural history of Gaucher disease. Am J Hematol. 2015;90 Suppl 1:S6–11.
Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, Wakefield LK, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82(3):192–207.
Alfonso P, Aznarez S, Giralt M, Pocovi M, Giraldo P, Spanish Gaucher’s Disease Registry. Mutation analysis and genotype/phenotype relationships of Gaucher disease patients in Spain. J Hum Genet 2007;52:391–396.
Giraldo P, Alfonso P, Irún P, Gort L, Chabás A, Vilageliu L, et al. Mapping the genetic and clinical characteristics of Gaucher disease in the Iberian Peninsula. Orphanet J Rare Dis. 2012;7:17.
Grabowski GA, Zimran A, Ida H. Gaucher disease types 1 and 3: phenotypic characterization of large populations from the ICGG Gaucher Registry. Am J Hematol. 2015;90(Suppl 1):S12–8.
Andrade-Campos M, Alfonso P, Irun P, Armstrong J, Calvo C, Dalmau J, et al. Diagnosis features of pediatric Gaucher disease patients in the era of enzymatic therapy, a national-base study from the Spanish Registry of Gaucher Disease. Orphanet J Rare Dis. 2017;12(1):84.
Di Rocco M, Andria G, Deodato F, Giona F, Micalizzi C, Pession A. Early diagnosis of Gaucher disease in pediatric patients: proposal for a diagnostic algorithm. Pediatr Blood Cancer. 2014;61(11):1905–9.
Mistry PK, Batista JL, Andersson HC, Balwani M, Burrow TA, Charrow J, et al. Transformation in pretreatment manifestations of Gaucher disease type 1 during two decades of alglucerase/imiglucerase enzyme replacement therapy in the International Collaborative Gaucher Group (ICGG) Gaucher Registry. Am J Hematol. 2017;92:929–39.
Roshan Lal T, Sidransky E. The spectrum of neurological manifestations associated with Gaucher disease. Diseases. 2017 Mar;2:5(1).
Barton NW, Furbish FS, Murray GJ, Garfield M, Brady RO. Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc Natl Acad Sci U S A. 1990;87:1913–6.
El-Beshlawy A, Tylki-Szymanska A, Vellodi A, Belmatoug N, Grabowski GA, Kolodny EH, et al. Long-term hematological, visceral, and growth outcomes in children with Gaucher disease type 3 treated with imiglucerase in the International Collaborative Gaucher Group Gaucher Registry. Mol Genet Metab. 2017;120:47–56.
Zimran A, Loveday K, Fratazzi C, Elstein D. A pharmacokinetic analysis of a novel enzyme replacement therapy with gene-activated human glucocerebrosidase (GA-GCB) in patients with type 1 Gaucher disease. Blood Cells Mol Dis. 2007;39:115–8.
Fox JL. First plant-made biologic approved. Nat Biotechnol. 2012;30:472.
Lee BH, Abdalla AF, Choi JH, Beshlawy AE, Kim GH, Heo SH, et al. A multicenter, open-label, phase III study of Abcertin in Gaucher disease. Medicine (Baltimore). 2017;96(45):e8492.
Gaucher Disease. A strategic collaborative approach from EMA and FDA. European Medicines Agency 2014;EMA/44410/2014.http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_ guideline/2014/05/WC500166587.pdf.
Andersson H, Kaplan P, Kacena K, Yee J. Eight-year clinical outcomes of long-term enzyme replacement therapy for 884 children with Gaucher disease type 1. Pediatrics. 2008;122(6):1182–90.
Pastores GM, Weinreb NJ, Aerts H, Andria G, Cox TM, Giralt M, et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41(4 Suppl 5):4–14.
Biegstraaten M, Cox TM, Belmatoug N, Berger MG, Collin-Histed T, Vom Dahl S, et al. Management goals for type 1 Gaucher disease: an expert consensus document from the European working group on Gaucher disease. Blood Cells Mol Dis. 2018;68:203–8.
