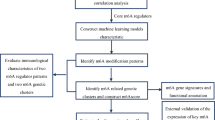
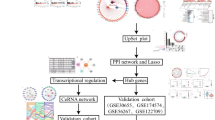
Abstract
Background
Ischemic stroke (IS) is a severe neurological disorder with a pathogenesis that remains incompletely understood. Recently, a novel form of cell death known as disulfidptosis has garnered significant attention in the field of ischemic stroke research. This study aims to investigate the mechanistic roles of disulfidptosis-related genes (DRGs) in the context of IS and to examine their correlation with immunopathological features.
Methods
To enhance our understanding of the mechanistic underpinnings of disulfidptosis in IS, we initially retrieved the expression profile of peripheral blood from human IS patients from the GEO database. We then utilized a suite of machine learning algorithms, including LASSO, random forest, and SVM-RFE, to identify and validate pivotal genes. Furthermore, we developed a predictive nomogram model, integrating multifactorial logistic regression analysis and calibration curves, to evaluate the risk of IS. For the analysis of single-cell sequencing data, we employed a range of analytical tools, such as "Monocle" and "CellChat," to assess the status of immune cell infiltration and to characterize intercellular communication networks. Additionally, we utilized an oxygen–glucose deprivation (OGD) model to investigate the effects of SLC7A11 overexpression on microglial polarization.
Results
This study successfully identified key genes associated with disulfidptosis and developed a reliable nomogram model using machine learning algorithms to predict the risk of ischemic stroke. Examination of single-cell sequencing data showed a robust correlation between disulfidptosis levels and the infiltration of immune cells. Furthermore, "CellChat" analysis elucidated the intricate characteristics of intercellular communication networks. Notably, the TNF signaling pathway was found to be intimately linked with the disulfidptosis signature in ischemic stroke. In an intriguing finding, the OGD model demonstrated that SLC7A11 expression suppresses M1 polarization while promoting M2 polarization in microglia.
Conclusion
The significance of our findings lies in their potential to shed light on the pathogenesis of ischemic stroke, particularly by underscoring the pivotal role of disulfidptosis-related genes (DRGs). These insights could pave the way for novel therapeutic strategies targeting DRGs to mitigate the impact of ischemic stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is a prevalent and globally distributed disease that poses a significant threat to the health and quality of life of populations worldwide (Muse et al., 2018). Typically, it is caused by the obstruction of a cerebral artery, leading to brain ischemia or hypoxia. The hallmark of the disease is the disruption or substantial reduction of blood supply to a brain region, which is often a fatal event (Zhao et al., 2009). Distinguishing between transient ischemic attack (TIA) and ischemic stroke within the thrombolysis time window has been a formidable challenge in medical research (Campbell & Khatri, 2020). Although advanced imaging techniques can assist in diagnosing TIA within a narrow time frame, their limited use is due to complexity and cost constraints. Recent studies have highlighted the broad diagnostic and prognostic potential of biomarkers related to oxidative stress, inflammation, and neurological damage for ischemic stroke, given their noninvasive, rapid, and cost-effective nature (Al-Bahrani et al., 2007).
Programmed cell death (PCD) is a process governed by specific molecular pathways and can be influenced by genetic or pharmacological interventions. PCD is essential for organism development and cellular homeostasis, and its dysregulation is linked to various diseases, including cancer and inflammatory conditions (Galluzzi et al., 2018). Recombinant Solute Carrier Family 7 Member 11 (SLC7A11) is crucial for cystine uptake, glutathione synthesis, and the attenuation of oxidative stress and disulfidptosis. However, a study from 2017 indicated that SLC7A11 significantly enhances cell death under conditions of glucose starvation (Koppula et al., 2017; Shin et al., 2017). Disulfidptosis, a novel form of cell death characterized by excessive intracellular cysteine accumulation and the subsequent generation of disulfide bond molecules, has been identified in recent research. Under glucose-starved conditions, the upregulation of SLC7A11 can deplete NADPH, leading to the abnormal accumulation of disulfide compounds like cystine, which induces disulfide stress and rapid cell death (Liu et al., 2020). Therefore, strategies to reduce disulfide compound formation and correct the sulfhydryl-disulfide bond imbalance may be pivotal in improving outcomes for ischemic stroke.
Ischemic stroke is marked by the cessation of blood flow to the brain, causing hypoxia and nutrient deprivation in the affected area, which can result in brain damage and cell death. The inflammatory response is a key player in this process, further exacerbating brain injury. The release of inflammatory mediators, such as cytokines and other chemical agents, can provoke an inflammatory reaction that leads to additional brain tissue damage (Tan et al., 2019). Moreover, the inflammatory response can compromise the integrity of the blood–brain barrier (BBB), a critical interface that protects the brain from harmful substances. A compromised BBB allows for the infiltration of inflammatory cells and other harmful agents, worsening the injury (Tan et al., 2017). The activation of inflammatory cells, including macrophages and astrocytes, and their release of mediators can initiate a cascade of reactions that damage brain tissue (Lee et al., 2016). While the immune response is crucial for clearing dead cells and facilitating tissue repair, it can also trigger an inflammatory reaction that amplifies tissue damage. Therefore, a thorough understanding of the interplay between the immune system and ischemic stroke is essential for developing effective preventative and therapeutic strategies.
The exploration of cellular demise pathways not only deepens our understanding of cellular equilibrium but also offers critical insights for therapeutic interventions in conditions like stroke. Emerging evidence suggests that disulfidptosis, potentially triggered by the inhibition of glucose transporters (GLUTs), may be an underappreciated mechanism in the pathogenesis of ischemic stroke. Future research will focus on elucidating the mechanisms of disulfidptosis and its interaction with the inflammatory response in ischemic stroke. Our study aims to identify key molecules and pathways involved in disulfidptosis and to explore potential therapeutic approaches that could modulate these pathways. We hope that our findings will contribute to mitigating tissue damage associated with disulfidptosis and ischemic stroke, thereby fostering innovative strategies for addressing these interconnected conditions.
