Abstract
As a key regulator of cell metabolism and survival, mechanistic target of rapamycin (mTOR) emerges as a novel therapeutic target for Parkinson’s disease (PD). A growing body of research indicates that restoring perturbed mTOR signaling in PD models can prevent neuronal cell death. Nevertheless, molecular mechanisms underlying mTOR-mediated effects in PD have not been fully understood yet. Here, we review recent progress in characterizing the association of mTOR signaling with PD risk factors and further discuss the potential roles of mTOR in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disorder characterized by loss of midbrain dopaminergic neurons in the substantia nigra pars compacta and the presence of intracytoplasmic inclusions called Lewy bodies (Lin et al. 2012; Dexter and Jenner 2013; Anderson and Maes 2014; Schapira et al. 2014). Aging is a primary risk factor for PD (Dauer and Przedborski 2003). It is estimated that PD afflicts about 1–2 % of persons 65 years and older. The economic burden of PD on national healthcare system continues to rise (Boland and Stacy 2012). Current therapeutic interventions for PD may be used to slow the worsening of clinical symptoms and improve the quality of life. However, no cure is currently available for PD. Investigations of the mechanisms underlying the etiology and pathogenesis of PD will facilitate the discovery of potential therapeutic targets for PD.
Mechanistic target of rapamycin (mTOR), a highly conserved serine/threonine kinase expressed in most of mammalian cell types, plays a central role in cell growth/proliferation, survival, metabolism, protein synthesis and autophagy (Laplante and Sabatini 2012; Cornu et al. 2013). There is increasing evidence that mTOR can regulate neuronal development, survival and differentiation, and it also plays a crucial role in synaptic plasticity (Jaworski and Sheng 2006; Swiech et al. 2008). Recent data have shown that deregulation of mTOR is implicated in the pathogenesis of PD (Xu et al. 2014; Zhou et al. 2015). Therefore, mTOR might be one of the potential therapeutic targets for PD. Here, we review recent understanding of mTOR signaling in PD.
mTOR Signaling Pathway
Components and Structure of mTOR
mTOR is a serine/threonine protein kinase, and it oversees multiple functions in cells that involve gene transcription, protein synthesis, cytoskeletal organization, cell metabolism, proliferation and survival (Laplante and Sabatini 2012; Maiese et al. 2013). In mammalian cells, there are two mTOR complexes, i.e., mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Fig. 1). mTORC1, which controls protein homeostasis and autophagy, contains mTOR, Raptor (regulatory-associated protein of mTOR), mLST8 (mammalian lethal with Sec-13 protein 8), PRAS40 (proline-rich Akt substrate of 40 kDa), Deptor (DEP domain-containing mTOR-interacting protein) and Tel2 (telomere maintenance 2)/Tti1 (Tel2 interacting protein 1). Both Raptor and mLST8 act as positive regulators to promote the mTORC1 signaling (Dennis et al. 2013). In contrast, PRAS40 can block mTORC1 activity by preventing the binding of mTORC1 to Raptor (Wang et al. 2012a). Phosphorylation of PRAS40 by protein kinase B (Akt) dissociates it from Raptor, which promotes the binding of PRAS40 to the cytoplasmic docking protein 14-3-3 and subsequent mTORC1 activation (Wang et al. 2012a; Chong et al. 2012; Shang et al. 2012b; Xiong et al. 2014). mTORC2, which regulates cellular survival and proliferation, contains mTOR, Deptor, mLST8, Rictor (rapamycin-insensitive companion of mTOR), mSin1 (mammalian stress-activated map kinase-interacting protein 1), Protor (the protein observed with Rictor) and Tti1/Tel2. Both Rictor and mSin1 are scaffold proteins regulating the assembly of mTORC2. mSin1 is also necessary for mTORC2 to activate Akt (Frias et al. 2006). Protor is involved in mTORC2-mediated activation of SGK1 (serum/glucocorticoid regulated kinase 1) (Pearce et al. 2011). mTORC1 and mTORC2 share three regulatory components in common, including Deptor, mLST8 and Tti1/Tel2. Tti1/Tel2 complex serves as scaffold proteins regulating the assembly of both mTORC1 and mTORC2. Besides, it has been suggested that mLST8 may be important for mTORC2 activity (Laplante and Sabatini 2012). In contrast, Deptor suppresses mTOR activity and negatively regulates both mTORC1 and mTORC2. Inhibition of Deptor increases the activities of Akt, mTORC1 and mTORC2 (Peterson et al. 2009). Despite the presence of catalytic mTOR subunit in both mTORC1 and mTORC2, mTORC1 is more sensitive than mTORC2 to the inhibition by canonical mTOR inhibitor rapamycin (Loewith et al. 2002).
