Abstract
Clinacanthus nutans (Lindau) (C. nutans) has diverse uses in traditional herbal medicine for treating skin rashes, insect and snake bites, lesions caused by herpes simplex virus, diabetes mellitus and gout in Singapore, Malaysia, Indonesia, Thailand and China. We previously showed that C. nutans has the ability to modulate the induction of cytosolic phospholipase A2 (cPLA2) expression in SH-SY5Y cells through the inhibition of histone deacetylases (HDACs). In the current study, we elucidated the effect of C. nutans on the hCMEC/D3 human brain endothelial cell line. Endothelial cells are exposed to high levels of the cholesterol oxidation product, 7-ketocholesterol (7KC), in patients with cardiovascular disease and diabetes, and this process is thought to mediate pathological inflammation. 7KC induced a dose-dependent loss of hCMEC/D3 cell viability, and such damage was significantly inhibited by C. nutans leaf extracts but not stem extracts. 7KC also induced a marked increase in mRNA expression of pro-inflammatory cytokines, IL-1β IL-6, IL-8, TNF-α and cyclooxygenase-2 (COX-2) in brain endothelial cells, and these increases were significantly inhibited by C. nutans leaf but not stem extracts. HPLC analyses showed that leaf extracts have a markedly different chemical profile compared to stem extracts, which might explain their different effects in counteracting 7KC-induced inflammation. Further study is necessary to identify the putative phytochemicals in C. nutans leaves that have anti-inflammatory properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinacanthus nutans (C. nutans) Lindau is colloquially known as Sabah Snake grass, or belalai gajah in Malay and you dun cao in Mandarin. It belongs to the Acanthaceae family and is native to Southeast Asia. The herb is commonly used for treatment of skin rashes, insect and snake bites, and for treating some symptoms caused by the herpes simplex virus, diabetes mellitus and gout. It is also prepared as a tea for treatment of diabetes and cancer (Kamarudin et al. 2017). Different methods of preparation affect the type and amounts of compounds obtained, as well as their range and extent of bioactivities. Analysis of this plant has revealed the identity of some bioactive compounds, such as shaftoside, stigmasterol, β-sitosterol and a triterpenoid lupeol. Among these compounds, stigmasterol and β-sitosterol were shown to have immunosuppressive properties (Le et al. 2017). C-glycosidic flavones such as shaftoside, isoorientin, orientin, isovitexin, and vitexin are the major flavonoids in the leaves of this plant (Chelyn et al. 2014). Some of the pharmacological effects of C. nutans have been established in laboratory studies, including analgesic, anti-inflammatory, anti-viral, anti-bacterial, anti-cancer, antioxidant, anti-venom, immunomodulatory, neuroprotective, and anti-hyperlipidemic effects (Khoo et al. 2018). C. nutans is highly regarded as a medical plant, and included in Thailand’s National List of Essential Medicines (Saokaew et al. 2015). Our previous study has shown that C. nutans extracts may have neuroprotective properties that ameliorated neuronal death and protected astrocytes and endothelial cells from hypoxic-induced cell death (Tsai et al. 2016). In another study C. nutans was found to modulate induction of cPLA2 expression in SH-SY5Y cells by histone deacetylase (HDAC) inhibitors, MS-275, MC-1568, and trichostatin A (TSA) (Tan et al. 2016). Moreover, C. nutans extracts were found to protect neurons and ameliorate brain ischemic injury through promoting the anti-apoptotic activity of peroxisome proliferator-activated receptor-gamma (PPAR-γ), a stress-induced transcription factor (Wu et al. 2018).
Thus far, however, little is known about the effect of C. nutans on endothelial cells. The latter are exposed to high levels of cholesterol oxidation product, 7-ketocholesterol (7KC), which can be found in the blood of patients with cardiovascular disease (Song et al. 2017; Wang et al. 2017) or diabetes mellitus (Murakami et al. 2000). 7KC is the most abundant oxysterol found in the blood and arterial plaques of coronary artery disease patients as well as various other disease tissues (Anderson et al. 2020). It has been found to induce ROS, cell cycle arrest and apoptosis, and increase the expression and secretion of the inflammatory cytokine IL-8 in endothelial cells (Chang et al. 2016). There is also evidence that 7KC could induce death of endothelial cells by necrosis (Ghelli et al. 2002; Hans et al. 2008). However, relatively little is known about whether 7KC-induced cellular injury could be modulated by phytochemicals. The present study was therefore carried out to determine whether C. nutans extracts could be useful in preventing 7KC-induced injury in a human brain endothelial cell line (hCMEC/D3 cells).
