Abstract
The present study was designed to determine the effect of different doses of oxytocin (OXT) on neuronal injury, spatial memory, blood-brain barrier (BBB) integrity and to explore possible underlying molecular mechanisms in the early stage of stroke in mice. Stroke model was generated by middle cerebral artery occlusion (MCAO) for 60 min and 24 h reperfusion in mice. OXT at doses of 1, 2, 4 and 8 IU/per mouse was administrated intranasally at the beginning of brain ischemia. Brain injury, BBB integrity, and spatial memory were evaluated by standard methods. Changes in the expression of nuclear factor-kappa B (NF-κB), and TUNEL positive cell were detected by immunohistochemistry. The levels of vascular endothelial growth factor (VEGF), aquaporin-4 (AQP4) and brain-derived neurotrophic factor (BDNF) proteins were determined by western blotting and ELISA methods. OXT at doses of 4 and 8 IU/per mouse reduced the infarct size by 42% and 52%, respectively, and improved spatial memory function (p < 0.001). OXT (8 IU/per mouse) significantly reduced brain edema, BBB disruption and upregulated the AQP4 expression (p < 0.001). Finally, OXT significantly diminished the number of apoptotic, NF-κB positive cells and enhanced the expression of BDNF and VEGF proteins in the brain tissue (p < 0.001). These findings provide important evidences that OXT significantly suppresses neuronal damage in the early stage of stroke by inhibiting apoptotic and NF-κB signaling pathway, increasing the expression of VEGF, AQP4 and BDNF proteins and reducing the BBB leakage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that oxytocin (OXT) is mainly synthesized in the hypothalamus, and acts as a neurotransmitter as well as hormone (Knobloch et al. 2012). OXT receptors are dispersed throughout the central nervous system such as amygdala, hippocampus and cortical regions (Boccia et al. 2013).Traditionally, OXT has an important function in labor and lactation processes. Moreover, central OXT plays a critical role in the regulation of several social behaviors such as emotion, social recognition, reward-based learning, pair bonding, maternal performance and reducing social anxiety/stress (Wigton et al. 2015; Bethlehem et al. 2013).
Since 1961, OXT has been used widely for research and therapeutic purposes (Melville and Varma 1961). Recent studies have found that OXT could inhibit apoptosis, oxidative stress and inflammatory cytokines in various tissues, and enhance the expression of vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) in the brain (Moghadam et al. 2018; Dayi et al. 2015; Jankowski et al. 2010; Yeniel et al. 2014; Gutkowska and Jankowski 2009; Al-Amran and Shahkolahi 2013; Akman et al. 2015). Also, findings of several experimental studies showed that exogenous OXT reduces ischemic tissue injury in ovary, liver, gastric, and heart tissues as well as sepsis-induced multiple organ damage (W. Zhang et al. 2007; Düşünceli et al. 2008; Ondrejcakova et al. 2009; Akdemir et al. 2014; İşeri et al. 2005). Moreover, Karelina et al. have demonstrated that OXT could protect the brain against stroke -induced injury via attenuating neuroinflammatory and oxidative stress pathways (Karelina et al. 2011). Additionally, It is reported that OXT has beneficial effects on cisplatin-induced neurotoxicity (Akman et al. 2015). Likewise, an in vitro study reported that OXT has neuroprotective effects against neuronal injury via GABA signaling (Kaneko et al. 2016). Moreover, OXT could reduce cerebral ischemic reperfusion injury via inhibiting calpain-1 in rats (Moghadam et al. 2018).
Damage to the blood-brain barrier (BBB) following stroke can exacerbate the primary damage via inducing cerebral edema and hemorrhagic transformation (Abdullahi et al. 2018). Therefore, one of the important clinical approach for stroke treatment is the monitoring of BBB dysfunction and reduction of brain edema. Many factors including free radicals, pre-inflammatory cytokines, Aquaporin-4 (AQP4) and VEGF are involved in the pathogenesis of BBB dysfunction after stroke (Abdullahi et al. 2018; Wang et al. 2020; Zhang and Chopp 2002). AQP4 is a water channel that is predominantly expressed in the astrocytes and has a essential function in the regulation of brain water homeostasis and pathogenesis of vasogenic brain edema following stroke in the central nervous system (Wang et al. 2020; Arcienega et al. 2010).
VEGF and BDNF are two important trophic factors that can promote neuroprotection and minimize the ischemic damage through enhancing the neuroplasticity, neurogenesis, angiogenesis and neural repair (Nowacka and Obuchowicz 2013; Houlton et al. 2019). In addition, experimental evidences have demonstrated that VEGF can increase the BBB permeability and accelerate vasogenic edema in acute phase cerebral stroke (Zhang and Chopp 2002). Moreover, it is approved that activation of the nuclear factor-kappa B (NF-κB) signaling pathway after stroke can exacerbate ischemic injury and BBB disruption (Behrouzifar et al. 2018; Zhao et al. 2018). Although two animal studies have shown that OXT can protect neurons against ischemic injury(Karelina et al. 2011; Moghadam et al. 2018), but its effect on the maintenance of the BBB integrity, and recovery of stroke induced memory loss and also the underlying molecular mechanisms of neuroprotection remain to be clarified.
Therefore, this study designed to investigation the effects of intranasal application of different doses of OXT on infarct size, brain edema, BBB integrity, neurological function and spatial memory in the early stage of stroke in mice. We also measured the expression of NF-κB, VEGF, AQP4 and BDNF proteins and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positive cells in the absence or presence of OXT treatment to determine the possible underlying mechanisms.
Materials and Methods
Animals and Intranasal Application of OXT
Adult male Swiss albino mice (35–40 g, 2–4 months old) were supplied from the animal services of Semnan University of Medical Sciences (SUMS). OXT (Sigma-Aldrich, O3251, Germany) or vehicle (saline) in a volume 10 µl gently infused into the bilateral nostrils immediately after middle cerebral artery occlusion (MCAO) by catheter (PE-10), which were inserted into the nasal cavity. Time of infusion of OXT or vehicle into nasal cavity was less than 30 s.