Materials and Methods
Sources and Pre-Processing of Raw Data
Two expression matrices of human peripheral blood from patients with ischemic stroke (GSE16561 and GSE58294) were obtained from the Gene Expression Omnibus (GEO). The GSE16561 cohort used the Illumina sequencing platform to study peripheral blood samples from 39 patients diagnosed with ischemic stroke (over 18 years old) and 24 healthy non-stroke control patients (Barr et al., 2010). The GSE58294 cohort included blood from cardiogenic stroke patients and controls, with RNA extracted and analyzed using the whole-genome U133 Affymetrix array. Researchers examined 23 control samples and 69 cardiogenic stroke samples, with the latter group analyzed at three time points post-event: within 3 h, 5 h, and 24 h (Stamova et al., 2014). RMA normalization is a processing method for Affymetrix microarray data that generates gene expression estimates through background correction, probe-level normalization, and calculation of average probe set expression values. The "affy" R package provides functions to perform RMA normalization, typically involving reading CEL files, applying the RMA algorithm, and extracting and using the normalized expression values for further analysis. Subsequently, we used the "limma" package for data normalization and batch correction, and the "ComBat" function from the "sva" package to further adjust for batch effects (Leek et al., 2012). In addition, we also downloaded single-cell RNA sequencing data of mouse brain tissue containing 58528 cells from GSE174574 (Zheng et al., 2022). In this dataset, three mice underwent middle cerebral artery occlusion (MCAO) surgery, while the other three underwent sham operations as a control group. The latest research article mentions 10 genes associated with disulfidptosis, which are SLC7A11, SLC3A2, RPN1, NCKAP1, NUBPL, NDUFA11, LRPPRC, OXSM, NDUFS1, and GYS1 (Liu et al., 2023). These genes are referred to as disulfidptosis-related genes (DRGs). A total of 29 inflammatory pathway-related genes were exported from the Molecular Signature Database (https://www.gsea-msigdb.org/gsea/msigdb/cards/BIOCARTA_INFLAM_PATHWAY).
Machine Learning-Based Screening Method for Key Genes
Three machine learning algorithms, Least Absolute Shrinkage and Selection Option (LASSO), Random Forest, and Support Vector Machine-Recursive Feature Elimination (SVM-RFE), were selected for their distinct capabilities in screening hub genes from high-dimensional data. The LASSO algorithm, known for its effectiveness in reducing dimensionality and enhancing model sparsity, was chosen for its ability to zero out irrelevant features, thereby simplifying the model and enhancing interpretability (Engebretsen & Bohlin, 2019). We utilized the "glmnet" R package to implement LASSO with a tuning parameter (lambda) optimized through tenfold cross-validation, which balances the trade-off between model complexity and goodness of fit.
The Random Forest algorithm, a robust supervised learning method, was chosen for its ability to handle a large number of variables without variable deletion and its capacity to identify the most important features. We employed the "random forest" R package to construct multiple decision trees and estimate the importance of each candidate hub gene based on the average decrease in impurity. By determining the importance scores, we identified the top ten centroid genes with the highest scores.
Lastly, the SVM-RFE algorithm was selected for its efficiency in feature selection, particularly in small sample size scenarios (Huang et al., 2014). It iteratively removes less important features, thereby reducing redundancy and focusing on the most relevant variables for the outcome. We applied SVM-RFE with tenfold cross-validation to ensure the stability and generalizability of the selected features, which is crucial for the predictive performance of the model. The combination of these algorithms, each with its unique strengths and hyperparameter tuning strategies, provided a comprehensive approach to identifying key genes in the dataset.
Construction and Validation of Nomograms
In this study, we implemented a meticulous variable selection and model-building process to ensure the accuracy and clinical utility of the nomogram. Initially, we conducted multivariate Logistic regression analysis using the "RMS" R package, integrating key genes with clinical features to construct a predictive model. During the model development, we not only considered the statistical significance of variables but also optimized their selection through stepwise regression to prevent overfitting and enhance the model's interpretability. To assess the model's predictive power, we calculated the area under the receiver operating characteristic curve (AUC) using the "ROCR" R package, a standard measure for evaluating the model's ability to discriminate between different outcomes. Additionally, we plotted calibration curves to evaluate the consistency between the model's predicted probabilities and actual occurrence rates, thereby validating the model's accuracy. To further assess the clinical applicability of the nomogram, we performed Decision Curve Analysis (DCA), which helps to determine the clinical benefits of the model at various threshold probabilities.
For internal validation, we employed the Bootstrap method, simulating different sample distributions through 100 repeated resamplings, thereby assessing the model's robustness. The rationale behind selecting 100 resamples was to ensure adequate sample variability while striking a balance between computational efficiency and model stability. This approach allowed us to more accurately estimate the model's performance on new data. Finally, we evaluated the model's consistency using the Hosmer–Lemeshow goodness-of-fit test, a statistical method to examine the differences between predicted probabilities and actual observed frequencies. Through these comprehensive assessment steps, we ensured the reliability of the nomogram model and its potential application in clinical decision-making.
Consensus Cluster Analysis and Prediction of Immune Cell Infiltration
In this study, we used the "IOBR" software package, which incorporates eight published techniques for quantifying the tumor microenvironment (TME). These techniques include CIBERSORT and MCPcounter, among others, and we used these tools to batch process and visualize the composition of the TME in order to more accurately capture the relative abundance of different cell type (Zeng et al., 2021).
In order to accurately determine the optimal number of clusters and to assess the clinicopathological characteristics of different subgroups of patients, we used the "ConsensusClusterPlus" software package to perform the cluster analysis. The parameters of the package were configured to refine the clustering process and to ensure the consistency and stability of the analysis results. In the parameter settings, the reps parameter is set to 100, which means that 100 cluster analyses are performed to evaluate the consistency of the results across different clusters. pItem and pFeature parameters are used to set the thresholds for the color of the cells in the consensus matrix, which are set to 0.8 and 1, respectively. In addition, the K-means algorithm is chosen as the core clustering method, and Euclidean distance as the most commonly used distance metric. The "ConsensusClusterPlus" software package demonstrates the consistency of the different clustering results by analyzing the clustering of multiple data subsets and constructing a consensus matrix. Consensus Cumulative Distribution Function (CDF) plots and the relative change in the area under the CDF curve provide a quantitative assessment metric for judging the reasonableness of the number of clusters (Wilkerson & Hayes, 2010).