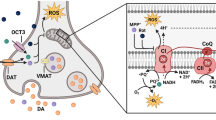
mTOR signaling in PD. As a negative regulator of autophagy, active mTORC1 could be associated with α-synuclein accumulation. In addition, Parkin regulates the activity of mTORC1 through REDD1. PINK1 activates mTORC2 via phosphorylation of Rictor. UCHL1 attenuates the kinase activity of mTORC1 for S6K1/4E-BP1, whereas it enhances mTORC2 activity for Akt. Similar to mTORC1, LRRK2 can also phosphorylate 4E-BP1
mTORC1 Signaling Pathway
mTOR lies downstream of insulin-like growth factor 1 (IGF-1) receptor (IGFR) (Ma and Blenis 2009). Previous studies have suggested tuberous sclerosis complex (TSC) 1/2 as an important regulator between PI3 K/Akt and mTORC1 (Gao et al. 2002; Inoki et al. 2002; Tee et al. 2002). TSC1/2 complex triggers the conversion of active GTP-bound form of GTPase Rheb to inactive GDP-bound state and thus negatively regulates mTORC1 activity (Tee et al. 2002; Manning and Cantley 2003). Furthermore, TSC1/2 can be activated by energy deficiency via AMP-activated kinase (AMPK), which is an important energy sensor (Wullschleger et al. 2006). It should be noted that AMPK can be phosphorylated by LKB1 (serine/threonine kinase 11) in response to the conditions of cellular energy deficiency (Kahn et al. 2005). Elevation of AMP levels or AMP/ATP ratio can induce AMPK phosphorylation and activation (Inoki et al. 2003). Phosphorylated AMPK subsequently activates the TSC1/2, which leads to increased GAP activity of TSC1/2 complex and inhibition of mTORC1 activity (Wullschleger et al. 2006). In addition, AMPK can modulate TSC1/2 activity through RTP801 (REDD1, DNA damage response 1). Upon hypoxia, AMPK activity can increase REDD1 expression to suppress mTORC1 activity through releasing TSC2 from protein 14-3-3 (DeYoung et al. 2008). Knockdown of REDD1 blocks the hypoxia-induced mTORC1 inhibition. mTORC1 exerts its role in regulation of protein translation mainly through controlling the ribosomal protein S6 kinase beta-1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), two well-known downstream substrates involved in translational regulation (Cornu et al. 2013; Laplante and Sabatini 2012). Activated mTORC1 phosphorylates and activates S6K1, which in turn phosphorylates and activates S6 protein, a component of the S40 ribosome subunit (Ma and Blenis 2009). In contrast, activated mTORC1 phosphorylates and inactivates 4E-BP1, a repressor of mRNA translation, by inhibiting the interaction between 4E-BP1 and eukaryotic translation initiation factor-4E (eIF-4E). Therefore, mTORC1 signaling mainly mediates the translation initiation and elongation, as well as the ribosome biogenesis (Ma and Blenis 2009).
mTORC2 Signaling Pathway
mTORC2, similar to mTORC1, also responds to growth factors through PI3 K pathway. In contrast to mTORC1, TSC1/2 may promote the mTORC2 activity through the N-terminal region of TSC2 and the C-terminal region of Rictor (Huang et al. 2009). Previous studies have suggested that removal of a functional TSC1/2 complex can lead to the loss of mTORC2 activity in vitro (Huang et al. 2008, 2009). In addition, mTORC2 exerts its role in regulating cell survival, metabolism and cytoskeleton organization mainly through its primary downstream targets, such as Akt, protein kinase C alpha (PKCα) and SGK1 (Guertin et al. 2006; Oh and Jacinto 2011). In addition to its substrate Akt, mTORC2 regulates cytoskeleton remodeling through PKCα phosphorylation (Sarbassov et al. 2004) and cell migration through the activation of Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 (Gulhati et al. 2011). Moreover, mTORC2 controls ion transport through phosphorylation and activation of SGK1, which is a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family (Garcia-Martinez and Alessi 2008).
mTOR Signaling in PD: Neuroprotective or Neurotoxic?
The mechanisms underlying the etiology and pathogenesis of PD remain largely unknown. In recent years, evidence has accumulated that mTOR signaling is altered during PD progression (Bockaert and Marin 2015; Dijkstra et al. 2015). However, the role of mTOR in PD seems to be controversial since it could be either neuroprotective or neurotoxic in different PD models (Table 1). For example, several studies have shown that PD toxins (rotenone, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), etc.) suppress mTOR signaling and reduce cell viability (Chen et al. 2010; Rieker et al. 2011; Rodriguez-Blanco et al. 2012; Selvaraj et al. 2012; Xu et al. 2014; Zhou et al. 2015), whereas over-expression of wild-type mTOR can partially prevent neuronal cell loss induced by PD toxins (Malagelada et al. 2010; Xu et al. 2014; Zhou et al. 2015). Moreover, Domanskyi and co-workers have reported that genetic deletion of PTEN, which encodes a lipid phosphatase, activates mTOR and protects dopaminergic neurons against neurotoxin insult in mouse models of PD (Domanskyi et al. 2011). By contrast, gene knockdown or oxidative stress-mediated down-regulation of Tnfaip8/Oxi-α, a potential mTOR activator, could prevent the activation of mTOR and mTOR inhibitor rapamycin could potentiate oxidative stress-induced neuronal cell death (Choi et al. 2010). In addition, REDD1, as an inhibitor of mTORC1 (DeYoung et al. 2008), is highly expressed in brains of patients with PD and up-regulated in neurotoxin-induced cellular models of PD (Malagelada et al. 2006). Consistent with these findings, it has been reported that elevated REDD1 expression suppresses mTOR signaling while inhibition of REDD1 translation is neuroprotective in cellular and animal models of PD (Malagelada et al. 2008, 2010; Bao et al. 2012). Interestingly, it has also been suggested that activation of Akt/mTOR signaling in dopaminergic neurons of substantia nigra may facilitate the regeneration of axons upon neurotoxin lesion (Kim et al. 2011). Taken together, these reports indicate that mTOR may be neuroprotective in certain PD models.