Focal Cerebral Ischemia
Focal cerebral ischemia was produced by MCAO in mice using a modified filament method (Behrouzifar et al. 2018). Mice were anesthetized with chloral hydrate (400 mg/kg, IP) and under Laser Doppler Flowmetry (LDF, Moor instruments DRT4, UK) guide; a silicone-coated 8–0 monofilament was inserted into the internal carotid artery to obstruction of MCA. The filament was removed after 60 min MCAO and the blood flow was restored for about 23 h in the brain. To diminish the animal pain, buprenorphine (0.01 mg/kg IP, Temad Co. Active Pharmaceutical Ingredients, Iran) was injected 30 min before and 8 h after the surgery.
Experimental Design
This research was done in the following plans. In the first step, the effect of intranasal injection OXT at different doses on brain damage, neurological function and spatial memory were investigated. For this purpose, 49 male mice were randomly alienated into seven equal groups (n = 7, each) as follows. Group 1(intact mice, no surgery + no MCAO) did not receive any drug or saline. Group 2 (sham operated, surgery + no MCAO) received saline (10 µl, intranasal) after surgery. Group 3 (control group, MCAO + saline) received saline (10 µl, intranasal) at the beginning of MCAO. Groups 4 to 7 (treatment groups, MCAO + OXT) received OXT at doses 1, 2, 4, and 8 IU/per mouse intranasal, at the commencement of MCAO. We used the dose of 8 IU of OXT per mouse as a most effective dose in next steps of experiment and exploring the molecular mechanisms. In all of these groups, 24 h after ischemia, neurological deficit scores and spatial memory were evaluated and then the animals were sacrificed to measure the infarct size. In this step, group 1 (intact mice) was used only to exclude the possible effect of surgery and anesthesia on the learning and spatial memory.
In the second step, effect of OXT at dose 8 (8 IU/per mouse) on percent of brain water content (BWC %) was investigated. For this aim, 24 male mice were randomly divided into three equal groups (n = 7, each) as follows: (1) Sham operated (surgery without MCAO), (2) control (MCAO + saline), (3) treatment (MCAO + OXT).
In the third step, effect of OXT (8 IU/per mouse) on BBB permeability was examined. For this aim, 18 male mice were randomly alienated into three equal groups (n = 6, each) as follows: (1) Sham operated (surgery without MCAO), (2) control (MCAO + saline), (3) treatment (MCAO + OXT).
To perform mechanistic studies, effect of OXT (8 IU/per mouse) on TUNEL positive cell (apoptosis) and NF-κB expression was investigated in the cortex and hippocampus. In this stage, 15 male mice were randomly divided into three equal groups (n = 5, each) as follows: (1) Sham operated (surgery without MCAO), (2) control (MCAO + saline), (3) treatment (MCAO + OXT).
In the final step of experiment, to evaluate effect of OXT (8 IU/per mouse) on levels of VEGF, AQ4 and BDNF proteins in the brain, 15 mice were randomly alienated into three groups (1) Sham operated (surgery without MCAO), (2) control (MCAO + saline), (3) treatment (MCAO + OXT).
Behavioral Test
A modified neurological severity score was applied to examine motor and sensory performance as previously described(Behrouzifar et al. 2018). The neurological scoring are as 10–14 severe; 5–9 moderate; and 1–4 mild injury. Behavioral test performed by a student who was blinded to experimental groups.
Spatial Memory
Spatial learning and memory were evaluated using a Radial Arm Water Maze (RAWM) task with six arms (Rahmati et al. 2019). The mice were confronted with the RAWM in 3 situation including habituation (1 day), training (4 days), and probe (1 day). Habituation: Mice were allowed to immerse in the pool and move freely for 2–3 min to adapt with the atmosphere of the RAWM. Training: In this period, mice given take five trials /day with a 30 s inter-trial interval. During training phase, the mice were given 60 s time to find the buried platform. If at this time mouse failing to discover the platform, it was manually guided to the platform. Probe: in this step, the platform was removed and mice were released from the same area in the water, and were allowed to swim for 60 s to find the position of the platform. The latency to reach the platform location (sec), the time spent in target zone (sec) and proximity (cm) to the platform were recorded and analyzed (NoldusEtho Vision XT7, the Netherlands).
Infarct Size
After deep anesthesia, the brain was removed and transferred to 4 °C saline solution for 5 min. Afterward, 5 consecutive 2-mm-thick slices of brain were prepared via a mice brain matrix. The slices were stained with 2,3,5-Triphenyltetrazolium chloride (TTC) solution (T8877, Sigma, Germany). After that, stained slices were transferred to the computer and the area of injury was measured using image analyzer software (Scion image, 4.0). The volume of damage was obtained by multiplying the area of injury in the thickness of each slice. The size of damage (mm3) of each brain was achieved with summing of the damage of five sections. Data were stated as infarct volume (mm3) corrected for edema(Vakili and Zahedi-Khorasani 2008).
Brain edema
At the end of the experiment, mice were sacrificed and their brains were removed and weighed to obtain their wet weight (WW). Afterward, the brains were dried at 110 °C for 24 h to find their dry weight (DW). Finally, brain edema is indirectly obtained by calculating the percentage of brain water content (BWC %) via the following formula: (WW − DW)/WW × 100.
Blood-Brain Barrier
Evans blue (EB) method (Asadi et al. 2018) was used to estimate the BBB disruption. Briefly, EB (E2129 Sigma, EB 2% in saline, 2 ml/kg) was given through the tail vein at one hour after MCAO. Twenty-four hours MCAO and under after deep anesthesia to eliminate EB from cerebral circulation thorax was opened and 200 ml normal saline was infused into heart. Subsequently, brains were detached, and hemispheres were isolated and weighed. Each hemisphere was homogenized in 2.5 ml PBS, and then 2.5 ml of 60% trichloroacetic acid (CAS 76–03-9 Merck, Germany) was added to precipitate the protein. Afterward, samples were centrifuged for 30 min at 3500 rpm. The supernatants were measured at 610 nm for the absorbance of EB using a spectrophotometer (UV VIS spectrophotometer, Spekol 2000, Analytik Jena, AG, and Germany). The results were reported in units of microgram per gram of the wet brain tissue.