Materials and Methods
C. nutans Extracts
C. nutans was purchased from a commercial source (Healing Plants Garden, Singapore), and prepared by methanol extraction. Leaves and stems were rinsed with dH2O and soaked in methanol for 1 h, blended using a Wiggenhauser homogenizer, and left to stand for 1 h. The extract was then filtered under vacuum, using P8 fluted filter paper (Fisher Scientific). The filtrate was then collected and concentrated in a Rotavapor R-144 rotary evaporator (BÜCHI Labortechnik AG) at 50 °C, and finally completely dried with a miVac DUO concentrator (Genevac). The resultant residue contained dark green to light brown solids. The approximate yield (w/w) was 2.12%. The extracts were stored at − 20 °C and reconstituted in fatty acid-free bovine serum albumin (FAFBSA) to make 10 mg/mL stock solution. It was used at a concentration of 100 μg/mL in experiments. Cells were pretreated with the C. nutans leaf or stem extracts for 1 h followed by co-incubation with 7 KC for 24 h before analyses.
Cells and Culture
The Human Cerebral Microvascular Endothelial Cell Line (hCMEC/D3 cells) was obtained from EMD Millipore (Temecula, CA, USA) and cultured as described previously (Weksler et al. 2005). This cell line was isolated from human temporal lobe microvessels and was found to preserve typical brain endothelial cell properties such as tight junction protein expression and localization, receptor expression and selective permeability, establishing its suitability as a simple blood–brain barrier model (Weksler et al. 2013). Cells were seeded in flasks coated with collagen and maintained humidified at 37 °C in 5% CO2 atmosphere in Endothelial Basal Medium (EBM-2, Lonza, USA) supplemented with HyClone™ Antibiotic/Antimycotic Solution (1x, Thermal Fisher Scientific, USA) and EGM-2MV SingleQuots Kit from Lonza (USA), which included Fetal Bovine Serum FBS (5%), Hydrocortisone, human Fibroblast Growth Factor-Beta (hFGF-β), Vascular Endothelial Growth Factor (VEGF), R3-Insulin-like Growth Factor-1 (R3-IGF-1), Ascorbic acid, human Epidermal Growth Factor (hEGF) and Gentamicin/Amphotericin-B (GA). All experiments use hCMEC/D3 cells from passages 5–15 and grown to 70–80% confluency. The cells were treated with C. nutans extracts for 1 h, followed by 7KC or ethanol (vehicle control). They were subsequently incubated for 24 h at 37 °C and 5% CO2 before analysis.
MTS Assay
The working concentration of 7KC was determined by treating cells at ascending concentrations of 7KC for 24 h. The appropriate working concentration of 7KC was determined to be 30 μM, which was a concentration that did not display excessive cytotoxicity to cells for subsequent experiments. Cells were pretreated with C. nutans extracts or vehicle for 1 h, followed by co-incubation with 7KC for 24 h. CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) (Promega, USA) was then added to each well. Absorbance was read at 490 nm using the Micro Read 1000 microplate reader (Global Diagnostics B, Belgium).
RT-PCR
Total RNA was extracted and purified with the RNeasy mini kit (QIAGEN, Germany) according to manufacturer’s protocol. Purified RNA was eluted from the QIAGEN mini spin columns in 20 μL of RNase-free water and quantified with a NanoDrop 2000 (Thermo Fisher Scientific, USA). 1000 ng RNA was used to make cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA) at 25 °C for 10 min, 37 °C for 30 min, then 85 °C for 5 min, in the T-Personal Thermocycler (Biometra, Germany). qPCR was carried out to quantify tight junction gene expression or pro-inflammatory gene expression. TaqMan Universal PCR Master Mix (Applied Biosystems, USA), with TaqMan Gene Expression Assay Probes obtained from Applied Biosystems (USA) were used for qPCR.