Nevertheless, increased mTOR protein levels have also been found in postmortem brains of PD (Wills et al. 2012). In En1 (engrailed 1) +/− mouse model of PD, heterozygous deletion of En1, a transcription factor important for the survival of mesencephalic dopaminergic neurons, results in up-regulation of mTOR signaling in dopaminergic neurons (Nordstrom et al. 2015). Additionally, maneb and paraquat, two environmental risk factors for PD, could significantly increase mTOR levels in mice (Wills et al. 2012). Moreover, accumulating evidence suggests that inhibition of mTOR with rapamycin or its derivatives could be neuroprotective in cellular and animal models of PD (Ravikumar et al. 2006; Pan et al. 2008, 2009; Tain et al. 2009; Spencer et al. 2009; Dehay et al. 2010; Malagelada et al. 2010; Cullen et al. 2011; Jiang et al. 2013a; Decressac and Bjorklund 2013). Interestingly, a common anti-Parkinsonian medication, l-DOPA (levodopa), elicits motor side effects (dyskinesia) via the activation of mTOR signaling in the striatum of mouse model of PD (Santini et al. 2009; Subramaniam et al. 2012). Inhibition of mTOR with rapamycin or its derivatives prevents the development of l-DOPA-induced dyskinesia without significantly compromising the anti-akinetic potency of l-DOPA in animal models of PD (Santini et al. 2009; Decressac and Bjorklund 2013). Collectively, these findings suggest that inhibition of mTOR in PD may be beneficial.
The neuroprotective function of mTOR activation seems to contradict with the beneficial effects of mTOR inhibition in PD. However, two caveats must be considered regarding the interpretation of these experimental data of mTOR in PD models. First, short-term treatment with rapamycin only partially inhibits mTORC1 activity and has limited effects on mTORC2 activity (Jacinto et al. 2004; Sarbassov et al. 2006; Thoreen and Sabatini 2009). In contrast, prolonged rapamycin treatment attenuates the activity of mTORC2, but not mTORC1, in a cell-type-dependent manner (Sarbassov et al. 2006; Choo et al. 2008). Therefore, the neuroprotective effect of rapamycin may be attributed to its inhibition of some but not all functions of mTOR. Certain mTOR functions could be still vital to the viability of neuronal cells upon rapamycin treatment. This notion can be supported by the finding that Torin1, a highly potent mTOR inhibitor (Thoreen et al. 2009), cannot replicate the neuroprotective effects of rapamycin in PD models (Malagelada et al. 2010). Second, it is not surprising that exogenous PD toxins can perturb mTOR signaling which is important for cell survival and metabolism. However, neurotoxin-induced cellular or animal models of PD only partially mimic neuropathological features of PD (Tieu 2011). Down-regulation of mTOR signaling by PD toxins cannot rule out the possibility that mTOR remains unaltered or is even more active in other PD risk factor-associated models. Suppression of hyperactive mTOR with rapamycin in these PD models is presumably neuroprotective. Therefore, we hypothesize that fine-tuned mTOR signaling may be crucial for the survival of dopaminergic neurons.
mTOR Signaling in Neuronal Apoptosis and Oxidative Stress
PD, as a progressive neurodegenerative disorder, is characterized by a selective loss of nigrostriatal dopaminergic neurons (Dawson and Dawson 2003). Accumulating experimental evidence suggests that persistent oxidative stress is a major contributor for degeneration of nigrostriatal dopaminergic neurons (Ciccone et al. 2013; Zeng et al. 2014; Zhang et al. 2015). The implication of oxidative stress in PD is also supported by postmortem analysis of the brains from patients with PD showing a deficiency in antioxidant glutathione (GSH), inhibited mitochondrial complex I activity and increased superoxide dismutase (SOD) activity in the substantia nigra (Mythri et al. 2011). The relation between oxidative stress and the loss of dopaminergic neurons is further supported by PD toxin-induced animal models (Perier et al. 2003; Richardson et al. 2005; Panov et al. 2005). Thus, oxidative stress may be a common mechanism contributing to neurodegeneration in PD.
Oxidative stress is defined as a surplus of oxidants or a deficit in antioxidants (Shulman et al. 2011). This may be due to either an overproduction of reactive oxygen species (ROS) or a failure of cellular antioxidant machinery (Yacoubian and Standaert 2009). Although normal levels of ROS produced by mitochondria are usually detoxified quickly, ROS overproduction can disrupt protein functions and activate or inactivate signaling pathways (such as mTOR), leading to neuronal cell death (Ruffels et al. 2004; Maiese et al. 2010; Jayaram et al. 2011; Yang et al. 2011).
mTOR, as a central controller in cell growth/proliferation, survival and metabolism, may play an important role in preventing neuronal cell death under oxidative stress (Shang et al. 2011). For example, previous studies reported by Chen et al. (2010) and Shang et al. (2011) demonstrated that hydrogen peroxide (H2O2)-generated oxidative stress induces neuronal cell death and inhibits mTOR activity. More recently, another study further indicated that rotenone induces neuronal cell death via the generation of H2O2 and the inhibition of mTOR (Zhou et al. 2015). Therefore, these studies suggest that mTOR activators may be exploited for prevention of oxidative stress-associated neuronal cell death. This notion has been supported by the report that a mTOR activating protein protects dopaminergic neurons against oxidative stress (Choi et al. 2010). Mechanistic studies indicated that mTOR activation prevents the apoptosis of neuronal cells upon oxidative stress via S6K1, 4E-BP1 or Akt. For instance, it has been reported that inhibition of mTOR activity by H2O2 or rotenone suppresses phosphorylation of both S6K1 and 4E-BP1, leading to neuronal cell death (Chen et al. 2010; Zhou et al. 2015). In addition, mTOR-mediated inhibition of neuronal cell death also relies upon Akt activation (Magri et al. 2011; Shang et al. 2011, 2012a). Xu et al. (2014) have reported that inactivation of Akt in a cellular model of PD results in suppression of mTOR signaling, leading to neuronal cell death. These studies suggest that mTOR activation may be required for cell survival during oxidative stress in dopaminergic neurons and loss of mTOR activity may lead to neurodegeneration. However, there are also other studies suggesting that mTOR activation mediates the apoptosis of neuronal cells under oxidative stress. For example, cadmium-induced ROS contributes to the activation of mTOR signaling, leading to neuronal cell death (Chen et al. 2008, 2011). In addition, mechanistic study indicated that calcium/calmodulin-dependent protein kinase II (CaMKII) and calcium signaling are involved in mTOR activation induced by cadmium (Chen et al. 2011; Xu et al. 2011). Taken together, it seems that the effects of mTOR activation on the neuronal cell survival or apoptosis during oxidative stress depend on the types of PD-related toxins. Thus, it is necessary to investigate the precise role of mTOR signaling pathway in oxidative stress induced by different PD toxins.