Western Blotting
Twenty-four hours after MCAO, the brain was removed, and the ischemic hemisphere was divided into two parts to western blotting of VEGF, AQP4 and ELISA measurement of BDNF.
For western blotting, collected samples of brain were utilized to AQP4, VEGF and GAPDH measurements. Protein components of brain samples were extracted. Tissue lysis was achieved in the RIPA lysis buffer, and common protease inhibitor cocktail was used to inhibit protein degradation during the process. Protein concentrations were measured using Bradford assay, and then transferred to PVDF membranes at 80 V for 90 min (Bio-Rad). After that, Proteins were separated by polyacrylamide gel electrophoresis (Bio-Rad) via 4–20% gradient polyacrylamide gels containing 0.1% sodium dodecyl sulfate for ~ 2 h at 95 V. After blocking with 5% non-fat milk in Tris-buffered saline and Tween 20 (pH 7.6) (TBST), the membranes were incubated with primary antibodies against AQP4(sc-32739, Santa Cruz Biotechnology, USA), VEGF( sc-7269, Santa Cruz Biotechnology, USA) and GAPDH (rabbit anti- (1:100, ab2350, U K) at 4 °C overnight. Then, the membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (HRP, 1:5000, sc-516102, Santa Cruz Biotechnology, USA,) for two hours at room temperature. The antibody incubations were followed by TBST washing three times and each time for ten minutes. Then, chromogenic substrate was added to the membranes. The protein bands were detected using DAB (3,3′-diaminobenzidine) substrate and images of the membrane were captured and analyzed using the Image J software. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization.
Measurement of BDNF
Twenty-four hours after MCAO, sample was collected and homogenized (1:10 w/v) in cold KCl 1.15% and centrifuged at 20,000 ×g at 4 °C for 10 min. The supernatant was used for measuring the BDNF protein level. The BDNF protein level was measured using an enzyme-linked immunosorbent assay (ELISA) and mouse BDNF ELISA kit (orb409268, biorbyt, UK).
Immunohistochemistry
NF-kB proteins expression and TUNEL positive cell were analyzed by immunofluorescent (IF) method. The samples of brains were collected and post-fixed overnight. Then, they were dehydrated in the ascending alcohol series, rinsed by xylene, and infiltrated with paraffin. Afterwards, all of the blocks were divided into 5 μm coronal sections. Accordingly, the sections were incubated in 50% formamide and 2x standard sodium citrate buffer at 65 °C for 2 h, and then were incubated twice in 100 mM of sodium borate (pH 8.5) and then DNA was denatured by incubating the sections in 2 N HCl at 37 °C, rinsed in phosphate-buffered saline (PBS), and blocked with 0.4% Triton X-100 in PBS and goat serum (10%, Gibco™ PCN5000 10,098,792, UK) for 30 min. The sections were incubated overnight at 4 °C with primary antibodies for NF-kB (rabbit anti-NF-kB (1:100, orb312399, biorbyt, UK)). The slices were then incubated with secondary antibody FITC anti-rabbit (1:200; ab6717) at 37 °C for 90 min in a dark place. DAPI (4′, 6-diamidino-2-phenylindole) was used to stain nuclei in IF. The samples were visualized with a fluorescent microscope (Olympus, Japan) at 400X magnification. The quantification of the immunohistochemical assay was based on the fluorescence intensity obtained by Image J software v1.8 (NIH, Wayne Rasband, USA).
TUNEL staining was accomplished using in situ cell death detection Kit, Fluorescein (Cat. No. 11684795910, Roche Diagnostic GmbH, Applied Science, Germany) according to the manufacturer's instructions. TUNEL positive cells were counted in three non-overlapping visual fields for each section by an unsighted investigator using a fluorescence microscopy (Olympus Corporation, Japan) at 50 × magnification in cortex and hippocampus. Data were analyzed by Image J software v1.8 (NIH, Wayne Rasband, USA). software. The numbers of nuclei stained by DAPI were computed to evaluate total cell number, and the number of TUNEL positive cells (green) was expressed as a percentage of the total number of DAPI -stained nuclei (blue).
Statistical Analyses
Data infarct size, neurological score, spatial memory, BBB permeability, brain edema, TUNEL positive cells, NF-κB, AQP4, VEGF and BDNF proteins were analyzed by one-way ANOVA and Bonferroni as post hoc t-test. (Sigma Stat/plot 12.3.0; Jandel Scientific, Erkmiceh, Germany). Results are presented as mean ± SEM. Value of p < 0.05 was considered statistically significant.
Results
Brain Circulation
Laser Doppler Flowmetry was used to monitor local cerebral blood flow (CBF) and guarantee obstruction of the MCA and ischemia. Reducing the blood flow to less than 20% of the initial ensures that brain ischemia has been created. In all experimental groups, after MCAO, CBF was decreased below 20% of the baseline and conserved during 60 min of MCAO (Fig. 1a). There was no significant difference among groups concerning CBF during 60 min ischemia and reperfusion (p > 0.05, Fig. 1a).
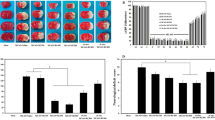
Local cerebral blood flow (LCBF) (a), TTC staining image (b), Infarct volume (c), and neurological deficit scores (d) in the sham operated (surgery + no MCAO), control ischemia (MCAO + saline) and OXT treated groups (MCAO + OXT) at doses 1 (OXT-1), 2 (OXT-2), 4 (OXT-4), and 8 (OXT-8) IU/kg. White color indicates injury and red color shows normal area. Values are as mean ± SEM (n = 7, each). *p < 0.01, compared to the saline group
Oxytocin Reduces Brain Damage and Recuperates Neurological Function and Spatial Learning and Memory
Creating stroke by the blocking of the MCAO for 60 min and 23 h reperfusion resulted in severe brain damage (153 ± 10 mm3) in mice (p < 0.001, Fig. 1b, c). Intranasal injection of OXT at doses 4 (89 ± 10 mm3, p < 0.01) and 8 IU/per mouse (65 ± 12 mm3, p < 0.001) significantly reduced the infarct size compared to the saline (control) group (Fig. 1b, c). Also, OXT at doses 1 (136 ± 14 mm3) and 2 IU/per mouse (135 ± 13 mm3) did not significantly alter infarct size (p > 0.05, Fig. 1b, c).
