Abstract
In the setting of renal disease and hyperparathyroidism, a near total parathyroidectomy with cryopreservation is a safe and effective treatment. Even with improved dialysis and medical management, parathyroid surgery remains effective therapy for patients with hyperparathyroidism associated with renal failure. Symptomatic patients particularly need to be identified earlier for parathyroidectomy to prevent progression of bone disease in chronic renal failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 5% of renal failure patients undergo parathyroidectomy for secondary hyperparathyroidism (sHPT) and 3% after a successful renal transplant. The most common cause of sHPT is chronic renal failure (CRF). Other causes of sHPT include osteomalacia, rickets, lithium toxicity, idiopathic hypercalciuria, vitamin D deficiency, and malnutrition. Pathogenic factors for sHPT include hyperphosphatemia, hypocalcemia, diminished synthesis of vitamin D metabolites (calcitriol), and bone resistance to parathyroid hormone (PTH). Additionally, changes in PTH set point render parathyroid cells relatively insensitive to increases in serum calcium levels and to calcitriol. With impending renal failure, PTH secretion is stimulated by a decline in 1,25-OH vitamin D levels, and a noticeable increase in PTH occurs when GFR falls below 30 cc/min. Phosphate excretion decreases as GFR decreases to 20 cc/min, thus raising serum phosphate levels. This results in hypocalcemia, which is a potent stimulus for PTH secretion. It has been postulated that the parathyroid glands enlarge under these chronic physiologic stimuli and eventually develop parathyroid neoplasia and autonomous hypersecretion of PTH (tertiary hyperparathyroidism (tHPT)). Both diffuse and nodular hyperplasia are found in enlarged parathyroid glands, with nodular hyperplasia being found predominantly in larger glands [1, 2].
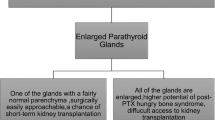
In normal parathyroid glands, negative feedback from increased serum calcium levels reduces secretion of PTH. However, hyperplastic parathyroid glands have decreased expression of the calcium-sensing receptor and this leads to an increased PTH secretion even with hypercalcemia. Nodular hyperplastic parathyroid tissue has also decreased sensitivity to vitamin D due to abnormalities of the vitamin D receptor, leading to an increase in PTH production. Elevated serum levels of PTH correlate with enlargement of the parathyroid glands in patients with sHPT (Fig. 1.) Improvements in dialysis, control of hyperphosphatemia with phosphate binders, normalizing calcium, and supplementation with vitamin D have constituted fairly a successful medical management of patients with sHPT, leading to fewer parathyroidectomies performed in these patients.
Indications for surgery
Parathyroidectomy is indicated when severe sHPT is unresponsive to medical therapy. Signs and symptoms of sHPT alerting to consideration of surgery include bone pain with fractures, osteoporosis, pruritus, hypercalcemia, extraskeletal nonvascular calcifications, tendon rupture, formation of bone cysts, loss of subperiosteal bone, and brown tumors. Coronary and peripheral artery calcifications and calciphylaxis are two complications that contribute to a high mortality in patients with sHPT. High PTH levels (>500 pg/ml) and calcium-phosphorus product >60 are usually found in these patients. Bone disorders in patients with CRF include osteitis fibrosa cystica, osteomalacia, aluminum osteodystrophy, and adynamic bone disease. Bone loss gives the skull a mottled, granular (salt and pepper) radiographic appearance. Parathyroidectomy is indicated in osteitis fibrosa cystica but may exacerbate adynamic bone disease. Parathyroidectomy may ameliorate hypertension, anemia, myocardial dysfunction, peripheral neuropathy, hyperlipidemia, insulin resistance, and mood disorders, and these are relative indications for surgery (Table 1) [3]. Rapid improvement in pain and pruritus is commonly seen after parathyroidectomy with slower improvement in bone density.