Processing of Single-Cell RNA Sequencing Data from Mouse Brain Tissue
We obtained single-cell gene expression profiles of mouse brain tissue from the GSE174574 dataset and pre-processed the scRNA-seq data using the "Seurat" R package (Hao, et al., 2021). In our single-cell sequencing data analysis process, we first meticulously selected a high-quality dataset using the "PercentageFeatureSet" function, excluding cells with abnormal mitochondrial gene proportions, low cell counts, or extreme gene expression levels. Subsequently, we applied the "NormalizeData" function to standardize the gene expression data of the selected cell population.
We calculated the expression variance coefficient or standard deviation for each gene to assess their expression variability within the cell population, and selected highly variable genes (HVGs) based on preset thresholds. Building on this, we conducted Principal Component Analysis (PCA) using these 2000 HVGs to reveal the main sources of variation among cells. By normalizing the scRNA-seq data and converting it into Seurat objects, we precisely identified these key HVGs using the "FindVariableFeatures" function. Furthermore, we performed cell clustering based on the top 20 principal components (PCs) extracted from PCA analysis. By combining the modularity optimization clustering algorithm based on shared nearest neighbors (SNN) and the Uniform Manifold Approximation and Projection (UMAP) technique, and using the recommended Neighbors parameter with an appropriate resolution, we effectively reduced the dimensionality of the data and visualized the cells in a two-dimensional space. To gain a deeper understanding of the characteristics of each cluster, we utilized the "FindAllMarkers" function to identify unique marker genes for each cluster. Finally, to interpret and annotate the different cell populations at the biological level, we employed the "SingleR" package, effectively correlating our cell classification results with known cell types.
Based on the expression of the 10 DRGs, we used a commonly used algorithm "AddModuleScore" to score the microglia data for gene sets (Tirosh et al., 2016). The pseudo-time trajectories of microglia were also analyzed using the "Monocle" R package (Borcherding et al., 2019). Finally, ""CellChat"" was used to analyze the intercellular communication network, assess the expression levels of receptors and ligands, and infer potential intercellular interactions (Jin et al., 2021).
Cell Culture and Cell Transfection
The human microglia (HMC3) were purchased from Pocell (Procell, China). Cells were cultured in Minimum essential medium(MEM including NEAA) (PM150467, Procell, China) supplemented with 10% fetal bovine serum (FBS)(Gibco). All the cells were maintained in a humidified incubator with a 5% CO2 atmosphere at 37 °C.
SLC7A11 overexpression plasmid based on pcDNA3.1/ + vector were designed and synthesized by GenePharma(Suzhou, China). The plasmids transfections were performed by using the Lipofectamine 3000 (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. HMC3 cells cultured in 6-well plates were transfected with SLC7A11-specific siRNAs (GenePharma, Suzhou, China) using Lipofectamine 3000 (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. After 48 h of transfection, the cells were collected and used for further analysis. The control siRNA treated or mock-transfected cells were used as negative control.
Immunofluorescence Staining
Cultured cells grown on coverslips were fixed with 4% paraformaldehyde in PBS for 15 min. After rinsing, cells were incubated with a blocking solution (10% normal goat serum (NGS) and 0.5% Triton X-100 in PBS) for 1 h at RT followed by incubation with primary antibodies overnight at 4 °C. After washing with 1 × PBS, the cells were incubated with cells were incubated with appropriate goat Alexa fluor 555/488 secondary antibodies (1:200, Thermo scientific Life Technologies Corporation) for 1 h at RT. The nuclei were counterstained with Hoechst (1:50, Thermo scientific Life Technologies Corporation). The image was shotting with EVOS (Thermo scientific Life Technologies Corporation).
Oxygen Glucose Deprivation/Reoxygenation (OGO/R) Cell Model
HMC3 cell were treated with OGD/R induction to cell model of ischemic stroke according to previous study (Huang et al., 2019). In brief, cells were washed and incubated in the OGD medium (glucose-free DMEM) in an anaerobic chamber containing a 95% N2 and 5% CO2 mixtures for 6 h at 37 °C. After OGD, cells were rinsed twice and returned to standard medium for 12 h.
Western Blots
Cells were lysed using RIPA buffer (#9806, CST) including the protease inhibitor. The extracted proteins were stored at − 80 ℃. For western blot analysis, 8–10% SDS–PAGE was used to resolve equal amounts of protein samples from both cell lysate and supernatant. Briefly, lysates were subjected to SDS–PAGE and transferred onto PVDF membranes (Millipore). The membranes were blocked with 5% nonfat milk and 0.05% Tween-20 (TBST) at room temperature for 1 h, followed by an overnight incubation at 4 °C with primary antibodies specific for the following proteins, as required by each experiment: iNOS (1:1000 dilution, ab178945, Abcam), Cox2 (1:1000 dilution, #12,282, CST), Gapdh (1:5000 dilution, 60,004–1-Ig, Proteintech). Next day, incubated membranes were washed three times with TBS-T, and then incubated with the horseradish peroxidase (HRP)-conjugated secondary antibodies at the dilution of 1:5000 for 1 h at room temperature. Finally, the antigen–antibody complex was screened by chemiluminescence using the Immobilon Western Chemiluminescent HRP Substrate (Millipore, USA). Housekeeping protein GAPDH was selected as an internal control. Each experiment was conducted in triplicate.
Quantitative RT-PCR
Total RNA was extracted by using TRIzol Reagent (Invitrogen, Carlsbad, USA). Total RNA concentration was measured by a Nanodrop spectrophotometer and 1 μg RNA was used for reverse transcription using cDNA Revert-Aid First Strand cDNA Synthesis Kit. All experiments were performed in triplicate and the specificity of the qPCR products was verified by melting curve analysis. GAPDH was considered as endogenous control. Sequences of all primers are provided (Supplementary Table 1).