Cerebral ischemia was associated with severe motor and sensory impairment in mice. These changes were significantly restored in the OXT treated (4 and 8 IU/per mouse) groups in comparison with the control group (p < 0.001, Fig. 1d). Other doses of OXT (1 and 2 IU/per mouse) did not alter the neurological deficit score (p > 0.05, Fig. 1d).
During the 4-day training prior to the experiment in RAWM learning tests, the time to find the hidden platform is gradually reduced in the animal under training (Fig. 2a). There was no significant difference between the groups in the training phase (p > 0.05, Fig. 2a). In the probe trials, after ischemic incident, time (sec) to find the position of the platform and the proximity (centimeters) to the platform were significantly extended, and time spent in the target area declined compared to intact and sham groups (p < 0.01, Fig. 2b–d). Intranasal administration of OXT only at doses 8 IU/per mouse significantly reduced the time, to find the place of the platform and proximity to the platform and increased the time spent in the target area (p < 0.01, Fig. 2b–d).
Escape latency (sec) in 4-day training in RAWM task (a), time (sec) to find the location of the platform (b) time spent in the target zone (c) and the proximity (cm) to the platform (d) in the intact mice (no surgery + no MCAO), sham operated (surgery + no MCAO), control ischemia (MCAO + saline) and OXT treated groups (MCAO + OXT) at doses 1 (OXT-1), 2 (OXT-2), 4 (OXT-4), and 8 (OXT-8) IU/kg. Values are mean ± SEM. *p < 0.01 compared to the saline group
Oxytocin Attenuated Brain Edema Formation, BBB Leakage and Enhanced AQP4 Expression
In the non-ischemic group (sham operated), percentage BWC % was 78.6 ± 0.2%, 24 h after surgery. Creating the brain ischemia significantly increased the percentage of BWC to 83.1 ± 0.2% (P < 0.001, Fig. 3a) at 24 h after MCAO. Intranasal exposure to OXT (8 IU/per mouse) significantly (80.7 ± 0.4%) reduced the percentage of BWC (as an index of brain edema) compared to the saline (control) group (p < 0.001, Fig. 3a). There was no significant difference among groups in terms of the BWC% in the non-ischemic hemisphere (p > 0.05, Fig. 3a).
Percentage of brain water content (BWC %) (a), EB leakage (µg/g tissue) (b), and photograph and quantitative analysis of AQP-4 (c and d) in the sham-operated (surgery + no MCAO) and control ischemia (MCAO + saline) and OXT (MCAO + OXT, 8 IU/kg) treated groups. #p < 0.001 compared to respective sham-operated group. *p < 0.01 compared to the saline group
The quantity of EB leakage to brain tissue was used as a marker of BBB damage. The quantity of EB in the ischemic brain tissue for the saline (control) group (39.40 ± 3.37 μg/g tissue) was considerably higher than the sham-operated group (1.69 ± 0.31 μg/g tissue; p < 0.001, Fig. 3b). Intranasal injection of OXT (8 IU/per mouse) significantly reduced (26.51 ± 3.10) the EB leakage into the ischemic tissue at 24 h after MCAO (p < 0.009, Fig. 3b).
Western blot analysis indicated that there is no significant difference in expression of AQP4 protein between sham and saline (control) groups (p > 0.05, Fig. 3c, d). Intranasal injection of OXT (8 IU/per mouse) at the beginning of MCAO significantly increased the expression of AQP4 (p < 0.01, Fig. 3c, d) protein in the brain tissue.
Oxytocin Enhanced Expression of VEGF and BDNF Proteins in the Brain Tissue
Western blot analysis indicated that there is no significant difference in expression of VEGF protein between sham and saline (control) groups (p > 0.05, Fig. 4a). Intranasal injection of OXT (8 IU/per mouse) at the beginning of MCAO considerably enhanced expression of VEGF (p < 0.001, Fig. 4a) protein in the brain tissue.
The photograph exhibits the levels of VEGF (western blotting) and BDNF (ELISA) in the sham-operated (surgery + no MCAO), control ischemia (MCAO + saline) and OXT (MCAO + OXT, 8 IU/kg) treated groups (a). The quantitative analysis shows VEGF/GAPDH ratio (b) and BDNF (ng/mg Pr) at 24 h after MCAO (n = 5, each). *p < 0.001 compared to the saline group. #p < 0.001 compared to respective sham-operated group
The ELISA assessment showed that intranasal injection of OXT (8 IU/per mouse) at the beginning of MCAO significantly increased the level of BDNF protein in the brain tissue (p < 0.001, Fig. 4b).
Oxytocin Suppressed TUNEL Positive Cell (Apoptosis) in the Cortex and Hippocampus
Cerebral ischemia resulted in a significant increment in apoptotic cells in TUNEL staining.
in the cortex and hippocampus compared to the sham group (p < 0.001, Fig. 5a, b). Intranasal injection of OXT (8 IU/per mouse) at the beginning of MCAO significantly declined the number of apoptotic cells in TUNEL staining in the cortex and hippocampus at 24 h after ischemia (p < 0.001, Fig. 5a, b).