PCR probes used are as follows: zonula occludens-1 (ZO-1/TJP1, Hs01551861_m1), claudin-5 (CLDN5, Hs01561351_m1) and occludin (OCLN, Hs01049880_m1); interleukin-1 beta (IL-1β, (Hs01555410_m1); interleukin-6 (IL-6, Hs00174131_m1); interleukin-8 (IL-8/CXCL8, Hs00174103_m1), tumor necrosis factor alpha (TNFα, Hs00174128_m1) and cyclooxygenase-2 (COX-2/PTGS2, Hs00153133_m1). The housekeeping gene used was β-Actin (Hs01060665_g1). Analysis was done using the 7500 Real-time PCR System (Applied Biosystems, USA). The PCR reaction conditions were 95 °C for 10 min, 40 cycles of: 95 °C for 15 s and 60 °C for 1 min. The comparative Ct (ΔΔCt) method was used to quantitative relative mRNA expression of all genes of interest. mRNA expression of genes of interest were normalized to mRNA expression of β-Actin.
High Performance Liquid Chromatography (HPLC)
HPLC was conducted with the Prominence HPLC system (Shimadzu, Japan) with a LC-20AD Solvent Delivery Unit, SIL-20AC Autosampler, CTO-20AC column oven, SPD-M20A Photodiode Array Detector, CBM-20A System Controller, RID-10A refractive index detector, and DGU-20A5 Degasser unit. The chromatographic separation was performed using a Zorbax SB-C18 column (9.4 × 150 mm, 5 μm; Agilent, USA) at 35 °C. The solvent system consisted of dH2O (A) and HPLC-grade methanol (B) in a low gradient mode, as follows: 10–100% B (10 min), 100% B (13 min), 100–10% B (1 min), and 10% B (2 min). C. nutans extract samples were dissolved in HPLC-grade methanol at 5 mg/mL. 100 μL of sample were injected and flow rate was 4.18 mL/min. Signal was monitored at 254 nm.
Statistical Analysis
The mean ± standard error of mean (SEM) was calculated for all groups. Comparisons made in the MTS cell proliferation assay were made using a Two-Way ANOVA with Bonferroni post hoc correction. Comparisons between groups for gene expression were made using One-Way ANOVA with Bonferroni post hoc correction. P < 0.05 was considered significant.
Results
MTS Assay
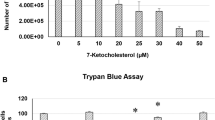
The toxicity of 7KC manifested as loss of viable cells by the MTS assay. 50 µM 7KC was found to significantly reduce cell viability in endothelial cells. C. nutans leaf extracts by themselves had no significant effect on cytotoxicity. However, C. nutans leaf extracts significantly attenuated the 7KC-mediated loss of cell viability in the MTS assay (Fig. 1).
a Effect of 7KC on endothelial cells in culture, determined using the MTS assay. hCMEC/D3 cells were treated with increasing concentrations of 7KC for 24 h. The oxysterol induced significant loss in viable cells from at 10 μM onwards. b Effect of C. nutans leaf or stem extracts on 7KC-induced endothelial cell damage, determined using the MTS assay. Cells were treated with C. nutans leaf and stem extracts (100 μg/mL) for 1 h, followed by 30 μM 7KC for 24 h. Co-treatment of cells with C. nutans leaf extracts (Leaf) results in modulation of 7KC-induced loss of viable cells. In contrast, no significant effect was observed with the stem extracts (Stem). For all experiments, endpoint 492 nm readings of each treatment groups were normalized against an untreated control and expressed as a percentage, where the viability of the untreated control = 100%. Data are expressed as mean ± SEM; n = 3. *P < 0.001 compared to 7KC
RT-PCR
The mRNA expression of tight junction proteins expressed by endothelial cells including zonula occludens-1, claudin-5 and occludin were largely unchanged with 7KC treatment (Fig. 2). However, 7KC induced significant increase in the mRNA expression of some pro-inflammatory genes, as determined by RT-PCR (Figs. 3, 4). 30 µM 7KC was found to significantly increase the expression of TNFα 17-fold, IL-6 22-fold, IL-8 ninefold, IL-1β 20-fold, and COX-2 tenfold. C. nutans leaf extracts by themselves had no significant effect on mRNA expression of pro-inflammatory genes. However, C. nutans leaf extracts significantly attenuated the increase in pro-inflammatory genes after addition of 7KC (Figs. 3, 4).