Statistical Analysis
All raw data processing was performed in R software (version 4.2.1). Nonparametric Wilcoxon rank sum test was used to test the relationship of continuous variables between two groups. Kruskal-Wallis test was used to explore the differences between more than two independent groups. Correlation coefficients were examined using Spearman correlation analysis. In all statistical investigations, P < 0.05 was considered statistically significant.
Results
Identification of Key Genes Associated with Disulfidptosis in Ischemic Stroke by Machine Learning
First, we obtained gene expression profile data containing 47 control samples and 108 IS samples by the "sva" R package after removing the batch effect between the two cohorts GSE16561 and GSE58294 (Supplementary Fig. 1A, B). By the ssGSEA approach, we assessed the disulfidptosis signature score for each sample, which was relatively higher in the IS group, suggesting that disulfidptosis is likely to be involved in the process of IS (Fig. 1A). The heatmap in Fig. 1B shows the relative expression of the 10 DRGs in the CT and IS groups.SLCA711 expression was highly up-regulated in the IS group whereas NDUFA11 expression was down-regulated (Fig. 1C, D). Figure 1E demonstrates the correlation of the expression of these DRGs. Subsequently, we used three machine learning algorithms to select key genes for IS. By LASSO regression analysis, seven variables were identified as markers for the diagnosis of IS (Fig. 1F, G). For the SVM-RFE algorithm, the classifier had the highest accuracy when the number of features was 6 (Fig. 1H). In the Random Forest algorithm, the error rate is usually stable when about 54 decision trees are used (Fig. 1I). We selected 8 DRGs with relative importance greater than 5 (Fig. 1J). We finally selected 5 key DRGs-SLC7A11, RPN1, NUBPL, NDUFA11, and NDUFS1-according to the LASSO algorithm, the random forest algorithm, and the SVM-RFE algorithm (Fig. 1K). These genes not only show high importance in machine learning, but also play a key role in the classification of IS samples. For example, high expression of SLC7A11 is closely associated with the pathogenesis of IS and may affect cellular responses to specific metabolic stresses.
Identification of disulfidptosis-related genes (DRGs) in ischemic stroke (IS). A Box line plots of disulfidptosis enrichment scores assessed by the ssGSEA method in normal control (CT) and IS groups, with relatively higher disulfidptosis scores in the IS group. B Heat map showing the relative expression of DRGs in CT and IS groups. C, D Volcano and box line plots showing the up- and down-regulated expression of DRGs in the IS group, respectively. E There is a correlation between the expression of DRGs. F Tenfold cross-validation for tuning parameter selection in the LASSO model. Each curve corresponds to one gene. G LASSO coefficient analysis. Vertical dashed lines are plotted at the best lambda. H SVM-RFE algorithm for key DRGs selection with the highest classifier accuracy when the number of features is 7. I Relationship between the number of random forest trees and the error rate. J Ranking of relative importance of DRGs. K Wayne diagram showing the selection of five feature genes based on the LASSO algorithm, Random Forest algorithm, and SVM-RFE algorithm
Construction of the Nomogram and Analysis of Immune Infiltration
To further explore the potential of disulfidptosis-related gene (DRG) features in diagnosing IS patients, based on the results of logistic regression (Supplementary Fig. 2A), we included five key DRGs in the construction of the nomogram (Fig. 2A). In the nomogram, each feature gene corresponds to a score, and the total score obtained by summing the scores of all genes corresponds to the risk of developing ischemic stroke. The calibration curve demonstrates that the nomogram can accurately estimate the incidence of IS (Fig. 2B). After integrating these five DRGs, the AUC of the ROC curve was 0.843 (Fig. 2C). This indicates that these five genes have good diagnostic efficiency in predicting the progression of ischemic stroke. As shown in the decision curve analysis (DCA), IS patients can benefit from the nomogram (Fig. 2D). Subsequently, we used the bootstrap method for internal validation of the accuracy of the nomogram, randomly sampling the dataset (n=100 bootstraps) and performing permutations (Supplementary Fig. 2B). The ranges of AUC, specificity and sensitivity distributions for the replacement cohort are shown in Supplementary Fig. 2C-E, respectively. Furthermore, the results of Spearman correlation analysis show that the five DRGs exhibit certain correlations with inflammation pathway-related genes. For instance, the expression of NUBPL shows a strong positive correlation with IL10 (Fig. 2E). According to the results of the CIBERSORT and MCP-Counter algorithms, we demonstrate the correlation between the expression of the five DRGs and the infiltration of different immune cells (Fig. 2F, G). For instance, in both algorithms, SLCA711 shows a significant negative correlation with CD8+T cells. In summary, the Nomogram has demonstrated significant clinical value in the risk assessment and management of ischemic stroke, not only improving the accuracy of risk prediction but also offering new perspectives for patient management and treatment. With further research and practice, the Nomogram is expected to become an important tool in clinical practice, improving the prognosis and quality of life for patients with IS.
Analysis of the correlation between the construction of the Nomogram and the immunization model. A Nomogram of 5 key genes in the diagnosis of OA patients. B Calibration curve of the diagnostic model. C Subject operating characteristic (ROC) curves based on 5 key genes in IS diagnosis. D Decision curve analysis showing the clinical benefit of the column-line graph. E Correlation between key gene expression and genes related to inflammatory pathways. F Correlation between key gene expression and 22 immune cell infiltration (CIBERSORT). G Correlation between key gene expression and abundance of 8 immune cells (MCP-Counter)
Identification of Disulfidptosis-Related Clusters in Ischemic Stroke
To thoroughly understand the patterns of disulfidptosis signaling involved in the occurrence of IS, we employed a consensus clustering algorithm to classify samples based on the expression of four key DRGs (Fig. 3A). The results of the cumulative distribution function (CDF) curve indicated that K = 4 was the optimal number of clusters (Fig. 3B). Therefore, the integrated cohort was divided into four disulfidptosis-related clusters. We integrated the gender, age, time after stroke occurrence, the probability of occurrence for each patient, and the expression levels of all DRGs, and the heatmap displayed significant heterogeneity in the expression of the four key genes (Fig. 3D). Furthermore, according to the results of the logistic regression algorithm, patients in cluster 2 exhibited a higher probability of occurrence (Fig. 3C). Interestingly, patients in cluster 2 also had a lower time after stroke occurrence (Fig. 3E). Subsequently, we observed significant heterogeneity in the expression of the five key DRGs among the four clusters, with the highest expression of SLC7A11 in cluster 2 (Fig. 3F). According to the results of the MCP-Counter algorithm, patients in group 2 also had relatively low levels of T cells (Fig. 3G). These findings suggest that patients with Cluster 2 may have specific biological and immunological characteristics that are associated with the onset and progression of ischemic stroke.