Giraldo P, Solano V, Pérez-Calvo JI, Giralt M, Rubio-Félix D. Spanish Group on Gaucher disease. Quality of life related to type 1 Gaucher disease: Spanish experience. Qual Life Res. 2005;14:453–62.
Di Rocco M, Andria G, Bembi B, Carubbi F, Giona F, Giuffrida G, et al. Minimal disease activity in Gaucher disease: criteria for definition. Mol Genet Metab. 2012;107:521–5.
Hurvitz N, Dinur T, Becker-Cohen M, Cozma C, Hovakimyan M, Oppermann S, et al. Glucosylsphingosine (lyso-Gb1) as a biomarker for monitoring treated and untreated children with Gaucher disease. Int J Mol Sci. 2019 Jun;21:20(12).
Starzyk K, Richards S, Yee J, Smith SE, Kingma W. The long-term international safety experience of imiglucerase therapy for Gaucher disease. Mol Genet Metab. 2007;90(2):157–63.
Langeveld M, Ghauharali KJ, Sauerwein HP, Ackermans MT, Groener JE, Hollak CE, et al. Type I Gaucher disease, a glycosphingolipid storage disorder, is associated with insulin resistance. J Clin Endocrinol Metab. 2008;93(3):845–51.
Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, et al. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med. 1991;324(21):1464–70.
Grabowski GA, Barton NW, Pastores G, Dambrosia JM, Banerjee TK, McKee MA, et al. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122(1):33–9.
Sims KB, Pastores GM, Weinreb NJ, Barranger J, Rosenbloom BE, Packman S, et al. Improvement of bone disease by imiglucerase (Cerezyme) therapy in patients with skeletal manifestations of type 1 Gaucher disease: results of a 48-month longitudinal cohort study. Clin Genet. 2008;73(5):430–40.
Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J. 2007;5(5):579–90.
Zimran A, Brill-Almon E, Chertkoff R, Petakov M, Blanco-Favela F, Muñoz ET, et al. Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood. 2011;118:5767–73.
Pastores GM, Petakov M, Giraldo P, Rosenbaum H, Szer J, Deegan PB, et al. A phase 3, multicenter, open-label, switchover trial to assess the safety and efficacy of taliglucerase alfa, a plant cell-expressed recombinant human glucocerebrosidase, in adult and pediatric patients with Gaucher disease previously treated with imiglucerase. Blood Cells Mol Dis. 2014;53:253–60.
Zimran A, Gonzalez-Rodriguez DE, Abrahamov A, Cooper PA, Varughese S, Giraldo P, et al. Long-term safety and efficacy of taliglucerase alfa in pediatric Gaucher disease patients who were treatment-naïve or previously treated with imiglucerase. Blood Cells Mol Dis. 2018;68:163–72.
Zimran A, Gonzalez-Rodriguez DE, Abrahamov A, Elstein D, Paz A, Brill-Almon E, et al. Safety and efficacy of two dose levels of taliglucerase alfa in pediatric patients with Gaucher disease. Blood Cells Mol Dis. 2015;54:9–16.
Abbas R, Park G, Damle B, Chertkoff R, Alon S. Pharmacokinetics of novel plant cell-expressed taliglucerase alfa in adult and pediatric patients with Gaucher disease. PLoS One. 2015;10(6):e0128986.
Zimran A, Wajnrajch M, Hernandez B, Pastores GM. Taliglucerase alfa: safety and efficacy across 6 clinical studies in adults and children with Gaucher disease. Orphanet J Rare Dis. 2018;13:36.
Zimran A, Durán G, Giraldo P, Rosenbaum H, Giona F, Petakov M, et al. Long-term efficacy and safety results of taliglucerase alfa through 5years in adult treatment-naïve patients with Gaucher disease. Blood Cells Mol Dis. 2019;78:14–21.