Kidney transplantation remains the most effective therapy for sHPT and usually results in a reduction of serum PTH levels. A few patients progress to develop hypercalcemia and tertiary hyperparathyroidism (tHPT). Transient hypercalcemia after a successful transplant occurs in 15% patients but serum calcium levels normalize within one year in approximately 95% of patients. Even though the primary stimulus of renal failure has been removed in these patients, hyperplastic parathyroids fail to involute. The causes of tHPT are not known, but may be due to impaired graft function, prolonged periods of pretransplant dialysis, parathyroid autonomy or impaired vitamin D metabolism. Pretransplant risk factors for tHPT include long duration of pretransplant dialysis and pretransplant hypercalcemia.
Calciphylaxis
Calciphylaxis is relatively a rare syndrome characterized by marked elevations of serum PTH, non-healing wounds, and tissue necrosis in patients with severe sHPT and tHPT. Although the pathogenesis of calciphylaxis is unknown, soft tissue, and large and small vessel calcifications are confirmed on biopsies and radiographs. Vessels in the extremities are often outlined on plain radiographs due to the extensive calcium deposition. Angiography will often show rolling calcium stones in the large vessels and areas of stenosis with limited collateralization. Distribution of necrosis varies depending on other coexisting vascular pathologies. With underlying large vessel disease, tissue necrosis/ischemia is usually seen in the distal lower extremities, and may require amputations. Often, patients have palpable pulses with minimal large vessel involvement and may have tissue involvement in more proximal areas, including abdominal wall, buttocks and thighs and non-healing surgical wounds [4, 5].
In most patients with suspected calciphylaxis, skin biopsy may be helpful to exclude other diagnoses, such as vasculitis, that would not benefit from parathyroidectomy.
Preoperative preparation
Patients with renal failure have many other comorbidities requiring careful assessment prior to pursuing a parathyroidectomy. Cardiovascular disease is the leading cause of perioperative mortality in renal failure patients and must be identified. Routinely, patients at our institution undergo dobutamine stress echocardiography, and many require cardiac optimization including coronary bypass or angioplasty. Dialysis should be done the day prior to surgery with control of hyperkalemia, hypermagnesemia and hypervolemia. Preoperative loading with vitamin D should be done to minimize postoperative hypocalcemia. 1-Deamino-8-D-Arginine Vasopressin should be available for patients with high-blood urea nitrogen levels to improve platelet function and hemostasis. Bone densiometry is routinely done to document baseline osteopenia and for follow-up. Bone biopsies are not performed routinely but may help to establish the diagnosis of osteitis fibrosa cystica in equivocal cases, and support intervention in patients who are otherwise asymptomatic (Table 2).
Localization
Localization studies such as 99Tc-sestamibi scans are efficacious for primary hyperparathyroidism (pHPT) but have a limited role in sHPT and tHPT. Recent analysis of our data (n = 28) shows that even when four large parathyroid glands are encountered during surgery, 99Tc-sestamibi scans show positive uptake in a single location in 75% of patients with sHPT and localize multiple glands in only 55%. In no patients were all parathyroid glands accurately localized. In only 30% of patients were mediastinal glands accurately predicted using 99Tc-sestamibi scan. Because of the expected universal presence of multiple enlarged parathyroids in patients with sHPT and tHPT, and because of the high-incidence of erroneous scans, we do not obtain scans preoperatively in our sHPT patients, and we perform routine bilateral explorations. Other modalities for preoperative localization are CT scan and ultrasonography, which are useful in reoperative surgery and for patient follow-up.