Microphotographs of TUNEL staining and quantitative analysis of percentage of TUNEL positive cells in the cortex (a) and hippocampus (b) in sham-operated (surgery + no MCAO), control ischemia (MCAO + saline) and OXT (MCAO + OXT, 8 IU/kg) treated groups. TUNEL positive cells (green) were expressed as a percentage of the total number of DAPI -stained nuclei (blue) (400 × fluorescent microscope). The percentage of TUNEL positive cells (apoptotic cells) was showed as mean ± SEM (n = 5, each). #p < 0.001 compared to respective sham-operated group. *p < 0.001 compared to the saline group
Oxytocin Diminished NF-κB Expression in the Cortex and Hippocampus
Immunohistochemistry analysis showed that the NF-κB expression in the sham group was low in the cortex and the hippocampus, but after ischemia, it considerably increased (p < 0.001; Fig. 6a, b). NF-κB expression was significantly decreased after intranasal OXT (8 IU/per mouse) administration compared to saline (control) group in the cortex and the hippocampus (p < 0.001; Fig. 6a, b).
Microphotographs of NF-kB proteins expression and quantitative analysis of percentage of NF-kB positive cells in the cortex (a) and hippocampus (b) in sham-operated (surgery + no MCAO), control ischemia (MCAO + saline) and OXT (MCAO + OXT, 8 IU/kg) treated groups. NF-kB positive cells (green) were expressed as percentages of the total number of DAPI -stained nuclei (blue) (400 × fluorescent microscope). The percentage of NF-kB positive cells was showed as mean ± SEM (n = 5, each). #p < 0.001 compared to respective sham-operated group. *p < 0.001 compared to the saline group
Discussion
In this study, the efficacy of intranasal injection of OXT on neuronal damage, BBB disruption, spatial memory, neurobehavioral outcomes and possible underlying molecular mechanisms were investigated in mouse model of stroke. The main findings of the present study were: 1. OXT at doses of 4 and 8 IU/per mouse restricted ischemic brain injury and recovered spatial memory impairment, 2. These OXT effects seem to be mediated by the decline in apoptosis, NF-κB signaling pathway and upregulation of VEGF and BDNF expression, 3. OXT also reduced the brain edema through diminishing BBB leakage and enhancing the AQP4 expression.
Synthetic oxytocin (syntocinon) is usually utilized intranasally or intravenously to initiate the labor and increase postpartum lactation in humans (Gimpl et al. 2008; Macdonald and Feifel 2013). Moreover, recent clinical studies have shown that OXT can be used in treating or controlling a variety of mental disorders such as stress, anxiety, social phobia, postpartum depression, bipolar disorder, autism and schizophrenia (Afzal et al. 2017; Cai et al. 2018). Also, a recent meta-analysis study confirmed the efficacy of intranasal OXT in the treatment of autism spectrum disorder (Cai et al. 2018). In addition, a systematic review paper showed that short-term intranasal application of OXT up to dose of 40 IU is safe and has no significant side-effects in humans (Macdonald and Feifel 2013). Our study indicated that intranasal application of OXT (4 and 8 IU/per mouse) at acute phase of stroke reduced the infarct size by 42% and 52%, respectively, which was accompanied by recuperation of neurological and memory functions. In line with our results, two animal studies reported that OXT has neuroprotective activity against cerebral ischemic injury (Karelina et al. 2011; Moghadam et al. 2018). However, our data additionally revealed a new application of OXT for the treatment of stroke in humans. Although this study indicated that OXT treatment is effective in the acute phase of stroke, future studies are necessary to clarify its efficacy in the late phase of stroke and/or its usefulness as a preventative treatment.
We also showed that intranasal administration of OXT (8 IU/kg) recuperated stroke induced spatial memory loss in the RAWM task. Although there is no data regarding the effect of OXT on memory loss following cerebral ischemia, consistent with our results, two studies have reported that intranasal application OXT reduced destructive effects of stress on the hippocampus spatial and recognition memory (Lee et al. 2015; Park et al. 2017).
In the current study, "intranasal method" was used to deliver the OXT to the brain. Intranasal application of OXT has been considered as the most reliable technique to transport this peptide into the brain for various therapeutic purposes. For instance, it has been shown that OXT reached the maximum peak in the hippocampus, 30 to 60 min after the intranasal injection in rats (Neumann et al. 2013). Recently, a study reported that after nasal injection more than 95% of OXT directly enters the brain (Tanaka et al. 2018).
Several studies have revealed that OXT receptors are widely expressed throughout the brain region such as hippocampus (Vaidyanathan and Hammock 2017; Lin and Hsu 2018). BDNF is the most important neurotrophic molecule that play a critical role in memory formation and cognitive processing (Andero et al. 2014; Bekinschtein et al. 2014; Greenberg et al. 2009). We found that administration of OXT prevented hippocampal BDNF reduction in the brain after ischemic stroke, suggesting that the protective effect of OXT against ischemia-induced memory loss might be mediated via increasing BDNF expression in the hippocampus. Future studies are needed to determine whether the inhibition of OXT receptors in the hippocampus would prevent the cognitive protective effects of OXT following brain ischemia.
BBB dysfunction and brain edema in response to ischemic injury is generated after stroke attack. In the clinical setting, cerebral edema is one of the main causes of neurological decline and death after stroke attacks. Hospital management of BBB dysfunction and cerebral edema is restricted and has not been altered in the past decades. It is well known that cytotoxic edema (cellular injury) in the early stages and vasogenic edema (BBB disruption) appear in the late phase of ischemic stroke (Stokum et al. 2016). We found that intranasal application of OXT (8 IU/per mouse) in therapeutic doses reduces the BBB damage by 33% and brain edema by 47% compared with control group. Although molecular mechanisms by which OXT protects the BBB against ischemia is not completely clear in the current study, one possible way is that OXT reduces the stroke induced BBB interruption through inhibiting the expression of NF-κB and pro-inflammatory cytokines, upregulation of APQ-4 and tight junction proteins. Tight junction proteins including occludin, and claudins (claudin-1, claudin-3, and claudin-5) play an essential role in the regulation of BBB integrity (Abdullahi et al. 2018). The present study did not examine the role of tight junction proteins (occludin, and claudins) in the protective effects of OXT against BBB dysfunction. Thus, further works are needed to elucidate the effect of OXT on the BBB integrity and the expression of tight junction proteins.