Zimran A, Dinur T, Revel-Vilk S, Akkerman EM, van Dussen L, Hollak CEM, et al. Improvement in bone marrow infiltration in patients with type I Gaucher disease treated with taliglucerase alfa. J Inherit Metab Dis. 2018;41(6):1259–65.
Zimran A, Altarescu G, Philips M, Attias D, Jmoudiak M, Deeb M, et al. Phase 1/2 and extension study of velaglucerase alfa replacement therapy in adults with type 1 Gaucher disease: 48-monthexperience. Blood. 2010;115(23):4651–6.
Zimran A, Wang N, Ogg C, Crombez E, Cohn GM, Elstein D. Seven-year safety and efficacy with velaglucerase alfa for treatment-naïve adult patients with type 1 Gaucher disease. Am J Hematol. 2015;90(7):577–83.
Zimran A, Pastores GM, Tylki-Szymanska A, Hughes DA, Elstein D, Mardach R, et al. Safety and efficacy of velaglucerase alfa in Gaucher disease type 1 patients previously treated with imiglucerase. Am J Hematol. 2013;88:172–8.
Ben Turkia H, Gonzalez DE, Barton NW, Zimran A, Kabra M, Lukina EA, et al. Velaglucerase alfa enzyme replacement therapy compared with imiglucerase in patients with Gaucher disease. Am J Hematol. 2013;88:179–84.
Hughes DA, Gonzalez DE, Lukina EA, Mehta A, Kabra M, Elstein D, et al. Velaglucerase alfa (VPRIV) enzyme replacement therapy in patients with Gaucher disease: long-term data from phase III clinical trials. Am J Hematol. 2015;90:584–91.
Pastores GM, Rosenbloom B, Weinreb N, Goker-Alpan O, Grabowski G. Cohn GM, et al multicenter open-label treatment protocol (HGT-GCB-058) of velaglucerase alfa enzyme replacement therapy in patients with Gaucher disease type 1: safety and tolerability. Genet Med. 2014 May;16(5):359–66.
Elstein D, Haims AH, Zahrieh D, Cohn GM, Zimran A. Impact of velaglucerase alfa on bone marrow burden score in adult patients with type 1 Gaucher disease:7-year follow-up. Blood Cells Mol Dis. 2014;53:56–60.
Elstein D, Foldes AJ, Zahrieh D, Cohn GM, Djordjevic M, Brutaru C, et al. Significant and continuous improvement in bone mineral density among type 1 Gaucher disease patients treated with velaglucerase alfa: 69-month experience, including dose reduction. Blood Cells Mol Dis. 2011;47:56–61.
Kaplan P, Baris H, De Meirleir L, Di Rocco M, El-Beshlawy A, Huemer M, et al. Revised recommendations for the management of Gaucher disease in children. Eur J Pediatr. 2013;172(4):447–58.
Bronstein S, Karpati M, Peleg L. An update of Gaucher mutations distribution in the Ashkenazi Jewish population: prevalence and country of origin of the mutation R496H. Isr Med Assoc J. 2014;16(11):683–5.
Alasmar D. Gaucher disease in Syrian children: common mutations identification, and clinical futures. Ann Saudi Med. 2015;35:127–32.
Feng Y, Huang Y, Tang C, Hu H, Zhao X, Sheng H, et al. Clinical and molecular characteristics of patients with Gaucher disease in southern China. Blood Cells Mol Dis. 2018;68:30–4.
Platt FM, Neises GR, Dwek RA, Butters TD. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J Biol Chem. 1994;269:8362–5.
Cox TM, Amato D, Hollak CE, Luzy C, Silkey M, Giorgino R, Steiner RD; Miglustat Maintenance Study Group. Evaluation of miglustat as maintenance therapy after enzyme therapy in adults with stable type 1 Gaucher disease: a prospective, open-label non-inferiority study. Orphanet J Rare Dis 2012 ;7:102.
Pastores GM, Elstein D, Hrebícek M, Zimran A. Effect of miglustat on bone disease in adults with type 1 Gaucher disease: a pooled analysis of three multinational, open-label studies. Clin Ther. 2007;29:1645–54.