Surgical management
Accepted surgical procedures for management of sHPT and tHPT are subtotal/near-total parathyroidectomy (STPTX), total parathyroidectomy (TPTX), and total parathyroidectomy with autograft (TPTX/AG). The procedure of choice at our institution is a variant of STPTX, the near-total parathyroidectomy (NTPTX) leaving in situ, and a vascularized remnant, which approximates the size of two normal parathyroid glands. All patients undergo a bilateral neck exploration using a transverse cervical incision and all parathyroid glands are identified prior to resection. This allows for the selection of the most appropriate parathyroid gland remnant. Usually this is an inferior parathyroid gland with a good vascular pedicle that is easily accessible at future reoperations. The viability of the remnant is ensured by observing its appearance prior to resection of the other parathyroid glands, and the remnant is expected to be functional immediately after surgery. Size determination of the parathyroid gland remnant is done intraoperatively to confirm that the parathyroid remnant is no larger than two normal parathyroid glands (60–80 mg). Parathyroid remnant mass is estimated by calculating the volume (Vol = π/6 × length × width × height) and assuming that remnant volume (mm3) approximates mass (mg). All parathyroid tissue is confirmed by histopathologic examination on frozen section. Intraoperative parathyroid hormone (IOPTH) levels are obtained after resection of each enlarged gland with the goal of a final IOPTH ≈ 100 pg/ml (Fig. 2). Final IOPTH levels correlate well with parathyroid remnant size and can be followed to determine recurrence. Routine cryopreservation of resected parathyroid tissue is done in the unlikely event that the in situ parathyroid remnant is nonviable or inadequate. Fragments of parathyroid tissue are minced into 1 × 2 mm slices and briefly suspended in sterile cold saline. Approximately 30–40 parathyroid fragments are transferred to each of four 2 ml cryopreservation vials containing a solution of RPMI (Sigma Chemical Co., St. Louis, Mo), 10% DMSO (Sigma) and 10% human serum albumin. These vials are then placed overnight in a −80°C freezer, after which they are transferred to a liquid nitrogen freeze, If the need arises for remiplantation of the cryopreserved parathyroid fragments, one vial is thawed and fragments are assessed for bacterial culture, histology and PTH secretion in vitro in tissue culture. If the culture is negative for bacterial growth, PTH levels are high in tissue culture, and the histology is consistent with >80% cell viability the parathyroid fragments from one to three vials may be reimplanted into a forearm muscle under local anesthesia.
Visual identification of the recurrent laryngeal nerve (RLN) is essential in avoiding injury. In contrast to parathyroid glands in primary hyperparathyroidism, the larger size of hyperplastic parathyroid glands in sHPT and tHPT can significantly alter the course of the RLN. The enlarged glands can displace the RLN laterally, or anteriorly, and can jeopardize a safe dissection for the unsuspecting surgeon. The course of RLN is highly variable near its insertion into the cricothyroid membrane and can be aberrantly displaced due to an enlarged superior parathyroid gland.
TPTX/AG is performed by many endocrine surgeons. This technique involves the removal of all parathyroid tissue and immediate reimplantation of minced fragments into an ectopic muscle, usually the brachioradialis or sternocleidomastoid muscle. The autograft may not immediately function adequately in the early postoperative period and this leads to transient hypocalcemia (Table 3) [6, 7]. TPTX alone has been advocated but has a high incidence of a permanent hypoparathyroid state. TPTX, however, does not completely exclude recurrent sHPT due to presence of supernumerary glands, ectopic parathyroid tissue in the thymus or spillage of parathyroid tissue during surgery. IOPTH measurements can minimize failure by potentially improving detection of supernumerary parathyroid glands. Many surgeons advocate TPTX in the setting of failed SPTX or NTPTX. Ethanol injection under ultrasound guidance has been advocated as a minimally invasive technique. This should be avoided except in the patients with extremely high surgical risk due to high rates of recurrent laryngeal nerve paresis and recurrent hyperparathyroidism.
The technique of parathyroid fragment autografting is important in achieving routine functional autografts. When possible, portions of the smallest, non-nodular glands should be selected. Mincing of tissue should be gentle to minimize all damage, but tiny 1–2 mm fragments should be the goal, to minimize necrosis of tissue after implantation and prior to revascularization. This recommendation is base upon the observations that tissue fragments >2 mm in diameter uniformly suffer central necrosis in in vitro tissue culture, and the knowledge that capillaries are rarely >2 mm apart in mammals. The majority of cells in the body survive by diffusion of nutrients into the interstitial fluid from adjacent capillaries. These observations may explain the 10–15% autograft failure rate, when large fragments are implanted manually.
Tiny 1–2 mm parathyroid fragments are quite difficult to manipulate and autograft using forceps. Therefore, we utilize a 14-gauge angiocatheter attached to a tuberculin syringe. The tiniest fragments are aspirated into the syringe preferentially, and transplanted directly into the body of the ipsilateral sternocleidomastoid muscle or nondominant forearm flexor muscle by inserting the angiocatheter and gently “seeding” the fragments into the muscle. In normal saline, 0.1 cc of tissue fragments approximates the volume (mass) of one normal parathyroid. Autografting of 0.2 cc of fragments is usual. The same syringe + angiocatheter technique is used to transfer additional parathyroid fragments to four cryopreservation vials (≈0.2 cc of fragments per 2 cc vial).