Also, we demonstrate, for the first time, that administration of OXT could upregulate the expression of AQP4 (a water-channel protein) by about 57% at 24 h after ischemia. This is an interesting finding that OXT may reduce the brain edema by increasing the expression of AQP4 in the late phase of stroke. Previous studies have shown that AQP4 plays a central role in the pathophysiology of cytotoxic and vasogenic brain edema (Filippidis et al. 2016). It has been shown that upregulation of AQP4 reduces vasogenic edema through accelerating transfer of water from parenchymal brain to CSF space and/or intraventricular compartments (Liu et al. 2020). In agreement with our data, an animal study recently reported that brain water content and BBB disruption are exacerbated following AQP4 knockout in a rat model of subarachnoid hemorrhage (Liu et al. 2020). Therefore, we can conclude that part of the anti-edematous effect of OXT might be related to the increased expression of AQP4 and declined BBB interruption.
The present study showed that administration of OXT caused a significant increase in the expression of BDNF and VEGF levels in the brain, which was associated with the reduction of brain damage and recover memory function. This is in line with findings of Dayi et al., who reported intranasal application OXT significantly increased the levels of BDNF and VEGF of hippocampus in a rat model chronic stress (Dayi et al. 2015). These findings together suggest that BDNF and VEGF mediate the protective effects of OXT against brain ischemic damage and promotion of cognitive functions and memory acquisition.
The mechanisms by which VEGF and BDNF can protect neurons against cerebral ischemia are not yet fully understood. The neuroprotective effects of BDNF might be exerted by inhibition of apoptosis, inflammatory signaling pathway, glutamate-induced neurotoxicity and promotion of neural regeneration (Chen et al. 2013). Likewise, VEGF can reduce neuronal death by suppression of apoptosis and induction of neurogenesis and angiogenesis (Sun et al. 2003; Jin et al. 2001). However, the overexpression of VEGF after cerebral ischemia might be associated with increase the BBB disruption and cerebral edema (Shen et al. 2011). The present study showed that OXT administration increased VEGF expression, and concurrently it maintained the integrity of the BBB and reduced cerebral edema. One possible explanation of these results is that the effect of upregulation of VFEG on the BBB might be counteracted by other observed effects of OXT such as the inhibition of inflammation and apoptosis and overexpression AQP-4.
NF-kB protein is a nuclear transcription factor that is located in the cytoplasm of unstimulated cells in the inactive form as binding to a specific inhibitors called IκB protein. As soon as IκB is destroyed, NF-κB is translocated into the nucleus and becomes functional. Activational form of NF-κB bonded with DNA and turn on the expression of specific genes that involved in the regulation of many physiological and harmful process such as inflammatory, cell survival and cellular proliferation, apoptosis, cytotoxic responses and tissue injury (Oeckinghaus and Ghosh 2009; M. H. Park and Hong 2016). It is approved that after cerebral ischemia event NF-κB overexpress and triggers the expression of many pro-inflammatory genes such as interleukin-1 (IL-1), IL-6 and tumor necrosis factor-a (TNF-α),which play important roles in the pathogenesis of neuronal injury(Berti et al. 2002; Zhao et al. 2018). In this study, activational form of NF-κB was detected by immunocytochemically with FITC-tagged antibody method that represented NF-κB-fluorescence localized predominantly within the nucleus. Results of our research indicated that OXT treatment reduced the expression of activational form of NF-κB in the cortex and hippocampus, showing a potential anti-inflammatory capacity. This finding demonstrated that the protective action of OXT, at least in part, might be associated with the decrease of activational form of NF-κB and inhibition of production of pro-inflammatory mediators. In agreement with our finding, several in vitro and in vivo investigations have demonstrated that OXT has anti-inflammatory activity by inhibiting the expression of NF-κB and pro-inflammatory cytokines (Ahmed and ELosaily 2011; Karelina et al. 2011; Düşünceli et al. 2008; Khori et al. 2018; Yuan et al. 2016).
In conclusion, our findings provide important evidences that OXT significantly suppresses neuronal damage and recovers memory impairment in the early stage of stroke by inhibiting apoptotic and NF-κB signaling pathway, increasing the expression of VEGF, AQP4 and BDNF proteins and reducing the BBB leakage. The results of our study may open a new approach and perspective for the inspection and use of OXT in clinical trials for stroke patients.
References
Abdullahi, W., Tripathi, D., & Ronaldson, P. T. (2018). Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. American Journal of Physiology-Cell Physiology, 315(3), C343–C356.
Afzal, M., Sidra, T., & Cheng, L. (2017). Oxytocin system in neuropsychiatric disorders: Old concept, new insights. Acta Physiologica Sinica, 69(2), 196–206.
Ahmed, M. A., & ELosaily, G. M. (2011). Role of oxytocin in deceleration of early atherosclerotic inflammatory processes in adult male rats. International Journal of Clinical and Experimental Medicine, 4(3), 169.
Akdemir, A., Erbas, O., Gode, F., Ergenoglu, M., Yeniel, O., Oltulu, F., et al. (2014). Protective effect of oxytocin on ovarian ischemia-reperfusion injury in rats. Peptides, 55, 126–130.
Akman, T., Akman, L., Erbas, O., Terek, M. C., Taskiran, D., & Ozsaran, A. (2015). The preventive effect of oxytocin to cisplatin-induced neurotoxicity: An experimental rat model. BioMed research international, 2015.
Al-Amran, F., & Shahkolahi, M. Oxytocin ameliorates the immediate myocardial injury in rat heart transplant through downregulation of neutrophil-dependent myocardial apoptosis. In Transplantation proceedings, 2013 (Vol. 6, 45, pp. 2506–2512). New York: Elsevier
Andero, R., Choi, D. C., & Ressler, K. J. (2014). BDNF–TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. In Progress in molecular biology and translational science (Vol. 122, pp. 169–192). New York: Elsevier.
Arcienega, I., Brunet, J., Bloch, J., & Badaut, J. (2010). Cell locations for AQP1, AQP4 and 9 in the non-human primate brain. Neuroscience, 167(4), 1103–1114.