Clustering typing and identification of subgroup features based on 5 key genes. A All samples in the IS cohort were categorized into 4 clusters using a consensus clustering algorithm (k = 4). B Cumulative distribution function (CDF) for k = 2–10. C The likelihood that a patient in 4 clusters will be diagnosed with IS. D Heterogeneity in the expression of DRGs across the 4 CLUSTERS was associated with various clinical traits. E Proportion plot of time since the onset of IS for patients in the 4 CLUSTERS. F Differential expression of key genes in the 4 clusters. G Differences in the abundance of 8 types of immune-related cells in the 4 clusters
Identification of Disulfidptosis Patterns in Ischemic Stroke Based on Single-Cell Sequencing Data
Single-cell gene expression profiles were obtained from the GSE174574 dataset for brain tissues of mice, three of which underwent middle cerebral artery occlusion (MCAO) and the other three underwent sham operations. We first normalized the sequencing depth and the number of genes to obtain 2000 highly variable genes after downscaling using the PCA method and further analyzed them according to the first 20 PCs for further analysis. After obtaining 21 clusters, we utilized the "SingleR" function to annotate cell subpopulations. A total of 9 cell types including fibroblasts, macrophages, endothelial cells, monocytes, epithelial cells, microglia, oligodendrocytes, astrocytes, and granulocytes were annotated. Figure 4A demonstrates the percentage of different cell types in the six samples, with endothelial cells comprising the major component. UMAP was used to display different samples, clusters, and cell types (Fig. 4B-D). The top 5 marker genes in each cell type are presented in the heatmap (Fig. 4F). Regarding, only HLA-DRA was not detected in single-cell sequencing, we demonstrated the expression of 5 key DRGs (SLC7A11, RPN1, NUBPL, NDUFA11, and NDUFS1) in the MCAO group and sham group by UMAP plot (Fig. 4E).
Classification of cell subpopulations in mouse brain tissue. A Histogram of cell type content for different samples. B-D UMAP plots of different samples, clusters of cells, and cell subpopulations correlated by color. E Expression of DRGs in sham samples and MCAO samples demonstrated by UMAP plots. F Heatmap showing the relative expression of marker genes for 8 cell subpopulations. Red color represents highly expressed genes and blue color represents lowly expressed genes
Based on the expression of all 10 DRGs, we evaluated the level of disulfidptosis in scRNA-seq using the algorithm AddModuleScore, which is commonly used for scoring gene sets. As shown in Figs. 5A and C, fibroblasts had relatively higher enrichment scores. Meanwhile, we also compared the enrichment scores of various cell types in MCAO and sham samples. Interestingly, microglia in the MCAO samples had relatively higher disulfidptosis enrichment scores, which was the focus of our next study (Fig. 5B). So we extracted the microglia in scRNA-seq for further clustering and dimensionality reduction analysis. Further clustering yielded a total of 12 microglia subpopulations. Sham samples had fewer microglia, mainly clustered in clusters 0, 1, 2, and 9, while MCAO samples had more microglia, mainly clustered in clusters 3, 4, 5, 6, 10, and 11 (Fig. 5D). Among them, subpopulation 3 had a relatively higher disulfidptosis enrichment score (Fig. 5E, G). Cell trajectory analysis can validate known cell differentiation relationships and tap into the dynamics of immune cells, screening for differentiation-critical cell subpopulations and genes. We observed that microglia in mouse brain tissue samples appeared in a total of three cell states during development, and the developmental trajectory of microglia also differentiated from sham to MCAO states (Fig. 5H). The heatmap in Fig. 5F demonstrates the expression of the 10 DRGs at different periods of microglia development, with SLC7A11 being expressed mainly at the end of microglia development.
Disulfidptosis enrichment scores of cell subpopulations and pseudo-time analysis. A The disulfidptosis enrichment scores of each cell type in ischemic stroke are demonstrated by bubble plots. B Differences in disulfidptosis enrichment scores for each cell type in sham and MACO samples. C The disulfidptosis enrichment scores for each cell type were demonstrated by UMAP plots. The darker the green color, the higher the score. D The clustering of microglia in sham and MACO samples is demonstrated by UMAP plots. A total of 12 cell subpopulations were obtained in microglia. F Heatmap showing the expression of 5 key DRGs during microglia development. Red color represents high expression and blue color represents low expression. E Differences in disulfidptosis enrichment scores of the 12 microglia subpopulations. G Dual-sulfur death enrichment scores of 12 microglia subpopulations demonstrated by UMAP plots. H Cell trajectory and pseudo-time analysis of microglia
To fully understand the immune microenvironmental features and signals associated with disulfidptosis in ischemic stroke, we used "CellChat" to visualize cellular communication in infiltrating cells in brain tissue. Microglia were first categorized into high and low-scoring groups based on the median value of their disulfidptosis enrichment score. Network plots demonstrated the number and weight of interactions between high-scoring and low-scoring microglia and other cells (Fig. 6A, B). We found that the TNF signaling pathway pathway may play an important role in the higher-scoring microglia and other cells (Fig. 6C, D). We evaluated the Outgoing signaling patterns and Incoming signaling patterns of various types of signaling pathways among different cells (Fig. 6E). However, inconsistent with our conjecture, immunofluorescence assays showed that overexpression of slc7a11 promoted M2 polarization of microglia (as indicated by the M2 marker Arg1) and inhibited M1 polarization (iNOS is an M1 marker) after OGD/R, thereby attenuating the inflammatory response (Fig. 6F).