Effect of 7KC and C. nutans leaf or stem extracts on mRNA expression of blood–brain barrier tight junction proteins, determined by real-time RT-PCR. 7KC had no significant effect on mRNA expression of junction proteins ZO-1, claudin-5 and occluding. Similarly, no significant effect of C. nutans leaf extracts was observed on these genes. Data are expressed as mean ± SEM; n = 3
Effect of 7KC and C. nutans leaf extracts on mRNA expression of selected pro-inflammatory genes determined by real-time RT-PCR. Quantitative RT-PCR results of gene expressions of pro-inflammatory cytokines IL-1β (a), IL-6 (b) and IL-8 (c) were determined from cells pretreated with C. nutans extract, then treated with 30 μM 7KC for 24 h. Data are represented as fold change values with housekeeping gene β-actin as a reference, using the ΔΔCt method. 7KC induced a significant increase in mRNA expression of IL-1β, IL-6, IL-8, and TNF-α. These increases were modulated by C. nutans methanolic leaf extracts (Leaf), but not stem extracts (Stem). Data are expressed as mean ± SEM; n = 3. * P < 0.05 compared to 7KC
Effect of 7KC and C. nutans leaf extracts on mRNA expression of selected pro-inflammatory genes determined by real-time RT-PCR. Quantitative RT-PCR results of gene expressions of pro-inflammatory cytokines a TNF-α and b COX-2 were determined from cells pretreated with C. nutans extract and 30 μM 7KC for 24 h. Data are represented as fold change with housekeeping gene β-actin as a reference, using the ΔΔCt method. 7KC induced significant increases in mRNA expression of TNFα and COX-2. These increases were modulated by C. nutans methanolic leaf extracts (Leaf), but not stem extracts (Stem). Data are expressed as mean ± SEM; n = 3. * P < 0.05 compared to 7KC
HPLC
Results show that leaf extracts have a completely different chemical profile from the stem extracts, which could explain the different properties observed in counteracting 7KC-induced toxicity and inflammation (Fig. 5).
Comparative analyses of components of C. nutans leaf and stem extracts. HPLC–UV/DAD chromatogram profiles obtained from 100 μL of 5 mg/mL methanolic leaf extracts (a) or stem extracts (b) of C. nutans, in water/methanol solvent system. Chromatograms are representative of at least 3 HPLC runs conducted per sample. λ = 254 nm. Marked differences between the chemical composition of leaf and stem extracts are found
Discussion
A common feature of metabolic and cardiovascular diseases is high levels of cholesterol oxidation products or oxysterols in the bloodstream (Lee et al. 2009). The present study was carried out to elucidate the effects of one of the oxysterols, 7KC, on brain endothelial cells, and possible protection by C. nutans methanolic leaf extracts. 7KC was found to be toxic to endothelial cells in culture. The oxysterol induced non-significant damage at 10 µM, but significant reduction in cell viability at higher concentrations, as shown by the MTS assay. A similar trend was observed in a previous study, where treatment for 24 h with a 7KC concentration of 25 μM (with serum restriction) led to a decrease in cell viability by 55%, and at 50 μM, cell viability dropped by nearly 75% (Rosa-Fernandes et al. 2017). 7KC-induced reduction of cell viability has also been reported in other endothelial cell lines including Eahy926 (EAHY) endothelial cells (Chang et al. 2016) and human aortic endothelial cells (Chalubinski et al. 2013). 7KC-induced loss of endothelial cell viability was inhibited by C. nutans leaf extracts, but not stem extracts. No change in mRNA expression of ZO-1, claudin-5 or occludin, which are key proteins involved in tight junction formation of brain endothelial cells was found, after 7KC treatment.
