Abstract
Kidney Disease (KD), has a high global prevalence and accounts for one of the most prominent causes of morbidity and mortality in the twenty-first century. Despite the advances in our understanding of its pathophysiology, the only available therapy options are dialysis and kidney transplantation. Mesenchymal stem cells (MSCs) have proven to be a viable choice for KD therapy due to their antiapoptotic, immunomodulatory, antioxidative, and pro-angiogenic activities. However, the low engraftment, low survival rate, diminished paracrine ability, and delayed delivery of MSCs are the major causes of the low clinical efficacy. A number of preconditioning regimens are being tested to increase the therapeutic capabilities of MSCs. In this review, we highlight the various strategies to prime MSCs and their protective effects in kidney diseases.
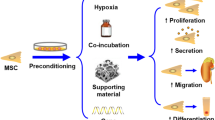
Graphical Abstract
Preconditioning Strategies of Mesenchymal Stem cell in Kidney Disease

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The regenerative capabilities of stem cells, especially mesenchymal stem/stromal cells (MSCs), are remarkable as they hold the potential of curing many degenerative/chronic diseases. However, the conflicting clinical outcomes of stem cell therapy originate from the large host specific variations found in the disease tissue niche.
In this review, we focus on kidney disease due to its high prevalence and high rate of mortality and morbidity. More than 750 million people around the world suffer from kidney disease. This results in 5 to 10 million deaths annually [67, 92]. There’s an established link between obesity [62], diabetes [5], hepatitis B virus infection [32] and the onset and progression of KD which in turn further increases the risk of cardiovascular disorders.
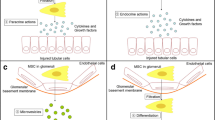
Acute kidney injury (AKI) and chronic kidney disease (CKD) are the two major renal disorders. Almost one-third of children and one-fifth of adult’s worldwide experience AKI. It is characterized by an abrupt loss of kidney function or kidney failure [102]. Numerous factors, such as renal ischemia, crush injuries, inflammation, and infections/obstructions of the urinary tract, can contribute to AKI [31, 86]. The diagnostic features include increased blood urea nitrogen (BUN) and creatinine concentrations, as well as decreased urine output [86]. Aside from a few initial symptoms, individuals experiencing AKI have a significant predisposition to CKD. CKD which is characterised by a progressive loss of kidney function which eventually leads to End-stage renal disease (ESRD). This progression is characterised by accumulation of collagen, which is brought on by inflammation, fibrosis initiation, vascular damage and tubular cell apoptosis [3] (Fig. 1). Diabetic Nephropathy (DN) is the most prevalent form of end-stage kidney disease (ESKD), which necessitates kidney replacement therapy (dialysis or transplant) worldwide [40, 68, 69].
Current interventions include drugs, hemodialysis, peritoneal dialysis, and renal transplantation as therapeutic options for kidney disease [18]. However, the efficacy of these interventions is variable due to the fact that kidney damage is irreversible, prevalence of drug induced severe side effects, inconsistencies in dialysis, and there is always a long waiting list for the donors [12]. As a result, there is an urgent need to investigate new therapeutic strategies to slow the progression of KD [1].
Stem cells have recently been used as a regenerative therapy for a variety of diseases, including KD. MSCs - based therapy for the treatment of KD has increased over the past ten years in a wide range of preclinical models. MSCs are multipotent fibroblast-like cells with strong self-renewal, regeneration, proliferation, and multilineage differentiation abilities. They can be obtained from a range of tissues, including bone marrow (BM), urine, umbilical cord blood, adipose tissue, wharton's jelly, umbilical tissue, and placenta. MSCs secrete a variety of chemokines, cytokines, growth factors, exosomes, and microvesicles (MVs) that have anti-inflammatory, anti-apoptotic, regenerative, angiogenic, anti-fibrotic, and immunomodulatory activities [27, 60, 70, 83]. In addition to the release of paracrine or endocrine chemicals, MSCs therapy involves direct cell-to-cell interactions [34]. Evidently, renal pericytes, which form a network around the microvasculature, are one of the source of MSCs [16]. They play a vital role in the regulation of capillary permeability, endothelial cell survival, renal blood flow, and immune responses [63]. As a result of their regenerative, angiogenic and immunomodulatory properties, MSCs are a promising therapeutic candidate for the recovery of damaged sites and the treatment of various pathological conditions, such as renal injury and renal failure.
However, clinical applications and experimental evidence differ majorly. Reduced cell function following transplantation is a major factor contributing to MSCs' subpar clinical outcomes in kidney disease [99]. Others include poor survival, limited engraftment, and diminished paracrine activity of MSCs following delivery [106].
Post cell transplantation, MSCs encounter an unfavourable environment as opposed to an ideal well-regulated conditions as found in in vitro cultures. This results in early cell apoptosis and loss of functions. Few studies pinpoint towards 80%-90% cell death, 72 hours post transplantation [113]. According to [38] only about 3% of MSCs those were transplanted remained alive 14 days following injection [38]. Additionally, MSCs exposed to uremic toxins released during AKI or CKD have diminished angiogenic potential, irregular migration patterns, and accelerated cellular senescence [33, 79].
The hostile endogenous environment that the cells face post transplantation is a major hurdle in the field of MSCs therapy [116]. To overcome this bottleneck, the preconditioning strategies for empowering the MSCs in a disease specific manner is the need of the hour. Pre-conditioning confers survival advantage to MSCs in adverse microenvironments by imposing conditions that are similar to the diseased or the injured tissue and enhance their paracrine responses during in vivo expansion.
There have been recent developments to improve MSCs' effectiveness in preclinical AKI models by subjecting them to a varied licensing environments in vitro and the findings so far are encouraging. This review focuses on describing the various preconditioning strategies used over the past ten years to increase the effectiveness of MSCs in KD models.
Strategies for Preconditioning MSCs
Advances in MSCs biology and bioengineering have revealed novel solutions with the potential to solve many of the difficulties associated with MSC-based therapy. In general, the preconditioning regimens include exposure to hypoxic conditions, pharmacological or chemical induction (cytokines, hormones and drugs) and physical factors. By a regulated licensing of MSCs before utilising them for transplantation, their properties can be enhanced, leading to beneficial outcomes in vivo.
Preconditioning with Hypoxia
Hypoxia is analogous to the natural microenvironment where stem cells thrive in contrast to the ambient oxygen tension (21%) in a typical cell culture environment. It is well known that low tissue oxygen levels provides a suitable environment for stem cells to survive, proliferate and renew [22, 91] and thereby pre-exposure expansion of the cells in an oxygen environment more similar to the environment in vivo has a considerable positive impact on paracrine capacity of MSCs [25]. Expression of genes encoding CXCR7, CXCR4, CX3CR1, and hypoxia-inducible factor (HIF), which play a role in cell migration and combating oxidative stress are shown to be activated when MSCs are first exposed to hypoxia [52, 71, 72].
Typically, the MSCs expanded under normoxic conditions, when transplanted to a diseased ischemic and hypoxic condition, leads to apoptosis of majority of MSCs. Pre-exposure of MSCs to hypoxia may aid in overcoming this challenge. While the ideal intensity of hypoxia varies throughout research studies, nevertheless the results are quite encouraging.
MSCs cultured for 24 hours under 1% O2 hypoxic conditions expressed higher levels of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF). These elevated factors exerted angiogenic, antioxidative, and antiapoptotic effects on ischemia-reperfusion induced (IRI-induced) AKI renal cells [114]. Preincubating MSCs in a hypoxic environment with 0.5% O2 reportedly influenced changes in the expression of 64 proteins, including bFGF, VEGF, and matrix metalloproteinase 12 (MMP12). These hypoxia conditioned MSCs dramatically decreased the levels of inflammatory cytokines and serum creatinine in a mouse model of cisplatin-induced AKI [81].
Interestingly, cobalt is an agent that aids in the generation of hypoxic conditions and has been shown to significantly improve the MSCs activity/migration by activating HIF-1 and up-regulating CXCR4. These cobalt conditioned MSCs have resulted in improved kidney function in AKI rats [111]. Administration of a high dose of hypoxia preconditioned Bone Marrow derived Mesenchymal Stem cells (BM-MSCs) via the renal artery, concurrently with induction of IRI results in optimal recovery of renal function after renal IRI [55].
Ishiuchi et al. [54] concluded that 1% O2 licensed MSCs significantly reduced inflammation and renal fibrosis in IRI rats compared to MSCs expanded in 21% O2. They also discovered that 1% O2 MSCs enables the upregulation of humoral factors like HGF, VEGF, and PGE2 that have a direct anti fibrotic effect. Collectively, these findings suggest that the allogeneic transplantation of hypoxia-preconditioned MSCs can be used as a cell therapy to attenuate the progression of AKI to CKD [54]. The mechanistic pathways as depicted by Tseng et al. [104] emphasize on tubular autophagy modulation in response to hypoxic MSCs in IRI injury rat models [104].
Hypoxia (5%) preconditioned MSCs have been shown to have a superior beneficial effect as compared to the normoxic MSCs mediating improvement in the renal function, and decrease in creatinine level and BUN in gentamicin- induced Acute renal failure (ARF) rats [84].
Likewise, in another IRI induced fibrosis model, Gao et al. [42] found that hypoxia pretreated human placenta derived MSCs extracellular vesicles (Hypo-EVs) display improvement in the repair of renal structure, the restoration of renal function, and the reduction of renal fibrosis. Hypo-EVs lead to an improved mitochondrial fatty acid oxidation (FAO) in the kidney, thereby reducing IRI damage, partially explained by the improvement of overall mitochondrial homeostasis [42].