mTOR-Mediated Autophagy and α-Synuclein Accumulation
Autophagy (self-eating) is a lysosome-dependent intracellular process that allows cells to recycle cytoplasmic components and remove defective cellular organelles such as endoplasmic reticulum (Gumy et al. 2010; Cheung and Ip 2011; Francois et al. 2014; Menzies et al. 2015; Vakifahmetoglu-Norberg et al. 2015; Nakka et al. 2016). Autophagy includes macroautophagy, microautophagy and chaperone-mediated autophagy (Yamada and Singh 2012). Macroautophagy is usually considered to represent autophagy in general. It involves four stages including induction, autophagosome formation, autophagosome fusion with lysosomes and autophagosome for degradation (Silva et al. 2011; He et al. 2013; Kim et al. 2014b; Lim et al. 2014; Maiese 2015). Usually, autophagy is maintained at basal levels in most tissues. It can be up-regulated by suppressing mTOR signaling (Wang et al. 2012b). As a negative regulator of autophagy, mTOR appears to modulate autophagy through the regulation of autophagy-related genes (Atg). It has been reported that loss of Atg5 or Atg7 induces abnormal intracellular protein accumulation/aggregation in neurons and causes neurodegeneration (Komatsu et al. 2006; Hara et al. 2006). These studies thus suggest that autophagy may help inhibit abnormal accumulation of protein aggregates in neurons. Increasing data suggest that autophagy inhibition during aging may contribute to the accumulation and aggregation of proteins (such as α-synuclein), resulting in cellular toxicity and eventual neurodegeneration as seen in PD (Cuervo et al. 2005; Wong and Cuervo 2010). In contrast, activation of autophagy promotes the clearance of cytoplasmic protein aggregates, such as α-synuclein (Menzies et al. 2015). Thus, autophagy constitutes a fundamental survival strategy for neurodegenerative diseases including PD.
The accumulation of α-synuclein and the formation of Lewy bodies are pathological hallmarks of PD (Lin et al. 2012). It has been reported that mutations of α-synuclein, such as A53T, A30P and E46K, and increase in concentrations of α-synuclein in dopaminergic neurons have been associated with the cause and progression of PD (Dehay et al. 2010; Wong and Cuervo 2010; Cannon et al. 2013; Dehay et al. 2015). In addition, lysosomal deficiency and autophagosome accumulation have been observed in postmortem brains of patients with PD (Anglade et al. 1997; Chu et al. 2009). Inhibition of autophagic functions may contribute to the accumulation and aggregation of proteins and eventual neurodegeneration as seen in PD (Wong and Cuervo 2010; Wills et al. 2012). In contrast, activation of autophagy by mTOR inhibition prevents the formation of cytoplasmic protein aggregates, such as α-synuclein (Webb et al. 2003; Sarkar et al. 2005, 2007; Yu et al. 2009; Spencer et al. 2009; Crews et al. 2010; Cullen et al. 2011; Jiang et al. 2013b; Decressac et al. 2013; Perez-Revuelta et al. 2014). Moreover, induction of autophagy with the inhibition of mTOR increases the survival of Drosophila melanogaster treated with the neurotoxin paraquat and attenuates rotenone-induced apoptosis of SH-SY5Y cells (Ravikumar et al. 2006; Pan et al. 2009; Bjedov et al. 2010). Autophagy may also protect neurons through the maintenance of mitochondrial homeostasis (Jeong et al. 2012; Williams et al. 2012). Impaired autophagic degradation leads to an accumulation of dysfunctional mitochondria that cannot be efficiently degraded through mitophagy in the cytosol of affected neurons (Vila et al. 2011). Accumulation of dysfunctional mitochondria was shown to contribute to death of dopaminergic neurons by the release of cytochrome C and subsequent activation of caspase-dependent apoptosis (Perier et al. 2005). However, autophagy may play a dual role that can either protect cell survival or potentiate neuronal lesion in PD. Under oxidative stress, autophagy induction with mTOR inhibition can lead to neuronal cell death (Choi et al. 2010). Therefore, a balance between autophagy-mediated inhibition of protein aggregation and apoptosis must be maintained for neuronal survival.