Analysis of cellular communication features related to disulfidptosis and polarization of microglial cells. A, B The number of cell–cell interactions and total interaction strength calculated by the R package "CellChat". C Strength of TNF cell signaling pathway between different cell types. D Likelihood of ligand-receptor association between different cell types. E A heat map displaying the intensity of incoming and outgoing interactions between different cell subpopulations. F HMC3 cell transfected with SLC7A11 after OGD/R iNOS immunostaining (green) and Arg1 immunostaining (red) following OGD 6 h/R 12 h. Scale bar, 50 μm
Validation of in vitro Experiments
To further investigate the effect of slc7a11 on m1/m2 polarization in hmc3 cells after ogd/r, we transfected hmc3 cells with overexpression plasmid and siRNA, respectively, and subjected them to OGD/R treatment, and collected proteins and RNAs to detect M1/M2 markers, respectively. After overexpression of slc7a11, the expression of iNOS/Cox2 protein level was decreased (Fig. 7A, B), and the level of microglia M1 polarization was reduced. The effect of SLC7A11 on the polarization of HMC3 cells was further verified by examining the mRNA expression levels. mRNA expression levels of the M1 polarization markers CD68/CD86 increased after OGD/R treatment and decreased after overexpression of slc7a11, while mRNA expression levels of the M2 polarization markers CD206/Arg-1 decreased after OGD/R treatment and decreased after overexpression of slc7a11. decreased after treatment and increased after overexpression of slc7a11 (Fig. 7E). Knockdown of slc7a11 expression using siRNA resulted in increased expression of iNOS/Cox2 protein levels (Fig. 7C, D), while mRNA expression levels of M1 polarization markers increased and M2 polarization markers decreased (Fig. 7F).
Effect of overexpression and knockdown of SLC7A11 on activation of HMC3 cells after OGD/R. A, B Representative images of western blot for M1 markers including iNOS and COX2 in HMC3 cells which transfected with negative control (NC) plasmid or overexpression (OE) plasmid. The quantitative analyses of western blots were shown as a ratio compared with the internal (n = 3, each group). *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant. C, D Representative images of western blot for M1 markers including iNOS and COX2 in HMC3 cells which transfected with siRNA negative control and siRNA. The quantitative analyses of western blots were shown as a ratio compared with the internal (n = 3, each group). *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant. E, F Real-time PCR findings elucidate the mRNA expression of M1/M2 markers (CD68, CD86, Arg-1, CD206) within hmc3 cells, both overexpressing and knocking down the SLC7A11 gene. The relative mRNA expression was calculated by 2 − △△CT value. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant
Discussion
Ischemic stroke is defined as necrosis of brain tissues due to stenosis or occlusion of arteries supplying blood to the brain (carotid and vertebral arteries) and insufficient blood supply to the brain, in which various kinds of programmed cell deaths, such as autophagy, ferroptosis, and necrotic apoptosis, are the important pathological endpoints induced by ischemic stroke injury (Zhou et al., 2021). In ischemic stroke, the occurrence of cell death is associated with multiple signaling pathways and molecular mechanisms. For example, ischemic stroke leads to a decrease in intracellular ATP levels, which in turn affects intracellular energy metabolism and cell membrane stability, ultimately leading to cell death. In addition, ischemic stroke triggers pathological processes such as inflammatory response and oxidative stress, which further promote cell death (Tuo et al., 2022). Disulfidptosis is an emerging form of programmed cell death that is distinct from other known mechanisms of programmed cell death such as apoptosis, autophagy, copper death, and ferroptosis. As a unique cell death mechanism, disulfidptosis offers new insights into how cells respond to metabolic stress and regulate cell fate. However, in the context of ischemic stroke, the interplay of disulfidptosis signaling pathways remains to be elucidated. Therefore, a profound understanding of the association between cell death and ischemic stroke is crucial for uncovering the pathophysiological mechanisms of the disease, seeking therapeutic strategies, and improving patient outcomes.
The application of machine learning to ischemic stroke transcriptomic data has become a hot area of current research. Ischemic stroke is a common and serious neurological disease, and the introduction of machine learning has provided researchers with an effective tool for identifying key molecular and biological processes associated with disease progression, as well as predicting disease risk and prognosis in patients (Heo et al., 2019). Our study identified key genes associated with ischemic stroke by LASSO regression analysis, SVM-RFE algorithm, and random forest algorithm and constructed corresponding diagnostic models. The role and significance of these algorithms in the study are explained and the identified key DRGs (SLC7A11, RPN1, NUBPL, NDUFA11, and NDUFS1) are emphasized as having important diagnostic and prognostic implications for ischemic stroke.
Among the genes we studied, SLC7A11 is the light chain subunit of System Xc-. It is a membrane transporter protein involved in intra- and extracellular cysteine/glutamate exchange to maintain intracellular glutathione synthesis (Lo et al., 2008). The main function of SLC7A11 is to regulate cellular sensitivity to Ferroptosis, thereby protecting cells from oxidative damage and lipid peroxidation-induced cell death.The expression level of SLC7A11 can be affect the sensitivity of cells to Ferroptosis through regulation at the transcriptional level or post-transcriptional level. Therefore, high expression of SLC7A11 can protect cells from Ferroptosis damage (Li et al., 2023). SLC7A11 was found to play an important role in a variety of acute injurious diseases, such as acute lung injury, acute kidney injury, acute liver injury, myocardial ischemia–reperfusion injury, and acute cerebral hemorrhage (Liu et al., 2022; Matthay et al., 2019; Zhang et al., 2016). Interestingly, a recent study points to another mode of cell death induced by SLC7A11. In the presence of high SLC7A11 expression, glucose starvation restricts NADPH production by the glucose-pentose phosphate pathway leading to a massive accumulation of small molecule disulfides, including cystine, causing a series of redox defects and cell death (Zhao et al., 2023). Based on our initial explorations and numerous previous studies, the SLC7A11-related signaling pathway is expected to be a target for therapeutic intervention in ischemic stroke. On the other hand, high expression of SLC7A11 can drive disulfidptosis to induce stroke. How to utilize the common mechanism of disulfidptosis and Ferroptosis to block both may become an important strategy for future ischemic stroke treatment.