Asadi, Y., Gorjipour, F., Behrouzifar, S., & Vakili, A. (2018). Irisin peptide protects brain against ischemic injury through reducing apoptosis and enhancing BDNF in a rodent model of stroke. Neurochemical Research, 43(8), 1549–1560.
Behrouzifar, S., Vakili, A., Bandegi, A. R., & Kokhaei, P. (2018). Neuroprotective nature of adipokine resistin in the early stages of focal cerebral ischemia in a stroke mouse model. Neurochemistry International, 114, 99–107.
Bekinschtein, P., Cammarota, M., & Medina, J. H. (2014). BDNF and memory processing. Neuropharmacology, 76, 677–683. https://doi.org/10.1016/j.neuropharm.2013.04.024.
Berti, R., Williams, A. J., Moffett, J. R., Hale, S. L., Velarde, L. C., Elliott, P. J., et al. (2002). Quantitative real-time RT—PCR analysis of inflammatory gene expression associated with ischemia—Reperfusion brain injury. Journal of Cerebral Blood Flow & Metabolism, 22(9), 1068–1079.
Bethlehem, R. A., van Honk, J., Auyeung, B., & Baron-Cohen, S. (2013). Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology, 38(7), 962–974.
Boccia, M., Petrusz, P., Suzuki, K., Marson, L., & Pedersen, C. (2013). Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience, 253, 155–164.
Cai, Q., Feng, L., & Yap, K. Z. (2018). Systematic review and meta-analysis of reported adverse events of long-term intranasal oxytocin treatment for autism spectrum disorder. Psychiatry and Clinical Neurosciences, 72(3), 140–151.
Chen, A., Xiong, L.-J., Tong, Y., & Mao, M. (2013). The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomedical Reports, 1(2), 167–176.
Dayi, A., Cetin, F., Sisman, A. R., Aksu, I., Tas, A., Gönenc, S., et al. (2015). The effects of oxytocin on cognitive defect caused by chronic restraint stress applied to adolescent rats and on hippocampal VEGF and BDNF levels. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 21, 69.
Düşünceli, F., İşeri, S. Ö., Ercan, F., Gedik, N., Yeğen, C., & Yeğen, B. Ç. (2008). Oxytocin alleviates hepatic ischemia–reperfusion injury in rats. Peptides, 29(7), 1216–1222.
Filippidis, A., Carozza, R., & Rekate, H. (2016). Aquaporins in brain edema and neuropathological conditions. International journal of molecular sciences, 18(1), 55.
Gimpl, G., Reitz, J., Brauer, S., & Trossen, C. (2008). Oxytocin receptors: Ligand binding, signalling and cholesterol dependence. Progress in Brain Research, 170, 193–204.
Greenberg, M. E., Xu, B., Lu, B., & Hempstead, B. L. (2009). New insights in the biology of BDNF synthesis and release: implications in CNS function. Journal of Neuroscience, 29(41), 12764–12767.
Gutkowska, J., & Jankowski, M. (2009). Oxytocin: Old hormone, new drug. Pharmaceuticals, 2(3), 168–183.
Houlton, J., Abumaria, N., Hinkley, S. F., & Clarkson, A. N. (2019). Therapeutic potential of neurotrophins for repair after brain injury: A helping hand from Biomaterials. Frontiers in neuroscience, 13, 790.
Işeri, S. Ö., Şener, G., Saǧlam, B., Gedik, N., Ercan, F., & Yeǧen, B. Ç. (2005). Oxytocin protects against sepsis-induced multiple organ damage: Role of neutrophils. Journal of Surgical Research, 126(1), 73–81.
Jankowski, M., Bissonauth, V., Gao, L., Gangal, M., Wang, D., Danalache, B., et al. (2010). Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Research in Cardiology, 105(2), 205–218.
Jin, K., Mao, X., Batteur, S., McEachron, E., Leahy, A., & Greenberg, D. (2001). Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience, 108(2), 351–358.
Kaneko, Y., Pappas, C., Tajiri, N., & Borlongan, C. V. (2016). Oxytocin modulates GABA A R subunits to confer neuroprotection in stroke in vitro. Scientific Reports, 6, 35659.
Karelina, K., Stuller, K. A., Jarrett, B., Zhang, N., Wells, J., Norman, G. J., et al. (2011). Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke, 42(12), 3606–3611.
Khori, V., Alizadeh, A. M., Khalighfard, S., Heidarian, Y., & Khodayari, H. (2018). Oxytocin effects on the inhibition of the NF-κB/miR195 pathway in mice breast cancer. Peptides, 107, 54–60.
Knobloch, H. S., Charlet, A., Hoffmann, L. C., Eliava, M., Khrulev, S., Cetin, A. H., et al. (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron, 73(3), 553–566.
Lee, S.-Y., Park, S.-H., Chung, C., Kim, J. J., Choi, S.-Y., & Han, J.-S. (2015). Oxytocin protects hippocampal memory and plasticity from uncontrollable stress. Scientific Reports, 5, 18540.
Lin, Y.-T., & Hsu, K.-S. (2018). Oxytocin receptor signaling in the hippocampus: Role in regulating neuronal excitability, network oscillatory activity, synaptic plasticity and social memory. Progress in Neurobiology.
Liu, E., Sun, L., Zhang, Y., Wang, A., & Yan, J. (2020). Aquaporin4 knockout aggravates early brain injury following subarachnoid hemorrhage through impairment of the glymphatic system in rat brain. Subarachnoid Hemorrhage (pp. 59–64). Berlin: Springer.
Macdonald, K., & Feifel, D. (2013). Helping oxytocin deliver: Considerations in the development of oxytocin-based therapeutics for brain disorders. Frontiers in Neuroscience, 7, 35.
Melville, K., & Varma, D. (1961). Synthetic oxytocin as an antagonist of experimental cardiac anoxic changes in rabbits. British Journal of Pharmacology and Chemotherapy, 17(2), 218–223.