Surgical findings and outcomes
During parathyroidectomy at Emory University Hospital, in 106 out of 133 patients (80%) 4 glands were identified, 8 patients had 5 glands, 4 patients had 2 glands and in 15 patients only 3 parathyroid glands were found [6]. Eight patients had mediastinal parathyroid glands, and 12 patients had ectopic, cervical glands. The mean total burden of parathyroid mass in the patients with renal HPT was 4,526 ± 4,515 g. After NTPTX, this mass was reduced to 77 ± 56 mg. Concomitant thyroid surgery occurred in 30 patients (21%) for thyroid nodules, thyroid cancer and bulky adherent or suspected intrathyroidal parathyroid glands.
Symptomatic improvement occurred virtually in all patients (Table 4) and many patients noted improvement within the first postoperative day. Baseline and follow-up bone density measurements were available in 16 patients with tHPT in this series. Bone densities and T-scores improved in all patients at follow-up 23 ± 25 months after parathyroidectomy. Bone density increased 7.1 ± 6.4%, with an absolute increase from 0.81 ± 0.22 g/cm2 to 0.87 ± 0.22 g/cm2 (P = 0.016) and T-score increased from −2.8 ± 1.3 to −2.1 ± 1.4 (P = 0.09). In 22 patients with calciphylaxis, all extremity wounds healed, and no patient required amputation. There was a dramatic reduction in pain from extremity ulcers with a decrease in narcotic requirement. There were no cases of perioperative death; one patient suffered a permanent RLN injury. Only one patient required a forearm parathyroid autograft from their own cryopreserved parathyroid tissue for persistent hypocalcemia. Six patients remained hyperparathyroid in follow-up. The overall cure rate was 96%, at 23 ± 26 months of follow-up.
Summary
In the setting of renal disease and hyperparathyroidism, a near total parathyroidectomy with cryopreservation is a safe and effective treatment. Even with improved dialysis and medical management, parathyroid surgery remains effective therapy for patients with hyperparathyroidism associated with renal failure. Symptomatic patients need to be identified earlier for parathyroidectomy to prevent progression of bone disease in chronic renal failure.
References
Goodman W. Recent developments in the management of secondary hyperparathyroidism. Kidney Int 2001;50:1187–201
Tominaga Y, Tanaka Y, Sato K, Nagasaka T, Takagi H. Histopathology, pathophysiology and indications for surgical treatment of renal hyperparathyroidism (To be published)
Olson JA, Leight GS. Surgical management of secondary hyperparathyroidism. Adv ren Replac Ther 2002;9:209–18
Beus KS, Stack BC. Calciphylaxis. Otolaryngol Clin N Am 2004;28:941–8
Milas M, Bush RL, Lin P, Brown K, Mackay G, Lumsden A, et.al. Calciphylaxis and non-healing wounds: the role fo the vascular surgeon in a multidisciplinary treatment. J Vasc Surg 2003;37:501–7
Tominaga Y, Uchida K, Haba T, Katayama A, Sato T, Hibi Y, et.al. More than a 1000 cases of total parathyroidectomy with forearm autograft for renal hyperparathyroidism. Am J Kidney Dis 2001;38:168–71
Rothmund M, Wagner PK, Schark C. Subtotal Parathyroidectomy versus total parathyroidectomy and autotransplantation in secondary hyperparathyroidism: a randomized trial. World J Surg 1991;15:745–50
Milas M Weber CJ. Near-total parathyroidectomy is beneficial for patients with secondary and tertiary hyperparathyroidism. Surgery 2004;136(6):1252–60
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, J., Weber, C.J. Surgical management of hyperparathyroidism in renal failure. Clinic Rev Bone Miner Metab 5, 103–107 (2007). https://doi.org/10.1007/s12018-007-0006-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-007-0006-8