Hypoxia (1%) preconditioned Adipose derived Mesenchymal Stem Cells (AD-MSCs) have also been shown to alter the properties of secreted EVs which restores the renal function after IRI by triggering different energy supply reactions, immunomodulatory, antiapoptotic, anti-oxidative stress and angiogenic responses in kidney tissue, compared with EVs secreted by MSCs in normoxic conditions [23]. Table 1 summarizes the effect of hypoxia preconditioned MSCs in KD disease preclinical models.
Preconditioning with Biological Compounds
Inspite of their hypoimmunogenic responses, MSCs respond with enhanced immunosuppressive abilities in an inflammatory milieu. Pre-activation with inflammatory agents or proinflammatory cytokines is well proven in simulating MSCs with an enhanced immunomodulatory action. In addition to priming of MSCs with different cytokines such as IFNγ, TNF-α, IL-17, IL-1β, TLR, ligands also have the capability to elevate the therapeutic effect of MSCs. Till date, no study has been published in which TNF-α primed MSCs has been used to in ameliorating kidney disease symptoms, however many studies depict that TNF-α preconditioned MSCs enhance the therapeutic ability of MSCs in other diseased conditions [11, 49, 65, 77]. Effect of other factors used for preconditioning MSCs in KD preclinical studies are compiled in Table 2.
Furthermore, combinatorial therapy with few hormones has been shown to influence renoprotection in a remarkable way in various animal models. Hormonal preconditioning of MSCs can provide novel insights into their therapeutic use in kidney diseases and the latest findings in the field are summarised in Table 3.
Cytokines
IL-17A
IL-17A is a potent cytokine and has capability to modulate immune functions of MSCs, without affecting their MHC expression [47, 82, 100]. Preconditioning of BM-MSCs with IL-17A increases the potential of MSCs to ameliorate the AKI in mice. Mechanistically, expansion of Regulatory T cells (Tregs) post IL-17a priming lead to enhanced immunosuppression resulting in improving the renal function. Contrarily, depletion of Tregs resulted in decrease suppression of T cell proliferation, and reverses the therapeutic potential of IL-17-A primed MSCs [9].
IFN-γ
Another preconditioning strategy involves treating MSCs with IFN-γ which is a cytokine secreted by natural killer cells, regulates array of immune responses. In a study, IFNγ pretreated BM-MSCs were administered in Ischemia- reperfusion injury (IRI) and Unilateral ureter obstruction (UUO) KD model of rats via abdominal aorta. It resulted in significant amelioration of renal fibrosis and suppression of immune cell infiltration. IFNγ primed MSCs have the potential to mediate their effect by inhibiting TGFβ smad signalling pathway- the main player for inducing renal fibrosis. Moreover, IFN-γ induces MSCs to release PGE2, which mediates the polarization of M1 macrophages into M2 macrophages. As a result, M2 macrophages enhance the production of anti-inflammatory cytokines attenuating inflammation at the injured site in above mentioned preclinical models [59].
IL-1β
IL-1β has also been shown to enhance the immunomodulation of MSCs. IL-1β conditioned MSCs when administered intravenously in haemorrhagic rat model resulted in lower serum creatinine levels hence improving the kidney function. These prelicensed MSCs potentiate reparative mechanisms and immunomodulation in haemorrhagic shock induced AKI by decreasing the levels of cytokines like IL-6, IL-10 and IL-1α [7].
TLR4
In a preclinical rat model, LPS (TLR-4 agonist) preconditioned AD-MSCs have been shown to reverse the DN pathophysiology including dysregulation in glomerular basement of glomeruli, tubules and mesangial cells [29]. These primed MSCs were effective in lowering blood glucose levels by restoring beta cell activity, as well as regulating inflammatory processes, fibrosis and angiogenesis, podocyte protection and decreasing serum urea levels. In addition, TLR-4 primed MSCs were responsible for attenuating the levels proinflammatory cytokines and down-regulate TGF-β expression involved in renal atrophy mediated by collagen accumulation and apoptosis of endothelial cell. As a result, primed TLR 4 primed MSCs were responsible in promoting kidney tissue regeneration in DN animal model [103].
Hormones
Melatonin
A neurohormone, melatonin is mainly secreted by the pineal gland. It is a fundamental regulator of circadian rhythms that regulate physiological function [90]. In animals, a sleep–wake cycle is ensured by a low blood level of melatonin during the day and an elevated level at night [88].
Melatonin is now believed to play many distinct pathophysiologic roles in addition to its conventional activity. Melatonin was found to be a potent free radical scavenger that has protective benefits in numerous malfunctioning organs, including the kidneys [58, 89]. Since all four key factors—inflammation, oxidative stress, thermal injury, and hypoxia—are the reason for the malfunction of implanted MSCs under disease settings, preconditioning with melatonin may be an effective tactic [94].
Animal models of myocardial infarction, cerebral ischemia, and limb ischemia all have shown that pre-transplant melatonin incubation of MSCs may improve therapeutic outcomes [45, 66]. It was considered, in general, that melatonin may adequately act as an antioxidant and shield MSCs from oxidative damage by physiologically reducing free radicals [41].
Melatonin receptors MT1 and MT2, which have also been discovered to be extensively expressed on the surface of MSCs, may possibly govern the fate of MSCs in a receptor-dependent manner in addition to receptor-independent mechanisms [30, 101].
Saberi et al. [94] transplanted MSCs into UUO rats after pre-treating them with melatonin. They observed that pre-treated MSCs could, in comparison to the control group, decrease the expression of TGF‐β1, TNF-α, and α-smooth muscle actin (α‐SMA) while increasing the expression of E-cadherin. As a result reduction in fibrosis of renal tissues were observed. Additionally, the prelicensed MSCs were found in the damaged kidneys post engraftment, pointing towards improved MSC migration and survival owing to melatonin incubation [94].
A noticeable subtype of acute kidney injury with a significant risk of death and morbidity is sepsis-induced AKI [15]. In a series of evaluations, Chen et al. [20, 21] compared melatonin and apoptotic adipose-derived MSCs (A-ADMSCs) with A-ADMSCs alone in a rat model of sepsis to determine whether the combination of both might have additional benefits in reducing sepsis-AKI. The combination treatment after caecal ligation and puncture (CLP) showed higher therapeutic results in terms of anti-inflammation, antioxidation, anti-apoptosis, anti-fibrosis activity, and the level of creatinine in the blood. Furthermore, haematoxylin and eosin (HE) staining revealed decreased kidney damage in the group receiving combination therapy [20, 21].
The effects of melatonin on ex vivo ADMSCs have also been assessed. Melatonin pretreatment substantially enhanced the proliferation of ADMSCs. Additionally, higher expression of P-Erk1/2, P-Akt, superoxide dismutase 1 (SOD-1), and heme oxygenase 1 (HO-1) was found, showing that AMSCs had improved survival, antiapoptotic, and antioxidative capability after being exposed to melatonin. Melatonin-pretreated AMSCs' conditioned media was also superior in promoting the proliferative, migratory, prosurvival, and antiapoptotic properties of human kidney epithelial cells (HK2) cells exposed to cisplatin [115]. Administration of melatonin-preconditioned BMMSCs to rats with IRI-AKI showed the protective mechanisms of the MSCs revealing that melatonin may have a role in guarding transplanted MSCs in the in vivo milieu. An in vitro examination showed that the preconditioning group had greater levels of catalase and SOD1 expression as well as overexpression of basic fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF). The melatonin receptor antagonist luzindole, however, abolished all of these positive effects, indicating that melatonin can increase the inbuilt prosurvival and paracrine properties of MSCs via their receptors [74].
When compared to the untreated DN animal model, the melatonin licensed MSCs treatment dramatically enhanced renal functioning while also ameliorating the underlying DN pathogenesis through elevating Beclin-1 protein levels [87].
The pretreatment of MSCs with melatonin has also been shown to inhibit ROS-dependent, AMPK pathway-mediated autophagy and prevented p-cresol-induced senescence of Human adipose tissue-derived MSCs by activating the PI3K and Akt pathways [112]. Likewise, melatonin and pioglitazone mixture conditioning was helpful in preventing the MSCs from enduring cell senescence induced by the uremic toxin IS. Co-treatment with melatonin and pioglitazone protected MSCs from uremic toxin-induced senescence through the regulation of the PPAR-γ-PrPC axis as compared to treatment with either melatonin or pioglitazone alone [48].
Erythropoietin
The proliferation and differentiation of hematopoietic stem cells (HSCs) in the bone marrow are stimulated by the glycoprotein hormone EPO through EPO receptor (EpoR) signalling. Both BMSCs and bone marrow HSCs express EpoR that are homologous to each other. Its evident that numerous disorders, including cerebral ischemia, myocardial infarction, chronic congestive heart failure, and renal damage, can be effectively treated with EPO mainly due to their anti-inflammatory and anti-oxidant properties [56, 110]. The in vitro pretreatment with EPO has substantial advantages in terms of clinical viability when compared to other pretreatments or methods that enhance the therapeutic efficacy of MSCs. For these effects, EPO is a prescribed widely with few adverse effects.
A pretreatment of BM MSCs with 500 IU/ml erythropoietin (EPO) for 48 hours before infusion significantly improved their capacity for homing and healing. This effect was mediated through improved dispersion of BMSCs in the target organs while decreased entrapment in the lungs. The apoptosis brought on by the toxicity of cyclosporine A (CsA) in HK2 cells was dramatically reduced by these BMSCs. Additionally, the EPO-BMSCs single infusion treatment dramatically restored the renal function in rats with chronic toxic renal injury brought on by CsA and encouraged the healing of renal fibrosis [119].
In an another study where IRI-AKI kidney homogenate supernatant (KHS) exposure led BMSCs to undergo apoptosis, was inhibited by pretreatment with EPO, demonstrating that EPO protected the cells against apoptosis. EPO was also shown to upregulate the expression of p53, Bcl-2, and silent information regulator 1 (SIRT1). The rats infused with EPO-BMSCs showed significantly greater levels of IL-10 and lower levels of serum IL-1β and TNF-α that led to decreased serum creatinine, blood urea nitrogen, and pathological scores in IRI-AKI rats [118, 120].