Moreover, there are many studies about the molecular mechanisms underlying autophagy induction through mTOR inhibition. For example, antagonizing neuronal toll-like receptor 2 activates autophagy through mTOR inhibition, resulting in prevention of synucleinopathy (Kim et al. 2015). mTOR-dependent protein phosphatase 2A activation is involved in the decrease in Ser-129 phosphorylated α-synuclein levels in neuronal cells through autophagy induction (Perez-Revuelta et al. 2014). Cooperative action of JNK and AKT/mTOR is involved in MPTP-induced autophagy of PC12 cells (Rodriguez-Blanco et al. 2012). Altered regulation of Tnfaip8 l1/Oxi-β may significantly contribute to deregulated autophagy observed in dopaminergic neurons under pathogenic oxidative stress condition (Ha et al. 2014). Over-expression of human E46K mutant α-synuclein impairs autophagy through inactivation of JNK1/Bcl-2 pathway (Yan et al. 2014). In addition, other studies suggested that the development of agents which can enhance autophagy activity might be a promising therapeutic strategy for PD. For example, curcumin is neuroprotective in A53T α-synuclein-associated cellular model of PD through down-regulation of mTOR/S6K1 signaling and recovery of autophagy (Jiang et al. 2013b). Corynoxine, a natural autophagy enhancer, promotes the clearance of α-synuclein via Akt/mTOR pathway (Chen et al. 2014). Chebulagic acid, the major constituent of Terminalia chebula and Phyllanthus emblica, protects neuronal cells against toxicity induced by MPTP via autophagy induction (Kim et al. 2014a). Onjisaponin B, derived from Radix polygalae, enhances autophagy and accelerates the degradation of mutant α-synuclein in PC12 cells (Wu et al. 2013).
The Association of mTOR with Other Genetic Risk Factors for PD
Besides α-synuclein, there are at least another five genes commonly associated with PD, including leucine-rich repeat kinase 2 (LRRK2), PTEN-induced putative kinase 1 (PINK1), RING domain-containing E3 ubiquitin ligase (Parkin), DJ-1 and ubiquitin carboxyl-terminal esterase L1 (UCHL1). It has been reported that REDD1 is a substrate of Parkin and Parkin knockout or loss-of-function mutations increase REDD1 levels in neurons (Romani-Aumedes et al. 2014). As mentioned above, REDD1 is a negative regulator of mTORC1. It is plausible that loss of Parkin causes neurodegeneration through up-regulation of REDD1 and subsequent down-regulation of mTORC1. Other than Parkin, PINK1 induces phosphorylation of Rictor, a unique element in mTORC2, leading to the activation of mTORC2 (Murata et al. 2011). Interestingly, UCHL1 regulates the activity of both mTOR complexes. It has been demonstrated that UCHL1 can attenuate the kinase activity of mTORC1 for S6K1 and 4E-BP1 and enhance mTORC2 activity for Akt (Hussain et al. 2013). However, it has also been reported that loss of UCHL1 function may induce the clearance of α-synuclein (Cartier et al. 2012). As inhibition of mTORC1 generally prevents α-synuclein aggregation, the effect of UCHL1-mediated mTORC1 inhibition on the clearance of α-synuclein needs to be clarified. As for LRRK2, it is the most common genetic risk factor for late-onset PD (Hu and Tong 2010). Although no direct link of LRRK2 to mTOR has been reported, LRRK2 can phosphorylate 4E-BP1, a substrate of mTORC1, resulting in deregulated protein translation and loss of dopaminergic neurons in Drosophila (Imai et al. 2008).
Conclusion and Future Perspectives
mTOR complexes (mTORC1 and mTORC2) are multi-functional in neuronal cells. It remains largely unexplored how mTOR modulates the survival of dopaminergic neurons. Both up-regulation and down-regulation of mTOR signaling have been reported in PD models. It thus raises a critical question of whether mTOR is neuroprotective or potentially promotes PD pathogenesis. To address this question, more mechanistic studies are required to elucidate how mTOR activity is perturbed in cellular and animal models of PD. It is envisaged that a balance between activation and inactivation of mTOR signaling is crucial for the survival of dopaminergic neurons. Restoration of perturbed mTOR signaling may be therapeutically beneficial for PD.
Compared with mTORC1, mTORC2 is a less studied protein complex in PD. As mTORC2 can regulate mTORC1 activity via Akt, the role of mTORC2 in PD also needs to be further investigated. To study the functions of both mTORC1 and mTORC2, Torin1, instead of rapamycin, should be used since Torin1 can potently inhibit both protein complexes. In addition, since rapamycin only partially inhibits mTORC1, the application of Torin1 as a control can help distinguish the effects of full inhibition of mTOR from those of rapamycin-mediated mTOR inhibition. The differential effects between partial and full inhibition of mTOR will be important for the drug development with mTOR as a therapeutic target.
mTOR is a negative regulator of autophagy. mTOR inhibition results in the activation of autophagy and consequently attenuates α-synuclein accumulation. However, the role of mTOR in PD may not be limited to autophagy–lysosome pathway. mTOR signaling in other cellular processes, such as protein synthesis and energy metabolism, needs to be further studied in PD models. In addition to α-synuclein, three other genetic risk factors for PD, including Parkin, PINK1 and UCHL1, can directly or indirectly regulate the activity of mTORC1 and/or mTORC2. Moreover, LRRK2 can also phosphorylate the substrate of mTORC1, 4E-BP1, suggesting that a crosstalk between LRRK2 and mTOR signaling may exist. These preliminary studies warrant further investigation of the contribution of both mTOR signaling and PD genes to the pathogenesis of PD.
References
Anderson, G., & Maes, M. (2014). Neurodegeneration in Parkinson’s disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Molecular Neurobiology, 49, 771–783.
Anglade, P., et al. (1997). Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histology and Histopathology, 12, 25–31.
Bao, X. Q., et al. (2012). FLZ protects dopaminergic neuron through activating protein kinase B/mammalian target of rapamycin pathway and inhibiting RTP801 expression in Parkinson’s disease models. Neuroscience, 202, 396–404.
Bjedov, I., et al. (2010). Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metabolism, 11, 35–46.
Bockaert, J., & Marin, P. (2015). mTOR in brain physiology and pathologies. Physiological Reviews, 95, 1157–1187.
Boland, D. F., & Stacy, M. (2012). The economic and quality of life burden associated with Parkinson’s disease: A focus on symptoms. The American Journal of Managed Care, 18, S168–S175.