7KC been shown in previous studies to induce cytokine expression and inflammation in cells. It induces the expression of VEGF, IL-6, and IL-8 through the AKT-PKCζ-NFκB, p38 MAPK, and ERK pathways in ARPE-19 cells (Larrayoz et al. 2010), and increases IL-1β, IL-6, TNF-α expression and inflammation in these cells (Yang et al. 2019). The oxysterol also markedly increases the formation and activation of NLRP3 inflammasomes and elevates IL-1β levels in mouse carotid arterial endothelial cells (Koka et al. 2017). We next sought to determine whether 7KC-induced increase in inflammation could be modulated by C. nutans extracts. Co-treatment with C. nutans methanolic leaf extracts but not stem extracts was found to significantly attenuate 7KC-induced upregulation of pro-inflammatory genes including IL-1β IL-6, IL-8 and TNFα mRNA expression, compared to cells treated with 7KC only, indicating an anti-inflammatory effect of the leaf extracts. We also elucidated the effect of C. nutans extracts on COX-2 expression. The cyclooxygenase (COX) family of enzymes catalyze the rate-limiting step in the synthesis of prostaglandins, and includes cyclooxygenase 1 (COX-1) which is constitutively expressed, and cyclooxygenase-2 (COX-2) which is induced by IL1β (Dinarello 2002) and TNF-α (Qiu et al. 2016). It was found that 7KC induced an increase in COX-2 mRNA expression, and that C. nutans leaf extracts, but not stem extracts significantly modulated the 7KC-induced increase in COX-2 expression. These findings further support the anti-inflammatory properties of C. nutans methanolic leaf extracts.
The striking differences between the ability of leaves and stems of C. nutans to modulate inflammation led us to the notion that there could be significant differences in chemical composition between these two plant parts. HPLC was carried out in a preliminary attempt at comparing the chemical composition of C. nutans leaves and stems. Most plant phenolics can be detected at UV–vis wavelengths between 210 and 370 nm, and 254 nm was chosen for this study because it showed the most well-resolved peaks. The separation was carried out with a reversed-phase system, which involves the adsorption of hydrophobic, or non-polar, molecule to a hydrophobic stationary phase, causing the more polar molecules to elute first. The C18 column offers retention and selectivity for a wide range of compounds with different polar or non-polar sidechains thus it makes a good choice for separation of the diverse class of compounds in C. nutans extracts. Results show that leaf extracts have a markedly different chemical profile compared to stem extracts, which might explain their different effects in counteracting 7KC-induced inflammation. Ethanol extraction of the leaves of C. nutans have yielded six C-glycosylflavones that are of relatively rare occurrence in plants (Teshima et al. 1997). Of these, schaftoside (or shaftoside) is present at the highest concentration with ranges from 2.55 mmol/g to 17.43 mmol/g. The other components are present in lower amounts, ranging from the highest for isovitexin (0.00–2.01 mmol/g), followed by orientin (0.00–0.86 mmol/g), to the lowest amount which was vitexin (0.00–0.91 mmol/g) (Chelyn et al. 2014).
Schaftoside has anti-inflammatory properties by suppressing the TLR4/Myd88 signaling pathway (De Melo et al. 2005; Zhou et al. 2019). Isovitexin inhibits MAPK phosphorylation, reduces NF-κB nuclear translocation, and upregulates nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase 1 (HO-1) expression in RAW 264.7 cells (Lv et al. 2016; Lin et al. 2005). Orientin suppresses LPS-induced TNF-α, IL-6, and NF-κB levels in endothelial cell lines (Lam et al. 2016) and decreases levels of TNF-α, IL-6, IL-1β, IL-18, and prostaglandin E2 (PGE2) together with reduction in expression levels of COX-2 and inducible nitric oxide synthase (iNOS) in RAW 264.7 cells. These effects are correlated with suppression of NF-κB pathway and nucleotide-binding domain- (NOD-) like receptor protein 3 (NLRP3) inflammasome activation (Xiao et al. 2017). The expression levels of angiopoietin-like 2 (angptl2) and NF-κB are also significantly upregulated in RAW 264.7 cells by oxidized LDL, and these effects are significantly reversed by orientin (Li et al. 2020). Vitexin decreases the activation of NF-κB key regulators, including p65, IκBα and IKKs in nasopharyngeal carcinoma cells (W. Wang et al. 2019). It also suppresses the expression of pro-inflammatory cytokines, including MCP-1, IL-6, IL-8, TNF-α, NF-κB p65, and reduces leukocyte-endothelial adhesion in a mouse model of septic encephalopathy (Cao et al. 2020).