Relaxin
In order to mediate the adaptive hemodynamic changes during pregnancy (improving cardiac output, renal blood flow, and arterial compliance), relaxin, an endogenous 6kDa peptide hormone, is primarily produced by the corpus luteum (ovary) during pregnancy. It is also produced in lesser amounts by the brain, heart, and kidney [13, 14, 24, 96, 97].
Various studies have analysed the combined effect of MSCs with synthetic relaxin [8, 53, 68, 69].This combinatorial therapy has been shown to prevent fibrosis progression in normotensive mice subjected to unilateral ureteric obstruction (UUO)-induced tubulointerstitial disease [53].
Badawi et al. [8] have identified a novel combined effect therapy for ESKD patients with synthetic relaxin (Serelaxin) and BM-MSCs which restored and enhanced the endothelial progenitor cells (EPCs) population [8]. BM-MSCs alone have been shown to attenuate BP to a similar degree as perindopril and decreased injury-induced fibrosis. Serelaxin alone significantly reduced tubular injury and modestly decreased renal fibrosis. Surprisingly, in comparison to either therapy alone and to the effects of perindopril, the combined effects of BM-MSCs (at both doses) and serelaxin dramatically reduced renal fibrosis and proximal tubular epithelial damage while restoring renal architecture [68, 69].
Preconditioning with Pharmacological Drugs
Calycosin
Calycosin (CA), an isoflavone, is a naturally occurring phytoestrogen. It is isolated from Astragalus membranaceus with kidney tonifying effect. It has a wide range of pharmacological activities such as anti-inflammatory, anti-cancer, anti-oxidant, pro-angiogenic properties [28]. Hu et al. [50], pretreated BM-MSCs with CA in mouse model of UUO (unilateral ureteral obstruction). It was demonstrated that pretreated MSCs could reduce renal fibrosis and prevent necroptosis in UUO mice, Additionally, by blocking the TGF-β1/TNF-α/TNFR1/Necroptosis pathway, CA pretreated MSCs improved the antifibrotic effects [50].
Chlorzoxane
For painful musculoskeletal diseases with central function, chlorzoxazone (5-chloro-3H-benzooxazol-2-one) is administered as a powerful muscle relaxant. The medication primarily affects the spinal cord and subcortical regions, suppressing multisynaptic reflex arcs that are involved in the development and maintenance of skeletal muscle spasms from a variety of sources. Spasms and pain related to musculoskeletal illnesses can be relieved by lowering muscle tone and tension through administering this drug [2]. Additionally, it appears in humans as a marker for hepatic cytochrome P4502E1 [80]. Deng et al. [28] demonstrated that Chlorzoxane (CZ) pretreated UC-MSCs can more effectively reduce inflammatory infiltration and tissue damage in the acute kidney injury rat model by inhibiting T cells activation and proliferative growth, enhancing the production of IDO and other immune mediators in vitro, and all of these effects in combination. It was found that CZ alters the phosphorylation of the transcriptional factor forkhead box O3 (FOXO3) without using the traditional AKT or ERK signalling pathways, promoting expression of downstream immune-related genes and enhancing the immunosuppressive potential of MSCs [28].
Metformin
A first-line treatment for type 2 diabetes is metformin [26]. Metformin is known to have pleiotropic effects in addition to its anti-diabetic effects, which include favorable effects on the kidney and cardiovascular systems as well as potentially lowering cancer risk [37]. Although many studies [19, 64, 75, 78, 98] have emphasised the renal protective effects of metformin, the risk of lactic acidosis makes it difficult to use metformin in patients with severe kidney dysfunction and absolutely contraindicated in those with an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 [26].
AD-MSCs from stage-5 CKD patients primed with metformin attenuated the senescence of CKD-MSCs. Moreover, metformin-treated AD-MSCs effectively reduced inflammation and fibrosis in UUO-CKD mice model concluding that treated CKD-MSCs can be used to develop patient-derived, autologous MSC-based therapeutics for CKD patients by optimising cell functionality [61].
Atorvastatin
Statins have been proven to have biological effects other than cholesterol reduction, including anti-apoptotic, antioxidant effect and anti-inflammatory responses. It has been shown that statins are responsible for improving microenvironment for MSCs and preventing them from apoptosis [85]. Cai et al. [17] strategize to provide combined therapy against IRI in rat model. 3 day prior pretreatment with statins before the administration of MSCs via left carotid artery immediately after IRI injury may be effective in improving the renal microenvironment. The resultant findings indicated that this combinatorial therapy downregulated the expression of oxidative stress and inflammation markers in the injured kidney, specifically MDA, MPO, IL-1β and TNF-α, while upregulating the expression of IL- 10 [17].
Darbepoetin-α
EPO receptors are shown to be present on the surfaces of tubular epithelial and mesangial cells. Animal studies indicate that EPO can act on these cells to protect the kidneys from acute damage. This protection appears to be linked to EPO's anti-apoptotic, antioxidant, and anti-inflammatory capabilities, as well as its proangiogenic potential [57]. Co-administration of Darbepoetin-α (DPO) had a greater renoprotective function. Histological studies also proved that MSCs and DPO have role in reducing tissue injury and serum levels of creatinine were significantly decreased. Hence, combination of DPO and MSCs can be explored further to treat AKI [6].
Vitamin E
Vitamin E is a fat-soluble vitamin with antioxidant properties. Its beneficial in the treatment line of AKI by decreasing oxygen-free radicals in renal tissue and preventing cell death [4, 73, 95]. Therapeutic effect of BM-MSCs and Vit E in the gentamicin-induced AKI model has been shown to lower the serum creatinine, reduce necrosis/apoptosis, and an overall improvement in renal pathological abnormalities [73]. Another study demonstrated the immunomodulatory effect of UC-MSCs and Vit E in AKI model. This combinatorial therapy lessened the damage in renal tissue of AKI mice and had a more favourable therapeutic outcome than using either UC-MSC or Vit E alone [44].
Recent studies reporting the usage of pharmacological drugs preconditioned MSCs in KD preclinical model are cited in Table 4.
3D Culture
Two-dimensional (2D) systems are the foundation of traditional method for MSC culture. However, 2D environment frequently leads to senescence and loss of cell functions. Whereas, a 3D microenvironment fills the gap between the conventional culture system and provides an intricate in vivo architecture in overcome these shortcomings. As a result of their greater ability to promote the survival and function of the transplanted cells, 3D techniques have also become a frontier in the delivery of stem cells [117]. These methods can be broadly divided into two categories: scaffold-based (hydrogel-focused) and scaffold-free (spheroid culture) techniques.
Hydrogel
One of the most promising candidates for 3D stem cell delivery and culture has been thought to be hydrogels. High water content and customizable mechanical, physical, and chemical properties define hydrogels, which are chemically or physically cross-linked within three-dimensional porous polymeric networks. The hydrogels' high moisture content and porous design enable the transportation of nutrients and metabolites in the networks [10, 109]. In this way, hydrogels can function as a synthetic extracellular matrix (ECM) that surrounds the cells, creating the ideal environment for cell-cell and cell-matrix interactions, which in turn affect MSC behaviour and functions [106]. So far, a wide range of hydrogels of natural and synthetic origins have been studied to imitate the native milieu in vivo [106]. Numerous preclinical KD models have shown the importance of hydrogels as scaffolds for promoting cell development and function.
A novel therapeutic approach for Rhabdomyolysis-Induced Acute Kidney Injury was developed using a hydrogel rich in mesenchymal stem cells and a programmable artificial kidney capsule (AKC). In particular, an elastic capsule with an interior chamber the same size and form as the kidney was created using 3D modelling and printing and used as the outer covering for kidney- and cell-filled hydrogels. Such a model offered a practical and adaptable technique for the repair and recovery of other organs in addition to offering a viable option for the treatment of AKI [39].
In an another interesting study, the bioactive motif Insulin like growth factor-1 (IGF-1C) and the D-form assembly motif Nap-DFDFG were combined with human placenta derived Mesenchymal stem cells (hp-MSCs) to develop a de novo β sheet supra-molecular self-assembling peptide hydrogel for AKI treatment. The results demonstrated the enhancement of cell engraftment; recovery of renal function; facilitation of angiogenesis; decrease in renal fibrosis in IRI induced AKI mice model [105].
A decellularized vascular matrix and collagen co-gel was tested to see if it enhanced the therapeutic potential of MSCs for AKI. The results depicted the survival and paracrine effects of MSCs in the injured kidney were improved when the co-gel and MSCs were co-transplanted into the kidney of model rats with IRI- AKI. More crucially, the co-gel enhanced the therapeutic effects of MSCs for AKI as demonstrated by reduced cell death & tissue damage, improved vascularization and renal function [51].
Duragen has also been used as a biological membrane to encapsulate MSCs on glycerol-injured renal tissue in an in vivo setting to avoid the escape of MSCs and enable their prolonged survival within the body. The encapsulation of MSCs using a biological membrane reduced kidney damage and cell death brought on by rhabdomyolysis [43].
Feng et al. immobilised the IGF-1 C domain peptide (IGF-1C) on chitosan to create a bioactive hydrogel. CS-IGF-1C hydrogel could shield the supplied ADSCs and further encourage the functional and structural recovery of the kidney when implanted into an ischemic kidney. In addition to giving ADSCs a synthetic niche, covalent immobilisation of IGF-1C peptide to CS hydrogel promoted the cell matrix anchoring and controlled cell proliferation and inflammation in kidney regeneration following AKI [35].