Ischemic stroke is a serious neurological disease, and due to the heterogeneity of brain tissues, we are unclear about the unique roles of different cell subpopulations within the neurovascular unit and peripheral immune cells in ischemic stroke. Compared with traditional transcriptome sequencing methods, single-cell sequencing possesses higher resolution and sensitivity in the study of stroke, which helps to deeply explore cellular heterogeneity, the functions of low-expressed genes, cell–cell interactions, and changes in cellular states, providing us with a more comprehensive insight that can help to unravel the pathomechanisms and potential therapeutic targets of stroke (Kiss et al., 2020).
Microglia are a class of immune cells in the central nervous system whose main functions are to remove cellular waste, regulate the neuronal environment, and participate in immune responses. Studies have shown that after stroke, microglia are activated and release inflammatory mediators, such as cytokines and oxygen free radicals, leading to neuroinflammatory responses and cellular damage. In addition, microglia are involved in neural repair processes, promoting neuronal regeneration and synaptic remodeling (Zhu et al., 2022). Stroke-induced ischemia and reperfusion injury lead to neuronal cell death. It was found that in stroke, cell death occurs mainly through the apoptotic pathway, while necrosis occurs mainly at the edges of ischemic regions. Analysis of single-cell sequencing data allowed us to gain a comprehensive understanding of the role of different cell types in brain tissue in ischemic stroke. In particular, the key role of microglia in the inflammatory process has received much attention. Our findings showed that the expression levels of disulfidptosis-related genes in microglia were significantly elevated during ischemic stroke, suggesting that disulfidptosis may play an important role in the inflammatory response to stroke. In addition, we further revealed the differences in the enrichment scores of disulfidptosis in different cell subpopulations, especially the relatively higher enrichment scores of microglial cells in MCAO samples, which provided key clues to gain insights into the role of microglia in ischemic stroke.
When ischemic stroke occurs, the microenvironment of microglia changes and polarizes in response to peripheral inflammation. In the early phase of ischemic stroke, microglia tend to present an M2 phenotype that is responsive to acute injury, and then microglia transform into an M1 phenotype that induces an inflammatory response (Perego et al., 2011). The mechanisms underlying microglia polarization during ischemic stroke involve multiple pathways that are not fully understood. By analyzing cell trajectories, we found that microglia show specific developmental state transitions during ischemia, which contributes to our understanding of the dynamic process of cellular changes in the inflammatory response.
Current research suggests that the type of microglia polarization is determined by signaling pathways. Understanding the precise mechanism of microglia polarization could lead to breakthroughs for treatment.TNF-α/IKKβ/NF-κB signaling pathway is a key signaling pathway mediating inflammatory response.TNF-α can bind to TNF receptor-related factors on the cell membrane surface and mediate multiple downstream signaling pathways such as the IKKβ/NF-κB signaling pathway, which promotes inflammatory response and stroke progression (Schneider et al., 1999). NF-κB signaling mediates the expression of matrix metalloproteinases (MMPs), leading to blood–brain barrier damage and brain inflammatory cell infiltration (Lenglet et al., 2015). And NF-κB p50 is a key redox signaling mechanism regulating M1/M2 balance in microglia (Porta et al., 2009). Based on our bioinformatics study, we speculated that inhibition of SLC7A11 expression and reduction of NF-κB expression could promote M2 phenotypic polarization of microglia and alleviate inflammation, which could potentially play a protective role against stroke. To this end, we constructed an oxygen–glucose deprivation (OGD) model to simulate isolated cerebral ischemia, and we overexpressed SLC7A11 in microglia to mimic the signaling signature of disulfidptosis. However, in our study, overexpression of SLC7A11 did not induce M1 polarization in microglia as we suspected. We speculate that the oxygen–glucose deprivation (OGD) model constructed in microglia did not accurately mimic the signaling signature of disulfidptosis, and that ferroptosis may play an important role in this.
Overall, our study preliminarily explored the characteristics and mechanisms in terms of disulfidptosis and inflammatory response in ischemic stroke. In response to the regulatory effects of SLC7A11 on microglia, we observed its ability to modulate the inflammatory response, which may provide new targets and strategies for future therapeutic strategies in ischemic stroke. Despite the limitations in sample size of our study, we have improved the quality of the research as much as possible through rigorous experimental design and statistical analysis. We hope that future studies can expand the sample size to further verify and consolidate our findings. The present study reveals the important role of the NF-κB-mediated TNF signaling pathway in regulating disulfidptosis-related genes in ischemic stroke. Although these findings provide important guidance for us to deeply understand the signaling mechanism of disulfidptosis in ischemic stroke and to find corresponding therapeutic strategies, further clinical validation and molecular mechanism studies are needed to consolidate and improve this finding.
Availability of Data and Materials
No datasets were generated or analyzed during the current study.
References
Al-Bahrani, A., et al. (2007). TNF-alpha and IL-8 in acute stroke and the modulation of these cytokines by antiplatelet agents. Current Neurovascular Research, 4(1), 31–37.
Barr, T. L., et al. (2010). Genomic biomarkers and cellular pathways of ischemic stroke by RNA gene expression profiling. Neurology, 75(11), 1009–1014.
Borcherding, N., et al. (2019). Single-cell profiling of cutaneous T-cell lymphoma reveals underlying heterogeneity associated with disease progression. Clinical Cancer Research, 25(10), 2996–3005.
Campbell, B. C. V., & Khatri, P. (2020). Stroke. Lancet, 396(10244), 129–142.
Engebretsen, S., & Bohlin, J. (2019). Statistical predictions with glmnet. Clin. Epigenetics, 11(1), 123.
Galluzzi, L., et al. (2018). Molecular mechanisms of cell death: Recommendations of the nomenclature committee on Cell Death 2018. Cell Death and Differentiation, 25(3), 486–541.
Hao, Y., et al. (2021). Integrated analysis of multimodal single-cell data. Cell, 184(13), 3573–3587.