Together, the above results show that 7KC could induce cell death and inflammation in brain endothelial cells, and the ability of C. nutans to reduce such damage. Further study is necessary to identify the effect of phytochemicals in C. nutans leaves on neurovascular disorders such as stroke and vascular dementia.
References
Anderson, A., Campo, A., Fulton, E., Corwin, A., Jerome, W. G., 3rd., & O’Connor, M. S. (2020). 7-Ketocholesterol in disease and aging. Redox Biology, 29, 101380. https://doi.org/10.1016/j.redox.2019.101380
Cao, H., Wang, X., Zhang, B., & Ren, M. (2020). The protective effect of vitexinin septic encephalopathy by reducing leukocyte-endothelial adhesion and inflammatory response. Annals of Palliative Medicine, 9(4), 2079–2089. https://doi.org/10.21037/apm-20-1211
Chalubinski, M., Zemanek, K., Skowron, W., Wojdan, K., Gorzelak, P., & Broncel, M. (2013). The effect of 7-ketocholesterol and 25-hydroxycholesterol on the integrity of the human aortic endothelial and intestinal epithelial barriers. Inflammation Research, 62(12), 1015–1023. https://doi.org/10.1007/s00011-013-0660-x
Chang, M. C., Chen, Y. J., Liou, E. J., Tseng, W. Y., Chan, C. P., Lin, H. J., et al. (2016). 7-Ketocholesterol induces ATM/ATR, Chk1/Chk2, PI3K/Akt signalings, cytotoxicity and IL-8 production in endothelial cells. Oncotarget, 7(46), 74473–74483. https://doi.org/10.18632/oncotarget.12578
Chelyn, J. L., Omar, M. H., Mohd Yousof, N. S., Ranggasamy, R., Wasiman, M. I., & Ismail, Z. (2014). Analysis of flavone C-glycosides in the leaves of Clinacanthus nutans (Burm f) Lindau by HPTLC and HPLC-UV/DAD. Scientific World Journal, 1, 1. https://doi.org/10.1155/2014/724267
De Melo, G. O., Muzitano, M. F., Legora-Machado, A., Almeida, T. A., De Oliveira, D. B., Kaiser, C. R., et al. (2005). C-glycosylflavones from the aerial parts of Eleusine indica inhibit LPS-induced mouse lung inflammation. Planta Medica, 71(4), 362–363. https://doi.org/10.1055/s-2005-864104
Dinarello, C. A. (2002). The IL-1 family and inflammatory diseases. Clinical and Experimental Rheumatology, 20(5 Suppl 27), S1-13.
Ghelli, A., Porcelli, A. M., Zanna, C., & Rugolo, M. (2002). 7-Ketocholesterol and staurosporine induce opposite changes in intracellular pH, associated with distinct types of cell death in ECV304 cells. Archives of Biochemistry and Biophysics, 402(2), 208–217. https://doi.org/10.1016/S0003-9861(02)00085-1
Hans, C. P., Zerfaoui, M., Naura, A. S., Catling, A., & Boulares, A. H. (2008). Differential effects of PARP inhibition on vascular cell survival and ACAT-1 expression favouring atherosclerotic plaque stability. Cardiovascular Research, 78(3), 429–439. https://doi.org/10.1093/cvr/cvn018
Kamarudin, M. N. A., Sarker, M. M. R., Kadir, H. A., & Ming, L. C. (2017). Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Clinacanthus nutans (Burm. f.) Lindau: A comprehensive review. Journal of Ethnopharmacology, 206, 245–266. https://doi.org/10.1016/j.jep.2017.05.007
Khoo, L. W., Audrey Kow, S., Lee, M. T., Tan, C. P., Shaari, K., Tham, C. L., et al. (2018). A comprehensive review on phytochemistry and pharmacological activities of Clinacanthus nutans (Burm.f.) Lindau. Evidence Based Complementary Alternative Medicine. https://doi.org/10.1155/2018/9276260
Koka, S., Xia, M., Chen, Y., Bhat, O. M., Yuan, X., Boini, K. M., et al. (2017). Endothelial NLRP3 inflammasome activation and arterial neointima formation associated with acid sphingomyelinase during hypercholesterolemia. Redox Biol, 13, 336–344. https://doi.org/10.1016/j.redox.2017.06.004
Lam, K. Y., Ling, A. P., Koh, R. Y., Wong, Y. P., & Say, Y. H. (2016). A Review on Medicinal Properties of Orientin. Advances in Pharmacological Sciences, 2016, 4104595. https://doi.org/10.1155/2016/4104595