Moghadam, S. E., Tameh, A. A., Vahidinia, Z., Atlasi, M. A., Bafrani, H. H., & Naderian, H. (2018). Neuroprotective effects of oxytocin hormone after an experimental stroke model and the possible role of calpain-1. Journal of Stroke and Cerebrovascular Diseases, 27(3), 724–732.
Neumann, I. D., Maloumby, R., Beiderbeck, D. I., Lukas, M., & Landgraf, R. (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology, 38(10), 1985–1993.
Nowacka, M., & Obuchowicz, E. (2013). BDNF and VEGF in the pathogenesis of stress-induced affective diseases: An insight from experimental studies. Pharmacological Reports, 65(3), 535–546.
Oeckinghaus, A., & Ghosh, S. (2009). The NF-κB family of transcription factors and its regulation. Cold Spring Harbor perspectives in biology, 1(4), a000034.
Ondrejcakova, M., Ravingerova, T., Bakos, J., Pancza, D., & Jezova, D. (2009). Oxytocin exerts protective effects on in vitro myocardial injury induced by ischemia and reperfusion. Canadian journal of physiology and pharmacology, 87(2), 137–142.
Park, M. H., & Hong, J. T. (2016). Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells, 5(2), 15.
Park, S.-H., Kim, Y.-J., Park, J.-C., Han, J.-S., & Choi, S.-Y. (2017). Intranasal oxytocin following uncontrollable stress blocks impairments in hippocampal plasticity and recognition memory in stressed rats. International Journal of Neuropsychopharmacology, 20(10), 861–866.
Rahmati, H., Momenabadi, S., Vafaei, A. A., Bandegi, A. R., Mazaheri, Z., & Vakili, A. (2019). Probiotic supplementation attenuates hippocampus injury and spatial learning and memory impairments in a cerebral hypoperfusion mouse model. Molecular biology reports, 1–11.
Shen, F., Walker, E. J., Jiang, L., Degos, V., Li, J., Sun, B., et al. (2011). Coexpression of angiopoietin-1 with VEGF increases the structural integrity of the blood–brain barrier and reduces atrophy volume. Journal of Cerebral Blood Flow & Metabolism, 31(12), 2343–2351.
Stokum, J. A., Gerzanich, V., & Simard, J. M. (2016). Molecular pathophysiology of cerebral edema. Journal of Cerebral Blood Flow & Metabolism, 36(3), 513–538.
Sun, Y., Jin, K., Xie, L., Childs, J., Mao, X. O., Logvinova, A., et al. (2003). VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. The Journal of Clinical Investigation, 111(12), 1843–1851.
Tanaka, A., Furubayashi, T., Arai, M., Inoue, D., Kimura, S., Kiriyama, A., et al. (2018). Delivery of oxytocin to the brain for the treatment of autism spectrum disorder by nasal application. Molecular Pharmaceutics, 15(3), 1105–1111.
Vaidyanathan, R., & Hammock, E. A. (2017). Oxytocin receptor dynamics in the brain across development and species. Developmental Neurobiology, 77(2), 143–157.
Vakili, A., & Zahedi-Khorasani, M. (2008). Effect of aminoguanidine on post-ischemic damage in rodent model of stroke. Pakistan Journal of Pharmaceutical Sciences, 21(1), 24–28.
Wang, H., Chen, H., Jin, J., Liu, Q., Zhong, D., & Li, G. (2020). Inhibition of the NLRP3 inflammasome reduces brain edema and regulates the distribution of aquaporin-4 after cerebral ischaemia-reperfusion. Life Sciences, 117638.
Wigton, R., Radua, J., Allen, P., Averbeck, B., Meyer-Lindenberg, A., McGuire, P., et al. (2015). Neurophysiological effects of acute oxytocin administration: Systematic review and meta-analysis of placebo-controlled imaging studies. Journal of Psychiatry & Neuroscience.
Yeniel, A. Ö., Erbas, O., Ergenoglu, A. M., Aktug, H., Taskiran, D., Yildirim, N., et al. (2014). Effect of oxytocin treatment on explant size, plasma and peritoneal levels of MCP-1, VEGF, TNF-α and histopathological parameters in a rat endometriosis model. European Journal of Obstetrics & Gynecology and Reproductive Biology, 175, 134–139.
Yuan, L., Liu, S., Bai, X., Gao, Y., Liu, G., Wang, X., et al. (2016). Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. Journal of Neuroinflammation, 13(1), 77.
Zhang, W., Zhang, J., Xu, M., & Zhang, Y. (2007). Effect of oxytocin on gastric ischemia-reperfusion injury in rats. Frontiers of Medicine in China, 1(4), 433–437.
Zhang, Z., & Chopp, M. (2002). Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends in Cardiovascular Medicine, 12(2), 62–66.
Zhao, H., Chen, Z., Xie, L.-J., & Liu, G.-F. (2018). Suppression of TLR4/NF-κB signaling pathway improves cerebral ischemia–reperfusion injury in rats. Molecular Neurobiology, 55(5), 4311–4319.
Acknowledgements
This study was funded by a research grant from Vice Chancellor for Research of the Semnan University of Medical Sciences (Grant Number: 1355). We thanks from Prof. Ali Rashidy-Pour for valuable comments and correction of manuscript and Mehrnoush Rahmani for help in doing of spatial memory evaluation. We also thank for some technical assistance provided by Reza Nasr form the Department of Biotechnology, Semnan University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All of procedures performed in this studies involving animals were in accordance with institutional animal care committee for animal surgery and ethical international guidelines for the care and use of laboratory animals. Research procedure and protocols were approved by the SUMS institutional Committee of Research Ethics (Ethical Code Number: IR.SUMUMS.REC. 1396.241).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Momenabadi, S., Vafaei, A.A., Bandegi, A.R. et al. Oxytocin Reduces Brain Injury and Maintains Blood–Brain Barrier Integrity After Ischemic Stroke in Mice. Neuromol Med 22, 557–571 (2020). https://doi.org/10.1007/s12017-020-08613-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-020-08613-3