Heo, J., et al. (2019). Machine learning-based model for prediction of outcomes in acute stroke. Stroke, 50(5), 1263–1265.
Huang, M. L., et al. (2014). SVM-RFE based feature selection and Taguchi parameters optimization for multiclass SVM classifier. ScientificWorldJournal, 2014, 795624.
Huang, W. Y., et al. (2019). miR-124 upregulates astrocytic glutamate transporter-1 via the Akt and mTOR signaling pathway post ischemic stroke. Brain Research Bulletin, 149, 231–239.
Jin, S., et al. (2021). Inference and analysis of cell-cell communication using Cell Chat. Nature Communications, 12(1), 1088.
Kiss, T., et al. (2020). Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience, 42(2), 429–444.
Koppula, P., et al. (2017). The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. Journal of Biological Chemistry, 292(34), 14240–14249.
Lee, H. K., et al. (2016). Natural allelic variation of the IL-21 receptor modulates ischemic stroke infarct volume. The Journal of Clinical Investigation, 126(8), 2827–2838.
Leek, J. T., et al. (2012). The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics, 28(6), 882–883.
Lenglet, S., Montecucco, F., & Mach, F. (2015). Role of matrix metalloproteinases in animal models of ischemic stroke. Current Vascular Pharmacology, 13(2), 161–166.
Li, P., et al. (2023). SLC7A11-associated ferroptosis in acute injury diseases: Mechanisms and strategies. European Review for Medical and Pharmacological Sciences, 27(10), 4386–4398.
Liu, J., et al. (2022). Resveratrol attenuate myocardial injury by inhibiting ferroptosis Via inducing KAT5/GPX4 in myocardial infarction. Frontiers in Pharmacology, 13, 906073.
Liu, X., et al. (2020). Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nature Cell Biology, 22(4), 476–486.
Liu, X., et al. (2023). Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nature Cell Biology, 25(3), 404–414.
Lo, M., Wang, Y. Z., & Gout, P. W. (2008). The x(c)- cystine/glutamate antiporter: A potential target for therapy of cancer and other diseases. Journal of Cellular Physiology, 215(3), 593–602.
Matthay, M. A., et al. (2019). Acute respiratory distress syndrome. Nature Reviews. Disease Primers, 5(1), 18.
Muse, E. D., et al. (2018). Validation of a genetic risk score for atrial fibrillation: A prospective multicenter cohort study. PLoS Medicine, 15(3), e1002525.
Perego, C., Fumagalli, S., & De Simoni, M. G. (2011). Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. Journal of Neuroinflammation, 8, 174.
Porta, C., et al. (2009). Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A, 106(35), 14978–14983.
Schneider, A., et al. (1999). NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nature Medicine, 5(5), 554–559.
Shin, C. S., et al. (2017). The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nature Communications, 8, 15074.
Stamova, B., et al. (2014). Gene expression in peripheral immune cells following cardioembolic stroke is sexually dimorphic. PLoS ONE, 9(7), e102550.
Tan, S., et al. (2017). Exacerbation of oxygen-glucose deprivation-induced blood-brain barrier disruption: Potential pathogenic role of interleukin-9 in ischemic stroke. Clinical Science (London, England), 131(13), 1499–1513.
Tan, S., et al. (2019). Neutralization of interleukin-9 ameliorates experimental stroke by repairing the blood-brain barrier via down-regulation of astrocyte-derived vascular endothelial growth factor-A. The FASEB Journal, 33(3), 4376–4387.
Tirosh, I., et al. (2016). Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science, 352(6282), 189–196.
Tuo, Q. Z., Zhang, S. T., & Lei, P. (2022). Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Medicinal Research Reviews, 42(1), 259–305.
Wilkerson, M. D., & Hayes, D. N. (2010). ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics, 26(12), 1572–1573.
Zeng, D., et al. (2021). IOBR: Multi-omics immuno-oncology biological research to decode tumor microenvironment and signatures. Frontiers in Immunology, 12, 687975.
Zhang, C. Y., et al. (2016). Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World Journal of Gastroenterology, 22(48), 10512–10522.
Zhao, B. Q., et al. (2009). von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood, 114(15), 3329–3334.
Zhao, S., et al. (2023). Crosstalk of disulfidptosis-related subtypes, establishment of a prognostic signature and immune infiltration characteristics in bladder cancer based on a machine learning survival framework. Front Endocrinol (Lausanne), 14, 1180404.
Zheng, K., et al. (2022). Single-cell RNA-seq reveals the transcriptional landscape in ischemic stroke. Journal of Cerebral Blood Flow and Metabolism, 42(1), 56–73.
Zhou, Y., et al. (2021). Insight into crosstalk between ferroptosis and necroptosis: Novel therapeutics in ischemic stroke. Oxidative Medicine and Cellular Longevity, 2021, 9991001.
Zhu, G., et al. (2022). Crosstalk between the oxidative stress and glia cells after stroke: From mechanism to therapies. Frontiers in Immunology, 13, 852416.
Acknowledgements
We thank all the participants involved in this study.
Funding
This work was supported by the Wuxi Taihu Lake Talent Plan, Supports for Leading Talents in Medical and Health Profession (2020THRC-DJ-SNW) and Basic Research Project of Wuxi Science and Technology Bureau (K20221024).
Author information
Authors and Affiliations
Contributions
Songyun Zhao and Hao Zhuang conceived and designed the experiments; Songyun Zhao and Wei Ji analyzed the data; Hao Zhuang and Chao Cheng performed the experiments; Songyun Zhao, Jun Sun, and Yuankun Liu wrote the paper: all authors read and approved the final draft.
Corresponding authors
Ethics declarations
Competing of interests
The authors declare no conflicts of interest.
Ethical Approval and Consent to Participate
This article does not contain any studies with human participants or animals.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, S., Zhuang, H., Ji, W. et al. Identification of Disulfidptosis-Related Genes in Ischemic Stroke by Combining Single-Cell Sequencing, Machine Learning Algorithms, and In Vitro Experiments. Neuromol Med 26, 39 (2024). https://doi.org/10.1007/s12017-024-08804-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12017-024-08804-2