Larrayoz, I. M., Huang, J. D., Lee, J. W., Pascual, I., & Rodriguez, I. R. (2010). 7-Ketocholesterol-induced inflammation: Involvement of multiple kinase signaling pathways via NFkappaB but independently of reactive oxygen species formation. Investigative Ophthalmology & Visual Science, 51(10), 4942–4955. https://doi.org/10.1167/iovs.09-4854
Le, C. F., Kailaivasan, T. H., Chow, S. C., Abdullah, Z., Ling, S. K., & Fang, C. M. (2017). Phytosterols isolated from Clinacanthus nutans induce immunosuppressive activity in murine cells. International Immunopharmacology, 44, 203–210. https://doi.org/10.1016/j.intimp.2017.01.013
Lee, C. Y., Seet, R. C., Huang, S. H., Long, L. H., & Halliwell, B. (2009). Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and Parkinson’s disease patients: Cautions in the use of biomarkers of oxidative stress. Antioxidants & Redox Signaling, 11(3), 407–420. https://doi.org/10.1089/ARS.2008.2179
Li, C., Cai, C., Zheng, X., Sun, J., & Ye, L. (2020). Orientin suppresses oxidized low-density lipoproteins induced inflammation and oxidative stress of macrophages in atherosclerosis. Bioscience, Biotechnology, and Biochemistry, 84(4), 774–779. https://doi.org/10.1080/09168451.2019.1702871
Lin, C. M., Huang, S. T., Liang, Y. C., Lin, M. S., Shih, C. M., Chang, Y. C., et al. (2005). Isovitexin suppresses lipopolysaccharide-mediated inducible nitric oxide synthase through inhibition of NF-kappa B in mouse macrophages. Planta Medica, 71(8), 748–753. https://doi.org/10.1055/s-2005-871287
Lv, H., Yu, Z., Zheng, Y., Wang, L., Qin, X., Cheng, G., et al. (2016). Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-kappaB and activating HO-1/Nrf2 pathways. International Journal of Biological Sciences, 12(1), 72–86. https://doi.org/10.7150/ijbs.13188
Murakami, H., Tamasawa, N., Matsui, J., Yasujima, M., & Suda, T. (2000). Plasma oxysterols and tocopherol in patients with diabetes mellitus and hyperlipidemia. Lipids, 35(3), 333–338. https://doi.org/10.1007/s11745-000-0530-1
Qiu, J., Yuan, H., Chen, S., Zhou, Y., Song, D., & Chen, R. (2016). TNFalpha up-regulates COX-2 in chronic progressive nephropathy through nuclear accumulation of RelB and NF-kappaB2. Archives of Physiology and Biochemistry, 122(2), 88–93. https://doi.org/10.3109/13813455.2016.1141961
Rosa-Fernandes, L., Maselli, L. M. F., Maeda, N. Y., Palmisano, G., & Bydlowski, S. P. (2017). Outside-in, inside-out: Proteomic analysis of endothelial stress mediated by 7-ketocholesterol. Chemistry and Physics of Lipids, 207(Pt B), 231–238. https://doi.org/10.1016/j.chemphyslip.2017.06.008
Saokaew, S., Sugimoto, T., Kamae, I., Pratoomsoot, C., & Chaiyakunapruk, N. (2015). Healthcare databases in Thailand and Japan: Potential sources for health technology assessment research. PLoS ONE, 10(11), e0141993. https://doi.org/10.1371/journal.pone.0141993
Song, J., Wang, D., Chen, H., Huang, X., Zhong, Y., Jiang, N., et al. (2017). Association of plasma 7-ketocholesterol with cardiovascular outcomes and total mortality in patients with coronary artery disease. Circulation Research, 120(10), 1622–1631. https://doi.org/10.1161/CIRCRESAHA.117.311049
Tan, C. S., Ho, C. F., Heng, S. S., Wu, J. S., Tan, B. K., Ng, Y. K., et al. (2016). Clinacanthus nutans extracts modulate epigenetic link to cytosolic phospholipase A2 expression in SH-SY5Y cells and primary cortical neurons. Neuromolecular Medicine, 18(3), 441–452. https://doi.org/10.1007/s12017-016-8404-z
Teshima, K.-I., Kaneko, T., & Ohtani, K. (1997). C-glycosyl flavones from Clinacanthus nutans. Natural Medicines, 51(6), 557.