Through physical crosslinking, an injectable NO-releasing hydrogel based on short aromatic dipeptides was recently created. The Fmoc-diphenylalanine S-nitroso-N-acetyl penicillamine hydrogel (Fmoc-FFSNAP) could more effectively mediate the regeneration of renal IRI injuries and improve renal function due to its localized and sustained release of NO. Recently, the effectiveness of therapy of Fmoc-FF+Fmoc-RGD-SNAP-MSCs in the renal IRI injury model was assessed in relation to free MSCs and Fmoc-FF+Fmoc-RGD-SNAP-MSCs. WJ-MSCs were used in combination with hydrogel as mentioned above and administered to IRI mice via intra-renal parenchymal injection at three different sites. As a result, kidney functional biomarkers, reactive oxygen production, histopathological changes and each parameter revealed positive results which signify, recovery of injured kidney and endothelial regeneration as compared with free MSCs [76]. In another study, in order to enhance the retention of AD-MSCs after transplantation, cells were embedded on kidney extracellular matrix hydrogel (EMCH). These embedded MSCs were injected into ischemic SD rats and it substantially elevated the retention and survival of cells at transplanted site. Also, ECM promote secretion of growth factors, decrease oxidative stress and apoptosis. Hence, has the potential to treat kidney injury [118, 120]. Yet, another study proves that administration of MSCs through injectable hydrogels enhances the therapeutic efficacy of MSCs. Unilateral delivery of MSCs via hyaluronic acid hydrogel below the kidney capsule in mice showed significant reduction in kidney studies. Further, proteomics studies also revealed the decrease expression of inflammatory and fibrosis markers [46]. Above mentioned studies provide a substantial evidence that injectable MSCs based hydrogel have the potential to mitigate the symptoms kidney injury.
Spheroid Culture
Another potential technique for 3D cell culture is spheroid culture. Without the use of a scaffold that mimics actual tissues, it is possible to create 3D aggregations of MSCs and their secreted ECM using this technique. Spheroid production methods include hanging drop, liquid overlay, spinner culture, pellet culture, and magnetic levitation, among others [36, 93]. Several studies have shown the therapeutic potential of spheroid MSCs application in kidney disease.
In order to treat acute kidney injury (AKI), Xu et al. [107] studied the curative properties of 3D spheroids of hAD-MSCs. Their set up revealed that 3D spheroids of MSCs produced more extracellular matrix proteins, such as collagen I, fibronectin, and laminin, and had stronger anti-apoptotic and anti-oxidative properties than 2D cultivated cells [107].
Furthermore, the paracrine secretion of cytokines by MSCs was also shown to be increased by 3D spheroid culture, including angiogenic factors (VEGF and basic fibroblast growth factor), anti-apoptotic factors (epidermal growth factor and hepatocyte growth factor), anti-oxidative factors (insulin-like growth factor) and anti-inflammatory proteins (tumor necrosis factor-alpha stimulated gene/protein 6). MSCs derived from 3D spheroids displayed improved survival and paracrine effects in vivo, which was consistent with in vitro findings. Additionally, 3D spheroids were superior to 2D cultured cells at protecting the IRI kidney against apoptosis, reducing tissue damage, promoting vascularization, and improving renal function when injected into the kidney of model rats with IRI- induced AKI [107].
MSCs, vascular endothelial cells, and podocytes were combined to create hybrid 3D cell spheroids that mimicked the glomerular milieu. Mice with hypertensive nephropathy had improved kidney function after receiving these hybrid 3D cell spheroids transplanted into their renal cortex. This suggests that podocyte transplantation using the newly developed hybrid 3D cell spheroids may hold great potential to further improve the effectiveness of podocyte replenishment and subsequent therapeutic function [108]. 3D cultured-MSCs in KD model are summarized in Table 5.
Discussion
The degree to which MSCs contribute clinically to KD is largely influenced by their capacity for paracrine secretion, survival and migration. However, there's still a long way to go before MSCs are used clinically, despite encouraging results in preclinical models. The heterogenous responsiveness is dependent upon various parameters like experimental models, dose-timings of infusion, intrinsic immune environment of the host. The relatively low quantity of transplanted MSCs to the kidneys following systemic delivery could explain the limited efficacy of MSC treatment in human AKI. The harsh in vivo milieu i.e. ischemia, inflammation, oxidative stress, and mechanical stress results in a low survival rate of administered MSCs. Therefore, it is challenging to assess the settings leading to the complete therapeutic competence of MSC-based therapies.
For the successful translation of MSCs applications from preclinical research to clinical trials, a full proof standard protocol using MSCs for KD therapy must be established. Different preconditioning techniques unquestionably increase MSCs survival and paracrine capacity, enhancing their positive effects. To date, there is a growing evidence that preconditioned MSCs indeed exhibit better therapeutic benefits than naive MSCs in a KD setting as documented in several studies cited above. Priming the MSCs with various agents especially the inflammatory cytokines or hypoxic agents prepares them to perform better in harsh environments, likewise it complements with various hormones, drugs etc which standalone are not sufficient for alleviating the disease but in a combinatorial way, the performance of both the MSCs and other factors increases markedly.
Inspite of these , it is challenging to establish which preconditioning technique is the most effective due to the significant variation in MSCs therapy regimens cited in literature. Inspite of the potential that preconditioned MSCs evidently possess, a few problems must be resolved before it can be successfully implemented in clinical settings. Some preconditioning techniques call for greater caution than others. For e.g., preconditioning with pharmacological/chemical agents or trophic factors/cytokines theoretically raises concerns about mode of action of these factors in a synergistic way. Every combination or preconditioning therapy must have a proof of concept study in preclinical models. Point of emphasis is to weigh the benefits and risks thoroughly. Furthermore,there are not enough studies comparing various preconditioning approaches thereby making it challenging to establish which preconditioning technique is the most effective. Different preconditioning techniques may be required depending on variable diseased niches that they are proposed to be transplanted into. Increased adoption of the preconditioning technique in KD and avoidance of potential adverse effects may be greatly aided by a greater knowledge of the molecular and cellular mechanisms underlying it. The best preconditioning technique for KD therapy is one that not only improves MSCs survival, paracrine function, and migration but poses no negative effects. With the development of new regimens for licensing MSCs, we expect to overcome the current barriers in therapeutic usage of MSCs.
To conclude, its pertinent to mention that new innovations combined with large scale futuristic clinical trials, disease specific licencing of MSCs can prove to be a major technology as treatment option for kidney diseases. On the basis of available literature and compilation of research as above, evidence-based approaches can be formulated and explored in the treatment of patients.
Summary
Stem cells therapy for kidney disease is a potential therapeutic strategy but it comes with various blind spots/loopholes when thought about in for clinical applications. We look forward to overcome existing hinderances and elevate the clinical use of MSCs in different kidney diseases by preconditioning the mesenchymal stem cells. Priming of MSCs apparently would elevate their therapeutic performance and renoprotective mechanism. Furthermore, combination of two or more preconditioning regimens may work in a synergistic way and aiding in ameliorating kidney disease. Nevertheless, a broader understanding of the mechanism of action of these preconditioning regimens and deciphering their precise signaling pathways will aid bench to bedside translation.
Data Availability
No new data were generated or analyzed in support of this research.