Cannon, J. R., et al. (2013). Expression of human E46K-mutated α-synuclein in BAC-transgenic rats replicates early-stage Parkinson’s disease features and enhances vulnerability to mitochondrial impairment. Experimental Neurology, 240, 44–56.
Cartier, A. E., et al. (2012). Differential effects of UCHL1 modulation on alpha-synuclein in PD-like models of alpha-synucleinopathy. PLoS ONE, 7, e34713.
Chen, L., et al. (2008). MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. Journal of Neurochemistry, 105, 251–261.
Chen, L., et al. (2010). Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Laboratory Investigation, 90, 762–773.
Chen, L., et al. (2011). Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radical Biology & Medicine, 50, 624–632.
Chen, L. L., et al. (2014). Corynoxine, a natural autophagy enhancer, promotes the clearance of alpha-synuclein via Akt/mTOR pathway. Journal of Neuroimmune Pharmacology, 9, 380–387.
Cheung, Z. H., & Ip, N. Y. (2011). Autophagy deregulation in neurodegenerative diseases—Recent advances and future perspectives. Journal of Neurochemistry, 118, 317–325.
Choi, K. C., et al. (2010). A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. Journal of Neurochemistry, 112, 366–376.
Chong, Z. Z., et al. (2012). PRAS40 is an integral regulatory component of erythropoietin mTOR signaling and cytoprotection. PLoS ONE, 7, e45456.
Choo, A. Y., et al. (2008). Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proceedings of the National Academy of Sciences of the United States of America, 105, 17414–17419.
Chu, Y., et al. (2009). Alterations in lysosomal and proteasomal markers in Parkinson’s disease: Relationship to alpha-synuclein inclusions. Neurobiology of Diseases, 35, 385–398.
Ciccone, S., et al. (2013). Parkinson’s disease: A complex interplay of mitochondrial DNA alterations and oxidative stress. International Journal of Molecular Sciences, 14, 2388–2409.
Cornu, M., Albert, V., & Hall, M. N. (2013). mTOR in aging, metabolism, and cancer. Current Opinion in Genetics & Development, 23, 53–62.
Crews, L., et al. (2010). Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS ONE, 5, e9313.
Cuervo, A. M., et al. (2005). Autophagy and aging: The importance of maintaining “clean” cells. Autophagy, 1, 131–140.
Cullen, V., et al. (2011). Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Annals of Neurology, 69, 940–953.
Dauer, W., & Przedborski, S. (2003). Parkinson’s disease: Mechanisms and models. Neuron, 39, 889–909.
Dawson, T. M., & Dawson, V. L. (2003). Molecular pathways of neurodegeneration in Parkinson’s disease. Science, 302, 819–822.
Decressac, M., & Bjorklund, A. (2013). mTOR inhibition alleviates L-DOPA-induced dyskinesia in parkinsonian rats. Journal of Parkinsons Disease, 3, 13–17.
Decressac, M., et al. (2013). TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proceedings of the National Academy of Sciences of the United States of America, 110, E1817–E1826.
Dehay, B., et al. (2010). Pathogenic lysosomal depletion in Parkinson’s disease. Journal of Neuroscience, 30, 12535–12544.
Dehay, B., et al. (2015). Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurology, 14, 855–866.
Dennis, M. D., Kimball, S. R., & Jefferson, L. S. (2013). Mechanistic target of rapamycin complex 1 (mTORC1)-mediated phosphorylation is governed by competition between substrates for interaction with raptor. Journal of Biological Chemistry, 288, 10–19.
Dexter, D. T., & Jenner, P. (2013). Parkinson disease: From pathology to molecular disease mechanisms. Free Radical Biology and Medicine, 62, 132–144.
DeYoung, M. P., et al. (2008). Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes & Development, 22, 239–251.
Dijkstra, A. A., et al. (2015). Evidence for immune response, axonal dysfunction and reduced endocytosis in the substantia nigra in early stage Parkinson’s disease. PLoS ONE, 10, e0128651.
Domanskyi, A., et al. (2011). Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson’s disease models. FASEB Journal, 25, 2898–2910.
Francois, A., et al. (2014). Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Molecular Brain, 7, 56.
Frias, M. A., et al. (2006). mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Current Biology, 16, 1865–1870.
Gao, X., et al. (2002). Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nature Cell Biology, 4, 699–704.
Garcia-Martinez, J. M., & Alessi, D. R. (2008). mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochemical Journal, 416, 375–385.
Guertin, D. A., et al. (2006). Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Developmental Cell, 11, 859–871.
Gulhati, P., et al. (2011). mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Research, 71, 3246–3256.
Gumy, L. F., Tan, C. L., & Fawcett, J. W. (2010). The role of local protein synthesis and degradation in axon regeneration. Experimental Neurology, 223, 28–37.
Ha, J. Y., et al. (2014). Tnfaip8l1/Oxi-beta binds to FBXW5, increasing autophagy through activation of TSC2 in a Parkinson’s disease model. Journal of Neurochemistry, 129, 527–538.
Hara, T., et al. (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature, 441, 885–889.
He, C., et al. (2013). Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes, 62, 1270–1281.
Hu, Y., & Tong, Y. (2010). A trojan horse for Parkinson’s disease. Science Signaling, 3, pe13.
Huang, J., et al. (2008). The TSC1–TSC2 complex is required for proper activation of mTOR complex 2. Molecular and Cellular Biology, 28, 4104–4115.
Huang, J., et al. (2009). Signaling events downstream of mammalian target of rapamycin complex 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors. Cancer Research, 69, 6107–6114.
Hussain, S., et al. (2013). Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Molecular and Cellular Biology, 33, 1188–1197.