Tsai, H. D., Wu, J. S., Kao, M. H., Chen, J. J., Sun, G. Y., Ong, W. Y., et al. (2016). Clinacanthus nutans protects cortical neurons against hypoxia-induced toxicity by downregulating HDAC1/6. Neuromolecular Medicine, 18(3), 274–282. https://doi.org/10.1007/s12017-016-8401-2
Wang, M., Long, W., Li, D., Wang, D., Zhong, Y., Mu, D., et al. (2017). Plasma 7-ketocholesterol levels and the risk of incident cardiovascular events. Heart, 103(22), 1788–1794. https://doi.org/10.1136/heartjnl-2016-310914
Wang, W., Cheng, H., Gu, X., & Yin, X. (2019). The natural flavonoid glycoside vitexin displays preclinical antitumor activity by suppressing NF-kappaB signaling in nasopharyngeal carcinoma. Onco Targets and Therapy, 12, 4461–4468. https://doi.org/10.2147/OTT.S210077
Weksler, B., Romero, I. A., & Couraud, P. O. (2013). The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS, 10(1), 16. https://doi.org/10.1186/2045-8118-10-16
Weksler, B. B., Subileau, E. A., Perriere, N., Charneau, P., Holloway, K., Leveque, M., et al. (2005). Blood-brain barrier-specific properties of a human adult brain endothelial cell line. The FASEB Journal, 19(13), 1872–1874. https://doi.org/10.1096/fj.04-3458fje
Wu, J. S., Kao, M. H., Tsai, H. D., Cheung, W. M., Chen, J. J., Ong, W. Y., et al. (2018). Clinacanthus nutans mitigates neuronal apoptosis and ischemic brain damage through augmenting the C/EBPbeta-driven PPAR-gamma transcription. Molecular Neurobiology, 55(7), 5425–5438. https://doi.org/10.1007/s12035-017-0776-z
Xiao, Q., Qu, Z., Zhao, Y., Yang, L., & Gao, P. (2017). Orientin ameliorates LPS-induced inflammatory responses through the inhibitory of the NF-kappaB pathway and NLRP3 inflammasome. Evidence-Based Complementary and Alternative Medicine, 2017, 2495496. https://doi.org/10.1155/2017/2495496
Yang, C., Xie, L., Gu, Q., Qiu, Q., Wu, X., & Yin, L. (2019). 7-Ketocholesterol disturbs RPE cells phagocytosis of the outer segment of photoreceptor and induces inflammation through ERK signaling pathway. Experimental Eye Research, 189, 107849. https://doi.org/10.1016/j.exer.2019.107849
Zhou, K., Wu, J., Chen, J., Zhou, Y., Chen, X., Wu, Q., et al. (2019). Schaftoside ameliorates oxygen glucose deprivation-induced inflammation associated with the TLR4/Myd88/Drp1-related mitochondrial fission in BV2 microglia cells. Journal of Pharmacological Sciences, 139(1), 15–22. https://doi.org/10.1016/j.jphs.2018.10.012
Acknowledgement
This work was supported by grants from the Ministry of Education, Singapore (R-181-000-183-114, WYO) and the National University Health System, Singapore (NUHSRO/2019/051/T1/Seed-Mar/04, WYO).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuo, X., Herr, D.R. & Ong, WY. Anti-inflammatory and Cytoprotective Effect of Clinacanthus nutans Leaf But Not Stem Extracts on 7-Ketocholesterol Induced Brain Endothelial Cell Injury. Neuromol Med 23, 176–183 (2021). https://doi.org/10.1007/s12017-020-08621-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-020-08621-3