Abbreviations
- KD:
-
Kidney disease
- AKI:
-
Acute Kidney Disease
- CKD:
-
Chronic Kidney Disease
- CsA:
-
Cyclosporine A
- CA:
-
Calycosin
- DPO:
-
Darbepoetin-α
- DKD:
-
Diabetic kidney disease
- DM:
-
Diabetes mellitus
- DN:
-
Diabetic nephropathy
- ESRD:
-
End Stage Renal Disease
- EPO:
-
Erythropoietin
- EpoR:
-
EPO receptor
- EPCs:
-
Endothelial progenitor cells
- FGF:
-
Fibroblast Growth Factor
- FOXO3:
-
Forkhead box O3
- GFR:
-
Glomerular Filtration Rate
- HGF:
-
Hepatocyte Growth Factor
- HSC:
-
Hematopoietic stem cells
- HE:
-
Haematoxylin and eosin
- HIF:
-
Hypoxia-inducible factor
- IGF-1:
-
Insulin like growth factor-1
- IRI:
-
Ischemia/reperfusion induced
- EVs:
-
Extracellular vesicles
- BM-MSCs:
-
Bone Marrow derived MSCs
- MSC:
-
Mesenchymal Stem Cell
- MV:
-
Microvesicles
- SOD-1:
-
Superoxide dismutase 1
- HO-1:
-
Heme oxygenase 1
- KHS:
-
Kidney homogenate supernatant
- MSCs:
-
Mesenchymal stem cells;
- A-ADMSCs:
-
Apoptotic adipose-derived MSCs
- bFGF:
-
Basic fibroblast growth factor
- UUO:
-
Unilateral ureteral obstruction
- hBM-MSCs:
-
Human bone marrow mesenchymal stem cells
- SOD-1:
-
Superoxide dismutase 1
- CPT1A:
-
Carnitine palmitoyl-transferase 1A
- ECM:
-
Extracellular matrix
- MMP12:
-
Matrix metalloproteinase 12
- UUO:
-
Unilateral ureteric obstruction
- SIRT1:
-
Silent information regulator 1
- TNF- α:
-
Tumour Necrosis Factor- alpha
- VEGF:
-
Vascular Endothelial Growth Factor
- IGF-1C:
-
C domain of insulin-like growth factor-1
- AKC:
-
Artificial kidney capsule
- ECM:
-
Extracellular matrix (ECM)
- hP-MSCs:
-
Human placenta-derived mesenchymal stem cells
- TGF‐β:
-
Transforming growth factor‐β
- RTECs':
-
Renal Tubular Epithelial Cells
- ROS:
-
Reactive Oxygen Species
- PDGF:
-
Platelet Derived Growth Factor
- Fmoc-FFSNAP:
-
Fmoc-diphenylalanine S-nitroso-N-acetyl penicillamine hydrogel
- ADSCs:
-
Adipose Derived Stem Cell
- UC MSCs:
-
Umbilical derived Mesenchymal Stem Cells
- CPT1A:
-
Carnitine palmitoyl-transferase 1A
- α‐SMA:
-
α-Smooth muscle actin
- eGFR:
-
Estimated glomerular filtration rate
- EMCH:
-
Extracellular matrix hydrogel
- CLP:
-
Caecal ligation and puncture
- WJ-MSCs :
-
Wharton Jelly Derived Mesenchymal Stem Cells
- hAD-MSCs:
-
Human Adipose Derived Mesenchymal Stem Cell
- hMSCs:
-
Human Mesenchymal Stem Cells
References
Ahmadi, A., Rad, N. K., & Vahid Ezzatizadeh, R. M. (2020). Kidney regeneration: Stem cells as a new trend. Current Stem Cell Research & Therapy, 15(3), 263–283. https://doi.org/10.2174/1574888X15666191218094513
Abbar, J. C., & Nandibewoor, S. T. (2012). Development of electrochemical method for the determination of chlorzoxazone drug and its analytical applications to pharmaceutical dosage form and human biological fluids. 51(1), 111–118. https://doi.org/10.1021/ie2021812
Aghajani Nargesi, A., Lerman, L. O., & Eirin, A. (2017). Mesenchymal stem cell-derived extracellular vesicles for kidney repair: Current status and looming challenges. Stem Cell Research and Therapy, 8(1), 1–12. https://doi.org/10.1186/s13287-017-0727-7
Ajith, T. A., Abhishek, G., Roshny, D., & Sudheesh, N. P. (2009). Co-supplementation of single and multi doses of vitamins C and E ameliorates cisplatin-induced acute renal failure in mice. Experimental and Toxicologic Pathology, 61(6), 565–571. https://doi.org/10.1016/j.etp.2008.12.002
Alicic, R. Z., Rooney, M. T., & Tuttle, K. R. (2017). Diabetic kidney disease: Challenges, progress, and possibilities. Clinical Journal of the American Society of Nephrology, 12(12), 2032–2045. https://doi.org/10.2215/CJN.11491116
Altun, B., Yilmaz, R., Aki, T., Akoglu, H., Zeybek, D., Piskinpasa, S., Uckan, D., Purali, N., Korkusuz, P., & Turgan, C. (2012). Use of mesenchymal stem cells and darbepoetin improve ischemia-induced acute kidney injury outcomes. American Journal of Nephrology, 35(6), 531–539. https://doi.org/10.1159/000339167
Aussel, C., Baudry, N., Grosbot, M., Caron, C., Vicaut, E., Banzet, S., & Peltzer, J. (2021). IL-1β primed mesenchymal stromal cells moderate hemorrhagic shock-induced organ injuries. Stem Cell Research and Therapy, 12(1), 1–16. https://doi.org/10.1186/s13287-021-02505-4
Badawi, A., Jefferson, O. C., Huuskes, B. M., Ricardo, S. D., Kerr, P. G., Samuel, C. S., & Murthi, P. (2022). A novel approach to enhance the regenerative potential of circulating endothelial progenitor cells in patients with end-stage kidney disease. Biomedicines, 10(4), 883. https://doi.org/10.3390/biomedicines10040883
Bai, M., Zhang, L., Fu, B., Bai, J., Zhang, Y., Cai, G., Bai, X., Feng, Z., Sun, S., & Chen, X. (2018). IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney International, 93(4), 814–825. https://doi.org/10.1016/j.kint.2017.08.030
Bai, R., Yang, J., & Suo, Z. (2019). Fatigue of hydrogels. European Journal of Mechanics, A/Solids, 74, 337–370. https://doi.org/10.1016/j.euromechsol.2018.12.001
Bai, X., Xi, J., Bi, Y., Zhao, X., Bing, W., Meng, X., Liu, Y., Zhu, Z., & Song, G. (2017). TNF-α promotes survival and migration of MSCs under oxidative stress via NF-κB pathway to attenuate intimal hyperplasia in vein grafts. Journal of Cellular and Molecular Medicine, 21(9), 2077–2091. https://doi.org/10.1111/jcmm.13131
Bastani, B. (2020). The present and future of transplant organ shortage: Some potential remedies. Journal of Nephrology, 33(2), 277–288. https://doi.org/10.1007/s40620-019-00634-x
Bathgate, R. A. D., Hsueh, A. J. W., & Sherwood, O. D. (2006). Physiology and molecular biology of the relaxin peptide family. In Knobil and Neill’s Physiology of Reproduction (Third Edit). Elsevier Inc. https://doi.org/10.1016/B978-0-12-515400-0.50021-X
Bathgate, R. A., Ivell, R., Sanborn, B. M., Sherwood, O. D., & Summers, R. J. (2006). International union of pharmacology LVII : Recommendations for the nomenclature of receptors for relaxin family peptides. 58(1), 7–31. https://doi.org/10.1124/pr.58.1.9.7
Brun-buisson, C., & Mondor, C. H. U. H. (2004). The EPISEPSIS Study Group EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Medicine, 30(4), 580–588. https://doi.org/10.1007/s00134-003-2121-4
Bruno, S., Chiabotto, G., & Camussi, G. (2014). Concise review: Different mesenchymal stromal/stem cell populations reside in the adult kidney. Stem Cells Translational Medicine, 3(12), 1451–1455. https://doi.org/10.5966/sctm.2014-0142
Cai, J., Yu, X., Zhang, B., Zhang, H., Fang, Y., Liu, S., Liu, T., & Ding, X. (2014). Atorvastatin improves survival of implanted stem cells in a rat model of renal ischemia-reperfusion injury. American Journal of Nephrology, 39(6), 466–475. https://doi.org/10.1159/000362623
Câmara, N. O. S., Iseki, K., Kramer, H., Liu, Z. H., & Sharma, K. (2017). Kidney disease and obesity: Epidemiology, mechanisms and treatment. Nature Reviews Nephrology, 13(3), 181–190. https://doi.org/10.1038/nrneph.2016.191
Cavaglieri, R. C., Day, R. T., Feliers, D., & Abboud, H. E. (2015). Molecular and cellular endocrinology metformin prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. Molecular and Cellular Endocrinology, 412, 116–122. https://doi.org/10.1016/j.mce.2015.06.006
Chen, H. H., Lin, K. C., Wallace, C. G., Chen, Y. T., Yang, C. C., Leu, S., Chen, Y. C., Sun, C. K., Tsai, T. H., Chen, Y. L., Chung, S. Y., Chang, C. L., & Yip, H. K. (2014). Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. Journal of Pineal Research, 57(1), 16–32. https://doi.org/10.1111/jpi.12140
Chen, H., Lin, K., Wallace, C. G., Yang, C., Chen, Y., Sun, K., & Tsai, T. (2014). Additional bene fi t of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. 1–17. https://doi.org/10.1111/jpi.12140
Cipolleschi, M. G., Dello Sbarba, P., & Olivotto, M. (1993). The role of hypoxia in the maintenance of hematopoietic stem cells. Blood, 82(7), 2031–2037. https://doi.org/10.1182/blood.V82.7.2031.2031
Collino, F., Lopes, J. A., Corrêa, S., Abdelhay, E., Takiya, C. M., Wendt, C. H. C., De Miranda, K. R., Vieyra, A., & Lindoso, R. S. (2019). Adipose-derived mesenchymal stromal cells under hypoxia: Changes in extracellular vesicles secretion and improvement of renal recovery after ischemic injury. Cellular Physiology and Biochemistry, 52(6), 1463–1483. https://doi.org/10.33594/000000102
Conard, K. P., von Versen- Hoynck, F., & Baker, V. L. (2022). Potential role of the corpus luteum in maternal cardiovascular adaptation to pregnancy and preeclampsia risk. The American Journal of Obstetrics & Gynecology, 226(5), 683–699. https://doi.org/10.1016/j.ajog.2021.08.018
Das, R., Jahr, H., Van Osch, G. J. V. M., & Farrell, E. (2010). The role of hypoxia in bone marrow – derived mesenchymal stem cells: Considerations. Tissue Engineering. Part B, Reviews, 16(2), 159–168. https://doi.org/10.1089/ten.TEB.2009.0296
Davies, M. J., Alessio, D. A. D., Fradkin, J., Kernan, W. N., Mathieu, C., & Mingrone, G. (2018). Management of Hyperglycemia in Type 2 Diabetes , 2018 . A Consensus Report by the American Diabetes Association ( ADA ) and the European Association for the Study of Diabetes ( EASD ). Diabetes Care, 41(December), 2669–2701. https://doi.org/10.2337/dci18-0033
de Almeida, D. C., Donizetti-Oliveira, C., Barbosa-Costa, P., Origassa, C. S., & Câmara, N. O. (2013). In search of mechanisms associated with mesenchymal stem cell-based therapies for acute kidney injury. The Clinical Biochemist. Reviews, 34(3), 131–144.
Deng, L., Li, H., Su, X., Zhang, Y., Xu, H., Fan, L., Fan, J., Han, Q., Bai, X., & Zhao, R. C. (2020). Chlorzoxazone, a small molecule drug, augments immunosuppressive capacity of mesenchymal stem cells via modulation of FOXO3 phosphorylation. Cell Death and Disease, 11(3). https://doi.org/10.1038/s41419-020-2357-8
Donate-Correa, J., Luis-Rodríguez, D., Martín-Núñez, E., Tagua, V. G., Hernández-Carballo, C., Ferri, C., Rodríguez-Rodríguez, A. E., Mora-Fernández, C., & Navarro-González, J. F. (2020). Inflammatory targets in diabetic nephropathy. Journal of Clinical Medicine, 9(2), 458. https://doi.org/10.3390/jcm9020458
Dubocovich, M. L., & Markowska, M. (2005). Functional MT 1 and MT 2 melatonin receptors in mammals. Endocrine, 27(2), 101–110.