Imai, Y., et al. (2008). Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO Journal, 27, 2432–2443.
Inoki, K., Zhu, T., & Guan, K. L. (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell, 115, 577–590.
Inoki, K., et al. (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biology, 4, 648–657.
Jacinto, E., et al. (2004). Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biology, 6, 1122–1128.
Jaworski, J., & Sheng, M. (2006). The growing role of mTOR in neuronal development and plasticity. Molecular Neurobiology, 34, 205–219.
Jayaram, H. N., Kusumanchi, P., & Yalowitz, J. A. (2011). NMNAT expression and its relation to NAD metabolism. Current Medicinal Chemistry, 18, 1962–1972.
Jeong, J. K., et al. (2012). Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neuroscience Research, 73, 99–105.
Jiang, J., et al. (2013a). Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson’s disease. International Journal of Molecular Medicine, 31, 825–832.
Jiang, T. F., et al. (2013b). Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. Journal of Neuroimmune Pharmacology, 8, 356–369.
Kahn, B. B., et al. (2005). AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism, 1, 15–25.
Kim, S. R., et al. (2011). Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Annals of Neurology, 70, 110–120.
Kim, H. J., et al. (2014a). Neuroprotective effect of chebulagic acid via autophagy induction in SH-SY5Y cells. Biomolecules & Therapeutics (Seoul), 22, 275–281.
Kim, K. A., et al. (2014b). High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biological and Pharmaceutical Bulletin, 37, 1248–1252.
Kim, C., et al. (2015). Antagonizing neuronal toll-like receptor 2 prevents synucleinopathy by activating autophagy. Cell Reports, 13, 771–782.
Komatsu, M., et al. (2006). Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature, 441, 880–884.
Laplante, M., & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149, 274–293.
Lim, Y. M., et al. (2014). Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nature Communications, 5, 4934.
Lin, X., et al. (2012). Conditional expression of Parkinson’s disease-related mutant a-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. Journal of Neuroscience, 32, 9248–9264.
Loewith, R., et al. (2002). Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Molecular Cell, 10, 457–468.
Ma, X. M., & Blenis, J. (2009). Molecular mechanisms of mTOR-mediated translational control. Nature Reviews Molecular Cell Biology, 10, 307–318.
Magri, L., et al. (2011). Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell, 9, 447–462.
Maiese, K. (2015). Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Current Neurovascular Research, 12, 173–188.
Maiese, K., et al. (2010). Oxidative stress: Biomarkers and novel therapeutic pathways. Experimental Gerontology, 45, 217–234.
Maiese, K., et al. (2013). mTOR: On target for novel therapeutic strategies in the nervous system. Trends in Molecular Medicine, 19, 51–60.
Malagelada, C., Jin, Z. H., & Greene, L. A. (2008). RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. Journal of Neuroscience, 28, 14363–14371.
Malagelada, C., et al. (2006). RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. Journal of Neuroscience, 26, 9996–10005.
Malagelada, C., et al. (2010). Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. Journal of Neuroscience, 30, 1166–1175.
Manning, B. D., & Cantley, L. C. (2003). Rheb fills a GAP between TSC and TOR. Trends in Biochemical Sciences, 28, 573–576.
Menzies, F. M., Fleming, A., & Rubinsztein, D. C. (2015). Compromised autophagy and neurodegenerative diseases. Nature Reviews Neuroscience, 16, 345–357.
Murata, H., et al. (2011). A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: Activation of AKT via mTORC2. Journal of Biological Chemistry, 286, 7182–7189.
Mythri, R. B., et al. (2011). Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochemical Research, 36, 1452–1463.
Nakka, V. P., Prakash-Babu, P., & Vemuganti, R. (2016). Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: Potential therapeutic targets for acute CNS injuries. Molecular Neurobiology, 53, 532–544.
Nordstrom, U., et al. (2015). Progressive nigrostriatal terminal dysfunction and degeneration in the engrailed1 heterozygous mouse model of Parkinson’s disease. Neurobiology of Diseases, 73, 70–82.
Oh, W. J., & Jacinto, E. (2011). mTOR complex 2 signaling and functions. Cell Cycle, 10, 2305–2316.
Pan, T., et al. (2008). Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiology of Diseases, 32, 16–25.
Pan, T., et al. (2009). Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience, 164, 541–551.
Panov, A., et al. (2005). Rotenone model of Parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. Journal of Biological Chemistry, 280, 42026–42035.
Pearce, L. R., et al. (2011). Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochemical Journal, 436, 169–179.
Perez-Revuelta, B. I., et al. (2014). Metformin lowers Ser-129 phosphorylated α-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death and Disease, 5, e1209.
Perier, C., et al. (2003). The rotenone model of Parkinson’s disease. Trends in Neurosciences, 26, 345–346.
Perier, C., et al. (2005). Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proceedings of the National Academy of Sciences of the United States of America, 102, 19126–19131.
Peterson, T. R., et al. (2009). DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell, 137, 873–886.
Ravikumar, B., et al. (2006). Rapamycin pre-treatment protects against apoptosis. Human Molecular Genetics, 15, 1209–1216.
Richardson, J. R., et al. (2005). Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicological Sciences, 88, 193–201.
Rieker, C., et al. (2011). Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. Journal of Neuroscience, 31, 453–460.
Rodriguez-Blanco, J., et al. (2012). Cooperative action of JNK and AKT/mTOR in 1-methyl-4-phenylpyridinium-induced autophagy of neuronal PC12 cells. Journal of Neuroscience Research, 90, 1850–1860.
Romani-Aumedes, J., et al. (2014). Parkin loss of function contributes to RTP801 elevation and neurodegeneration in Parkinson’s disease. Cell Death and Disease, 5, e1364.