Belmatoug N, Burlina A, Giraldo P, Hendriksz CJ, Kuter DJ, Mengel E, et al. Gastrointestinal disturbances and their management in miglustat-treated patients. J Inherit Metab Dis. 2011;34(5):991–1001.
Lukina E, Watman N, Arreguin EA, Banikazemi M, Dragosky M, Iastrebner M, et al. A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type 1. Blood. 2010;116:893–9.
Mistry PK, Lukina E, Ben Turkia H, Amato D, Baris H, Dasouki M, et al. Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial. JAMA. 2015;313(7):695–706.
Cox TM, Drelichman G, Cravo R, Balwani M, Burrow TA, Martins AM, et al. Eliglustat compared with imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015;385(9985):2355–62.
Charrow J, Fraga C, Gu X, Ida H, Longo N, Lukina E, et al. Once- versus twice-daily dosing of eliglustat in adults with Gaucher disease type 1: the phase 3, randomized, double-blind EDGE trial. Mol Genet Metab. 2018;123(3):347–56.
Peterschmitt MJ, Cox GF, Ibrahim J, MacDougall J, Underhill LH, Patel P, et al. A pooled analysis of adverse events in 393 adults with Gaucher disease type 1 from four clinical trials of oral eliglustat: evaluation of frequency, timing, and duration. Blood Cells Mol Dis. 2018;68:185–91.
Sawkar AR, Adamski-Werner SL, Cheng WC, Wong CH, Beutler E, Zimmer KP, et al. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem Biol. 2005;12(11):1235–44.
Tropak MB, Kornhaber GJ, Rigat BA, Maegawa GH, Buttner JD, Blanchard JE, et al. Identification of pharmacological chaperones for Gaucher disease and characterization of their effects on beta-glucocerebrosidase by hydrogen/ deuterium exchange mass spectrometry. Chembiochem. 2008;9:2650–62.
Yilmazer B, Yagci ZB, Bakar E, Ozden B, Ulgen K, Ozkirimli E. Investigation of novel pharmacological chaperones for Gaucher Disease. J Mol Graph Model. 2017;76:364–78.
Maegawa GH, Tropak MB, Buttner JD, Rigat BA, Fuller M, Pandit D, et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem. 2009;284:23502–16.
Luan Z, Li L, Higaki K, Nanba E, Suzuki Y, Ohno K. The chaperone activity and toxicity of ambroxol on Gaucher cells and normal mice. Brain Dev. 2013;35:317–22.
Narita A, Shirai K, Itamura S, Matsuda A, Ishihara A, Matsushita K, et al. Ambroxol chaperone therapy for neuronopathic Gaucher disease: a pilot study. Ann Clin Transl Neurol. 2016;3:200–15.
High KA, Roncarolo MG. Gene therapy. N Engl J Med. 2019;381:455–64.
Dunbar CE, High KA, Joung JE, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359:175.
Dahl M, Doyle A, Olsson K, Månsson JE, Marques ARA, Mirzaian M, et al. Lentiviral gene therapy using cellular promoters cures type 1 Gaucher disease in mice. Mol Ther. 2015;23:835–44.
Cohn G, Ciotti R, Golipour A, Pfeifer R, Yang J, Kerner J et al. A first-in-human ex-vivo lentiviral gene therapy using AVR-RD-02 for Gaucher disease: phase 1/2 study protocol. Presented in the 13th European Working Group on Gaucher Disease Congress (EWGGD 2019), Clermont-Ferrand, France, 4–6 July 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author has received research grants and speaker honorarium from Company Takeda, Sanofi-Genzyme, Pfizer. All fees are donated to the FEETEG for research support.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giraldo, P. Current and Emerging Pharmacotherapy for Gaucher Disease. Clinic Rev Bone Miner Metab 17, 142–151 (2019). https://doi.org/10.1007/s12018-019-09267-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-019-09267-x