English, P. B. (1974). Acute renal failure in the dog and cat. Australian Veterinary Journal, 50(9), 384–392. https://doi.org/10.1111/j.1751-0813.1974.tb05343.x
Fabrizi, F., Cerutti, R., & Ridruejo, E. (2019). Hepatitis B virus infection as a risk factor for chronic kidney disease. Expert Review of Clinical Pharmacology, 12(9), 867–874. https://doi.org/10.1080/17512433.2019.1657828
Fadini, G. P., Ciciliot, S., & Albiero, M. (2017). Concise review: Perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells, 35(1), 106–116. https://doi.org/10.1002/stem.2445
Fan, M., Zhang, J., Xin, H., He, X., & Zhang, X. (2018). Current perspectives on role of MSC in renal pathophysiology. Frontiers in Physiology, 9(SEP), 1–8. https://doi.org/10.3389/fphys.2018.01323
Feng, G., Zhang, J., Li, Y., Nie, Y., Zhu, D., Wang, R., Liu, J., Gao, J., Liu, N., He, N., Du, W., Tao, H., Che, Y., Xu, Y., Kong, D., Zhao, Q., & Li, Z. (2016). IGF-1 C domain-modified hydrogel enhances cell therapy for AKI. Journal of the American Society of Nephrology, 27(8), 2357–2369. https://doi.org/10.1681/ASN.2015050578
Fennema, E., Rivron, N., Rouwkema, J., van Blitterswijk, C., & De Boer, J. (2013). Spheroid culture as a tool for creating 3D complex tissues. Trends in Biotechnology, 31(2), 108–115. https://doi.org/10.1016/j.tibtech.2012.12.003
Foretz, M., Guigas, B., Bertrand, L., Pollak, M., & Viollet, B. (2014). Review metformin: From mechanisms of action to therapies. Cell Metabolism, 20(6), 953–966. https://doi.org/10.1016/j.cmet.2014.09.018
Freyman, T., Polin, G., Osman, H., Crary, J., Lu, M. M., Cheng, L., Palasis, M., & Wilensky, R. L. (2006). A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. European Heart Journal, 27(9), 1114–1122. https://doi.org/10.1093/eurheartj/ehi818
Fu, Z., Chu, Y., Geng, X., Ma, Y., Chi, K., Song, C., Liao, S., Hong, Q., Wu, D., & Wang, Y. (2022). Artificial kidney capsule packed with mesenchymal stem cell-laden hydrogel for the treatment of rhabdomyolysis-induced acute kidney injury. ACS Biomaterials Science & Engineering. https://doi.org/10.1021/acsbiomaterials.1c01595
Fu, H., Liu, S., Bastacky, S. I., Wang, X., Tian, X. J., & Zhou, D. (2019). Diabetic kidney diseases revisited: A new perspective for a new era. Molecular Metabolism, 30(October), 250–263. https://doi.org/10.1016/j.molmet.2019.10.005
Galano, A., Tan, D. X., & Reiter, R. J. (2011). Melatonin as a natural ally against oxidative stress: A physicochemical examination. Journal of Pineal Research, 51(1), 1–16. https://doi.org/10.1111/j.1600-079X.2011.00916.x
Gao, Z., Zhang, C., Peng, F., Chen, Q., Zhao, Y., Chen, L., Wang, X., & Chen, X. (2022). Hypoxic mesenchymal stem cell-derived extracellular vesicles ameliorate renal fibrosis after ischemia–reperfusion injure by restoring CPT1A mediated fatty acid oxidation. Stem Cell Research and Therapy, 13(1), 1–15. https://doi.org/10.1186/s13287-022-02861-9
Geng, X., Hong, Q., Wang, W., Zheng, W., Li, O., Cai, G., Chen, X., & Wu, D. (2017). Biological membrane-packed mesenchymal stem cells treat acute kidney disease by ameliorating mitochondrial-related apoptosis. Scientific Reports, 7(1), 1–12. https://doi.org/10.1038/srep41136
Guo, Q., & Wang, J. (2018). Effect of combination of vitamin E and umbilical cord-derived mesenchymal stem cells on inflammation in mice with acute kidney injury. Immunopharmacology and Immunotoxicology, 40(2), 168–172. https://doi.org/10.1080/08923973.2018.1424898
Han, D., Huang, W., Li, X., Gao, L., Su, T., Li, X., Ma, S., Liu, T., Li, C., Chen, J., Gao, E., & Cao, F. (2016). Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. Journal of Pineal Research, 60(2), 178–192. https://doi.org/10.1111/jpi.12299
Han, D. S., Erickson, C., Hansen, K. C., Kirkbride-Romeo, L., He, Z., Rodell, C. B., & Soranno, D. E. (2023). Mesenchymal stem cells delivered locally to ischemia-reperfused kidneys via injectable hyaluronic acid hydrogels decrease extracellular matrix remodeling 1 month after injury in male mice. Cells, 12(13), 1771. https://doi.org/10.3390/cells12131771
Han, X., Yang, Q., Lin, L., Xu, C., Zheng, C., Chen, X., Han, Y., Li, M., Cao, W., Cao, K., Chen, Q., Xu, G., Zhang, Y., Zhang, J., Schneider, R. J., Qian, Y., Wang, Y., Brewer, G., & Shi, Y. (2014). Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death and Differentiation, 21(11), 1758–1768. https://doi.org/10.1038/cdd.2014.85
Han, Y. S., Kim, S. M., Lee, J. H., & Lee, S. H. (2018). Co-administration of melatonin effectively enhances the therapeutic effects of pioglitazone on mesenchymal stem cells undergoing indoxyl sulfate-induced senescence through modulation of cellular prion protein expression. International Journal of Molecular Sciences, 19(5), 1–15. https://doi.org/10.3390/ijms19051367
Heo, S. C., Jeon, E. S., Lee, I. H., Kim, H. S., Kim, M. B., & Kim, J. H. (2011). Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. Journal of Investigative Dermatology, 131(7), 1559–1567. https://doi.org/10.1038/jid.2011.64
Hu, Q., Zhu, B., Yang, G., Jia, J., Wang, H., Tan, R., Zhang, Q., Wang, L., & Kantawong, F. (2023). Calycosin pretreatment enhanced the therapeutic efficacy of mesenchymal stem cells to alleviate unilateral ureteral obstruction-induced renal fibrosis by inhibiting necroptosis. Journal of Pharmacological Sciences, 151(2), 72–83. https://doi.org/10.1016/j.jphs.2022.12.001
Huang, S., Li, Y., Wang, X., Ma, X., & Zhang, X. (2017). Injectable co-gels of collagen and decellularized vascular matrix improve MSC-based therapy for acute kidney injury. Journal of Biomaterials Science, Polymer Edition, 28(18), 2186–2195. https://doi.org/10.1080/09205063.2017.1388556
Hung, S. C., Pochampally, R. R., Hsu, S. C., Sanchez, C. C., Chen, S. C., Spees, J., & Prockop, D. J. (2007). Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE, 2(5), e416. https://doi.org/10.1371/journal.pone.0000416
Huuskes, B. M., Wise, A. F., Cox, A. J., Lim, E. X., Payne, N. L., Kelly, D. J., Samuel, C. S., & Ricardo, S. D. (2014). Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fi brosis in obstructive nephropathy. 1–14. https://doi.org/10.1096/fj.14-254789
Ishiuchi, N., Nakashima, A., Doi, S., Yoshida, K., Maeda, S., Kanai, R., Yamada, Y., Ike, T., Doi, T., Kato, Y., & Masaki, T. (2020). Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Research and Therapy, 11(1), 1–15. https://doi.org/10.1186/s13287-020-01642-6
Jang, M. J., You, D., Park, J. Y., Kim, K., Aum, J., Lee, C., Song, G., Shin, H. C., Suh, N., Kim, Y. M., & Kim, C. S. (2018). Hypoxic preconditioned mesenchymal stromal cell therapy in a rat model of renal ischemia-reperfusion injury: Development of optimal protocol to potentiate therapeutic efficacy. International Journal of Stem Cells, 11(2), 157–167. https://doi.org/10.15283/ijsc18073
Jun, J. H., Jun, N. H., Shim, J. K., Shin, E. J., & Kwak, Y. L. (2014). Erythropoietin protects myocardium against ischemia – reperfusion injury under moderate hyperglycemia. European Journal of Pharmacology, 745, 1–9. https://doi.org/10.1016/j.ejphar.2014.09.038
Junk, A. K., Mammis, A., Savitz, S. I., Singh, M., Roth, S., Malhotra, S., Rosenbaum, P. S., Cerami, A., Brines, M., & Rosenbaum, D. M. (2002). Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America, 99(16), 10659–10664. https://doi.org/10.1073/pnas.152321399
Kaçmaz, A., User, E. Y., Şehirli, A. Ö., Tilki, M., Ozkan, S., & Şener, G. (2005). Protective effect of melatonin against ischemia/reperfusion-induced oxidative remote organ injury in the rat. Surgery Today, 35(9), 744–750. https://doi.org/10.1007/s00595-005-3027-2