Ruffels, J., Griffin, M., & Dickenson, J. M. (2004). Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. European Journal of Pharmacology, 483, 163–173.
Santini, E., et al. (2009). Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Science Signaling, 2, 36.
Sarbassov, D. D., et al. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current Biology, 14, 1296–1302.
Sarbassov, D. D., et al. (2006). Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular Cell, 22, 159–168.
Sarkar, S., et al. (2005). Lithium induces autophagy by inhibiting inositol monophosphatase. Journal of Cell Biology, 170, 1101–1111.
Sarkar, S., et al. (2007). Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. Journal of Biological Chemistry, 282, 5641–5652.
Schapira, A. H., et al. (2014). Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet, 384, 545–555.
Selvaraj, S., et al. (2012). Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. The Journal of Clinical Investigation, 122, 1354–1367.
Shang, Y. C., et al. (2011). Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Current Neurovascular Research, 8, 270–285.
Shang, Y. C., et al. (2012a). Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging, 4, 187–201.
Shang, Y. C., et al. (2012b). Wnt1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern beta-amyloid apoptotic injury of microglia. Current Neurovascular Research, 9, 239–249.
Shulman, J. M., De Jager, P. L., & Feany, M. B. (2011). Parkinson’s disease: Genetics and pathogenesis. Annual Review of Pathology: Mechanisms of Disease, 6, 193–222.
Silva, D. F., et al. (2011). Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer’s disease. Current Alzheimer Research, 8, 563–572.
Spencer, B., et al. (2009). Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. Journal of Neuroscience, 29, 13578–13588.
Subramaniam, S., et al. (2012). Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nature Neuroscience, 15, 191–193.
Swiech, L., et al. (2008). Role of mTOR in physiology and pathology of the nervous system. Biochimica et Biophysica Acta, 1784, 116–132.
Tain, L. S., et al. (2009). Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nature Neuroscience, 12, 1129–1135.
Tee, A. R., et al. (2002). Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proceedings of the National Academy of Sciences of the United States of America, 99, 13571–13576.
Thoreen, C. C., & Sabatini, D. M. (2009). Rapamycin inhibits mTORC1, but not completely. Autophagy, 5, 725–726.
Thoreen, C. C., et al. (2009). An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. Journal of Biological Chemistry, 284, 8023–8032.
Tieu, K. (2011). A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harbor Perspectives in Medicine, 1, a009316.
Vakifahmetoglu-Norberg, H., Xia, H. G., & Yuan, J. (2015). Pharmacologic agents targeting autophagy. Journal of Clinical Investigation, 125, 5–13.
Vila, M., et al. (2011). Lysosomal membrane permeabilization in Parkinson disease. Autophagy, 7, 98–100.
Wang, H., et al. (2012a). Proline-rich Akt substrate of 40 kDa (PRAS40): A novel downstream target of PI3 k/Akt signaling pathway. Cellular Signalling, 24, 17–24.
Wang, Y., et al. (2012b). Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. American Journal of Translational Research, 4, 44–51.
Webb, J. L., et al. (2003). Alpha-synuclein is degraded by both autophagy and the proteasome. Journal of Biological Chemistry, 278, 25009–25013.
Williams, A. C., et al. (2012). Nicotinamide, NAD(P)(H), and methyl-group homeostasis evolved and became a determinant of ageing diseases: Hypotheses and lessons from pellagra. Current Gerontology and Geriatrics Research, 2012, 302875.
Wills, J., et al. (2012). Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS ONE, 7, e30745.
Wong, E., & Cuervo, A. M. (2010). Autophagy gone awry in neurodegenerative diseases. Nature Neuroscience, 13, 805–811.
Wu, A. G., et al. (2013). Onjisaponin B derived from Radix Polygalae enhances autophagy and accelerates the degradation of mutant a-synuclein and huntingtin in PC-12 cells. International Journal of Molecular Sciences, 14, 22618–22641.
Wullschleger, S., Loewith, R., & Hall, M. N. (2006). TOR signaling in growth and metabolism. Cell, 124, 471–484.
Xiong, X., et al. (2014). PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiology of Diseases, 66, 43–52.
Xu, B., et al. (2011). Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS ONE, 6, e19052.
Xu, Y., et al. (2014). Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cellular Signalling, 26, 1680–1689.
Yacoubian, T. A., & Standaert, D. G. (2009). Targets for neuroprotection in Parkinson’s disease. Biochimica et Biophysica Acta, 1792, 676–687.
Yamada, E., & Singh, R. (2012). Mapping autophagy on to your metabolic radar. Diabetes, 61, 272–280.
Yan, J. Q., et al. (2014). Overexpression of human E46K mutant a-synuclein impairs macroautophagy via inactivation of JNK1-Bcl-2 pathway. Molecular Neurobiology, 50, 685–701.
Yang, H., et al. (2011). Oxidative stress and diabetes mellitus. Clinical Chemistry and Laboratory Medicine, 49, 1773–1782.
Yu, W. H., et al. (2009). Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. American Journal of Pathology, 175, 736–747.
Zeng, X. S., et al. (2014). The role of thioredoxin-1 in suppression of endoplasmic reticulum stress in Parkinson disease. Free Radical Biology and Medicine, 67, 10–18.
Zhang, Z., et al. (2015). Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radical Biology and Medicine, 84, 331–343.
Zhou, Q., et al. (2015). Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicological Sciences, 143, 81–96.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 11375213, 21390411) and Hundred Talents Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lan, Ap., Chen, J., Zhao, Y. et al. mTOR Signaling in Parkinson’s Disease. Neuromol Med 19, 1–10 (2017). https://doi.org/10.1007/s12017-016-8417-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-016-8417-7