Kanai, R., Nakashima, A., Doi, S., Kimura, T., Yoshida, K., Maeda, S., Ishiuchi, N., Yamada, Y., Ike, T., Doi, T., Kato, Y., & Masaki, T. (2021). Interferon-γ enhances the therapeutic effect of mesenchymal stem cells on experimental renal fibrosis. Scientific Reports, 11(1), 1–14. https://doi.org/10.1038/s41598-020-79664-6
Khubutiya, M. S., Vagabov, A. V., Temnov, A. A., & Sklifas, A. N. (2014). Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models ofacute organ injury. Cytotherapy, 16(5), 579–585. https://doi.org/10.1016/j.jcyt.2013.07.017
Kim, H., Yu, M. R., Lee, H., Kwon, S. H., Jeon, J. S., Han, D. C., & Noh, H. (2021). Metformin inhibits chronic kidney disease-induced DNA damage and senescence of mesenchymal stem cells. Aging Cell, 20(2), 1–12. https://doi.org/10.1111/acel.13317
Kovesdy, C. P., Furth, S. L., & Zoccali, C. (2017). Obesity and kidney disease: Hidden consequences of the epidemic. Journal of Nephrology, 30(1), 1–10. https://doi.org/10.1007/s40620-017-0377-y
Kramann, R., & Humphreys, B. D. (2014). Kidney pericytes: Roles in regeneration and fibrosis. Seminars in Nephrology, 34(4), 374–383. https://doi.org/10.1016/j.semnephrol.2014.06.004
Kwon, S., Kim, Y. C., Park, J. Y., Lee, J., An, J. N., Kim, C. T., Oh, S., Park, S., & Kim, D. K. (2020). The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care, 43(May), 948–955. https://doi.org/10.2337/dc19-0936
Kwon, Y. W., Heo, S. C., Jeong, G. O., Yoon, J. W., Mo, W. M., Lee, M. J., Jang, I. H., Kwon, S. M., Lee, J. S., & Kim, J. H. (2013). Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1832(12), 2136–2144. https://doi.org/10.1016/j.bbadis.2013.08.002
Lee, J. H., Han, Y., & Lee, S. H. (2017). Potentiation of biological effects of mesenchymal stem cells in ischemic conditions by melatonin via upregulation of cellular prion protein expression. 0–2. https://doi.org/10.1111/ijlh.12426
Levin, A., Tonelli, M., Bonventre, J., Coresh, J., Donner, J. A., Fogo, A. B., Fox, C. S., Gansevoort, R. T., Heerspink, H. J. L., Jardine, M., Kasiske, B., Köttgen, A., Kretzler, M., Levey, A. S., Luyckx, V. A., Mehta, R., Moe, O., Obrador, G., Pannu, N., … Yang, C. W. (2017). Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. The Lancet, 390(10105), 1888–1917. https://doi.org/10.1016/S0140-6736(17)30788-2
Li, H., Lu, W., Wang, A., Jiang, H., & Lyu, J. (2021). Changing epidemiology of chronic kidney disease as a result of type 2 diabetes mellitus from 1990 to 2017: Estimates from Global Burden of Disease 2017. Journal of Diabetes Investigation, 12(3), 346–356. https://doi.org/10.1111/jdi.13355
Li, Y., Shen, M., Ferens, D., Broughton, B. R. S., Murthi, P., Saini, S., Widdop, R. E., Ricardo, S. D., Pinar, A. A., & Samuel, C. S. (2021). Combining mesenchymal stem cells with serelaxin provides enhanced renoprotection against 1K/DOCA/salt-induced hypertension. British Journal of Pharmacology, 178(5), 1164–1181. https://doi.org/10.1111/bph.15361
Liang, X., Ding, Y., Zhang, Y., Tse, H. F., & Lian, Q. (2014). Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplantation, 23(9), 1045–1059. https://doi.org/10.3727/096368913X667709
Liu, H., Liu, S., Li, Y., Wang, X., Xue, W., Ge, G., & Luo, X. (2012). The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PloS One, 7(4), e34608. https://doi.org/10.1371/journal.pone.0034608
Liu, H., Xue, W., Ge, G., Luo, X., Li, Y., Xiang, H., Ding, X., Tian, P., & Tian, X. (2010). Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochemical and Biophysical Research Communications, 401(4), 509–515. https://doi.org/10.1016/j.bbrc.2010.09.076
Liu, P., Feng, Y., Dong, C., Liu, D., Wu, X., Wu, H., Lv, P., & Zhou, Y. (2013). Study on therapeutic action of bone marrow derived mesenchymal stem cell combined with vitamin e against acute kidney injury in rats. Life Sciences, 92(14–16), 829–837. https://doi.org/10.1016/j.lfs.2013.02.016
Mias, C., Trouche, E., Seguelas, M.-H., Calcagno, F., Dignat-George, F., Sabatier, F., Piercecchi-Marti, M.-D., Daniel, L., Bianchi, P., Calise, D., Bourin, P., Parini, A., & Cussac, D. (2008). Ex Vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells, 26(7), 1749–1757. https://doi.org/10.1634/stemcells.2007-1000
Morales, A. I., Detaille, D., Prieto, M., Puente, A., Briones, E., Are, M., Lo, M., & Leverve, X. (2010). Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney International, 77(10), 861–869. https://doi.org/10.1038/ki.2010.11
Najafi, H., Abolmaali, S. S., Heidari, R., Valizadeh, H., Tamaddon, A. M., & Azarpira, N. (2022). Integrin receptor-binding nanofibrous peptide hydrogel for combined mesenchymal stem cell therapy and nitric oxide delivery in renal ischemia/reperfusion injury. Stem Cell Research and Therapy, 13(1), 1–17. https://doi.org/10.1186/s13287-022-03045-1
Nakao, Y., Fukuda, T., Zhang, Q., Sanui, T., Shinjo, T., Kou, X., Chen, C., Liu, D., Watanabe, Y., Hayashi, C., Yamato, H., Yotsumoto, K., Tanaka, U., Taketomi, T., Uchiumi, T., Le, A. D., Shi, S., & Nishimura, F. (2021). Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomaterialia, 122, 306–324. https://doi.org/10.1016/j.actbio.2020.12.046
Neven, E., Vervaet, B., Brand, K., Gottwald-Hostalek, U., Opdebeeck, B., De Maré, A., ... & D’Haese, P. C. (2018). Metformin prevents the development of severe chronic kidney disease and its associated mineral and bone disorder. Kidney International, 94(1), 102–113. https://doi.org/10.1016/j.kint.2018.01.027
Noh, H., Yu, M. R., Kim, H. J., Jeon, J. S., Kwon, S. H., Jin, S. Y., Lee, J., Jang, J., Park, J. O., Ziyadeh, F., Han, D. C., & Lee, H. B. (2012). Uremia induces functional incompetence of bone marrow-derived stromal cells. Nephrology Dialysis Transplantation, 27(1), 218–225. https://doi.org/10.1093/ndt/gfr267
Mishin, V. M., Rosman, A. S., Basu, P., Kessova, I., Oneta, C. M., & Lieber, C. S. (1998). Chlorzoxazone pharmacokinetics as a marker of hepatic cytochrome P4502E1 in humans. The American Journal of Gastroenterology, 93(11), 2154–2161. https://doi.org/10.1111/j.1572-0241.1998.00612.x
Overath, J. M., Gauer, S., Obermüller, N., Schubert, R., Schäfer, R., Geiger, H., & Baer, P. C. (2016). Short-term preconditioning enhances the therapeutic potential of adipose-derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Experimental Cell Research, 342(2), 175–183. https://doi.org/10.1016/j.yexcr.2016.03.002
Park, H., Li, Z., Yang, X. O., Chang, S. H., Nurieva, R., Wang, Y. H., Wang, Y., Hood, L., Zhu, Z., Tian, Q., & Dong, C. (2005). A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology, 6(11), 1133–1141. https://doi.org/10.1038/ni1261
Pittenger, M. F., Discher, D. E., Péault, B. M., Phinney, D. G., Hare, J. M., & Caplan, A. I. (2019). Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regenerative Medicine, 4(1). https://doi.org/10.1038/s41536-019-0083-6
Putra, A., Pertiwi, D., Milla, M. N., Indrayani, U. D., Jannah, D., Sahariyani, M., Trisnadi, S., & Wibowo, J. W. (2019). Hypoxia-preconditioned MSCs have superior effect in ameliorating renal function on acute renal failure animal model. Open Access Macedonian Journal of Medical Sciences, 7(3), 305–310. https://doi.org/10.3889/oamjms.2019.049
Qu, Z., Xu, H., Tian, Y., & Jiang, X. (2013). Atorvastatin improves microenvironment to enhance the beneficial effects of BMSCS therapy in a rabbit model of acute myocardial infarction. Cellular Physiology and Biochemistry, 32(2), 380–389. https://doi.org/10.1159/000354445
Rahman, M., Shad, F., & Smith, M. C. (2012). Acute kidney injury: A guide to diagnosis and management. American Family Physician, 86(7), 631–639.
Rashed, L. A., Elattar, S., Eltablawy, N., Ashour, H., Mahmoud, L. M., & El-Esawy, Y. (2018). Mesenchymal stem cells pretreated with melatonin ameliorate kidney functions in a rat model of diabetic nephropathy. Biochemistry and Cell Biology, 96(5), 564–571. https://doi.org/10.1139/bcb-2017-0230
Reiter, R. J. (1991). Melatonin: The chemical expression of darkness. Molecular and Cellular Endocrinology, 79(1–3), C153–C158. https://doi.org/10.1016/0303-7207(91)90087-9
Reiter, R. J., Tan, D.-X., Poeggeler, B., Menendez-Pelaez, A., Chen, L.-D., & Saarela, S. (1994). Melatonin as a free radical scavenger: Implications for aging and age-related diseases. Annals of the New York Academy of Sciences, 719(1), 1–12. https://doi.org/10.1111/j.1749-6632.1994.tb56817.x
Reiter, R. J., Tan, D. X., & Lorena, F. B. (2010). Melatonin: A multitasking molecule. In Progress in Brain Research (First edit, Vol. 181, Issue C). Elsevier. https://doi.org/10.1016/S0079-6123(08)81008-4
Rochefort, G. Y., Delorme, B., Lopez, A., Hérault, O., Bonnet, P., Charbord, P., Eder, V., & Domenech, J. (2006). Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells, 24(10), 2202–2208. https://doi.org/10.1634/stemcells.2006-0164
Rota, C., Morigi, M., & Imberti, B. (2019). Stem cell therapies in kidney diseases: Progress and challenges. International Journal of Molecular Sciences, 20(11). https://doi.org/10.3390/ijms20112790
Ryu, N. E., Lee, S. H., & Park, H. (2019). Spheroid culture system methods and applications for mesenchymal stem cells. Cells, 8(12), 1–13. https://doi.org/10.3390/cells8121620
Saberi, K., Pasbakhsh, P., Omidi, A., Borhani-Haghighi, M., Nekoonam, S., Omidi, N., Ghasemi, S., & Kashani, I. R. (2019). Melatonin preconditioning of bone marrow-derived mesenchymal stem cells promotes their engraftment and improves renal regeneration in a rat model of chronic kidney disease. Journal of Molecular Histology, 50(2), 129–140. https://doi.org/10.1007/s10735-019-09812-4
Salehipour, M., Monabbati, A., Salahi, H., Nikeghbalian, S., Bahador, A., Marvasti, V. E., Rezaei, H., Kazemi, K., Dehghani, M., Mohammadian, R., & Malek-Hosseini, S. A. (2010). Protective effect of parenteral vitamin E on ischemia-reperfusion injury of rabbit kidney. Urology, 75(4), 858–861. https://doi.org/10.1016/j.urology.2009.04.062
Samuel, C. S., & Hewitson, T. D. (2006). Relaxin in cardiovascular and renal disease. Kidney International, 69(9), 1498–1502. https://doi.org/10.1038/sj.ki.5000264
Samuel, C. S., & Hewitson, T. D. (2009). Relaxin and the progression of kidney disease. https://doi.org/10.1097/MNH.0b013e32831b7096
Satriano, J. (2013). Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. 305(5), F727–F733. https://doi.org/10.1152/ajprenal.00293.2013
Silva, L. H. A., Antunes, M. A., Dos Santos, C. C., Weiss, D. J., Cruz, F. F., & Rocco, P. R. M. (2018). Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Research and Therapy, 9(1), 1–9. https://doi.org/10.1186/s13287-018-0802-8
Sivanathan, K. N., Rojas-Canales, D. M., Hope, C. M., Krishnan, R., Carroll, R. P., Gronthos, S., Grey, S. T., & Coates, P. T. (2015). Interleukin-17A-induced human mesenchymal stem cells are superior modulators of immunological function. Stem Cells, 33(9), 2850–2863. https://doi.org/10.1002/stem.2075
Slominski, R. M., Reiter, R. J., Schlabritz-Loutsevitch, N., Ostrom, R. S., & Slominski, A. T. (2013). Melatonin membrane receptors in peripheral tissues: Distribution and functions. 351(2), 152–166. https://doi.org/10.1016/j.mce.2012.01.004.Melatonin
Susantitaphong, P., Cruz, D. N., Cerda, J., Abulfaraj, M., Alqahtani, F., Koulouridis, I., & Jaber, B. L. (2013). World incidence of AKI: A meta-analysis. Clinical Journal of the American Society of Nephrology, 8(9), 1482–1493. https://doi.org/10.2215/CJN.00710113
Tollabi, M., Ghasemzadeh, N., & Dehghani Firoozabadi, A. (2022). Potential therapeutic effect of TLR4-primed mesenchymal stem cells in lessening kidney damages in rat model of diabetic nephropathy. International Journal of Medical Laboratory, 9(3), 169–186. https://doi.org/10.18502/ijml.v9i3.10903
Tseng, W. C., Lee, P. Y., Tsai, M. T., Chang, F. P., Chen, N. J., Chien, C. T., Hung, S. C., & Tarng, D. C. (2021). Hypoxic mesenchymal stem cells ameliorate acute kidney ischemia-reperfusion injury via enhancing renal tubular autophagy. Stem Cell Research and Therapy, 12(1), 1–22. https://doi.org/10.1186/s13287-021-02374-x
Wang, H., Shang, Y., Chen, X., Wang, Z., Zhu, D., Liu, Y., Zhang, C., Chen, P., Wu, J., Wu, L., Kong, D., Yang, Z., Li, Z., & Chen, X. (2020). Delivery of mscs with a hybrid β-sheet peptide hydrogel consisting igf-1c domain and d-form peptide for acute kidney injury therapy. International Journal of Nanomedicine, 15, 4311–4324. https://doi.org/10.2147/IJN.S254635
Wechsler, M. E., Rao, V. V., Borelli, A. N., & Anseth, K. S. (2021). Engineering the MSC secretome: A hydrogel focused approach. Advanced Healthcare Materials, 10(7), 1–17. https://doi.org/10.1002/adhm.202001948
Xu, Y., Shi, T., Xu, A., & Zhang, L. (2016). 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. Journal of Cellular and Molecular Medicine, 20(7), 1203–1213. https://doi.org/10.1111/jcmm.12651
Yang, W., Chen, L., Jhuang, Y., Lin, Y., Hsu, L., Ko, P. H. Y., Lee, M. T. Y., Hsu, C. Y. H., & Huang, C. (2021). Injection of hybrid 3D spheroids composed of podocytes, mesenchymal stem cells, and vascular endothelial cells into the renal cortex improves kidney function and replenishes glomerular podocytes. Bioengineering & Translational Medicine, November 2020, 1–12. https://doi.org/10.1002/btm2.10212
Yap, J. X., Leo, C. P., Mohd Yasin, N. H., Show, P. L., Chu, D. T., Singh, V., & Derek, C. J. C. (2022). Recent advances of natural biopolymeric culture scaffold: Synthesis and modification. Bioengineered, 13(2), 2226–2247. https://doi.org/10.1080/21655979.2021.2024322
Yin, J., Guo, J., Zhang, Q., Cui, L., Zhang, L., & Peng, S. (2018). Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicology in Vitro, 51, 1–10. https://doi.org/10.1016/j.tiv.2018.05.001
Yu, X., Lu, C., Liu, H., Rao, S., Cai, J., Liu, S., Kriegel, A. J., Greene, A. S., Liang, M., & Ding, X. (2013). Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS ONE, 8(5), e62703. https://doi.org/10.1371/journal.pone.0062703
Yun, S. P., Han, Y. S., Lee, J. H., Kim, S. M., & Lee, S. H. (2018). Melatonin rescues mesenchymal stem cells from senescence induced by the uremic toxin p-cresol via inhibiting mTOR-dependent autophagy. Biomolecules and Therapeutics, 26(4), 389–398. https://doi.org/10.4062/biomolther.2017.071
Zhang, M., Methot, D., Poppa, V., Fujio, Y., Walsh, K., & Murry, C. E. (2001). Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. Journal of Molecular and Cellular Cardiology, 33(5), 907–921. https://doi.org/10.1006/jmcc.2001.1367
Zhang, W., Liu, L., Huo, Y., Yang, Y., & Wang, Y. (2014). Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. BioMed Research International, 2014. https://doi.org/10.1155/2014/462472
Zhao, J., Young, Y. K., Fradette, J., & Eliopoulos, N. (2023). Melatonin pretreatment of human adipose tissue-derived mesenchymal stromal cells enhances their prosurvival and protective effects on human kidney cells. 8, 1474–1483. https://doi.org/10.1152/ajprenal.00512.2014
Zhao, L., Hu, C., Zhang, P., Jiang, H., & Chen, J. (2019). Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury. Journal of Cellular and Molecular Medicine, 23(2), 720–730. https://doi.org/10.1111/jcmm.14035
Zhao, Y., Song, S., Wang, D., Liu, H., Zhang, J., Li, Z., Wang, J., Ren, X., & Zhao, Y. (2022). Nanozyme-reinforced hydrogel as a H2O2-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nature Communications, 13(1), 1–14. https://doi.org/10.1038/s41467-022-34481-5
Zhou, C., Zhou, L., Liu, J., Xu, L., Xu, Z., Chen, Z., Ge, Y., Zhao, F., Wu, R., Wang, X., Jiang, N., Mao, L., & Jia, R. (2020). Kidney extracellular matrix hydrogel enhances therapeutic potential of adipose-derived mesenchymal stem cells for renal ischemia reperfusion injury. Acta Biomaterialia, 115, 250–263. https://doi.org/10.1016/j.actbio.2020.07.056
Zhou, S., Liu, Y.-G., Zhang, Y., Hu, J.-M., Liu, D., Chen, H., Li, M., Guo, Y., Fan, L.-P., Li, L. Y., & Zhao, M. (2018). Bone mesenchymal stem cells pretreated with erythropoietin enhance the effect to ameliorate cyclosporine A-induced nephrotoxicity in rats. Journal of Cellular Biochemistry, 119(10), 8220–8232. https://doi.org/10.1002/jcb.26833
Zhou, S., Qiao, Y., Liu, Y., Liu, D., Hu, J., Liao, J., Li, M., Guo, Y., Fan, L., Li, L.-Y., & Zhao, M. (2020). Bone marrow derived mesenchymal stem cells pretreated with erythropoietin accelerate the repair of acute kidney injury. Cell & Bioscience, 10(1), 1–12. https://doi.org/10.1186/s13578-020-00492-2
Acknowledgements
Figure 1 and graphical abstract figure created with BioRender.com.
Funding
None.
Author information
Authors and Affiliations
Contributions
AR contributed to design of the manuscript, critical evaluation of various drafts, DM contributed to design of figures, DM, DG, VM have made substantial contributions to accumulation of the data, the drafting of the manuscript and its critical revision for important intellectual content and the final approval of the version to be submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical Approval
Not Applicable.
Consent To Participate
Not Applicable.
Consent To Publish
All authors consent to publication of the present manuscript.
Competing Interests
The authors declare that they have no competing interests.
Conflict of Interest
The authors listed in the manuscript have no conflict-of-interest w.r.t financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Makkar, D., Gakhar, D., Mishra, V. et al. Fine Tuning Mesenchymal Stromal Cells – Code For Mitigating Kidney Diseases. Stem Cell Rev and Rep 20, 738–754 (2024). https://doi.org/10.1007/s12015-024-10684-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-024-10684-9