Abstract
The methyltransferase-like (METTL) family is a diverse group of methyltransferases that can methylate nucleotides, proteins, and small molecules. Despite this diverse array of substrates, they all share a characteristic seven-beta-strand catalytic domain, and recent evidence suggests many also share an important role in stem cell biology. The most well characterized family members METTL3 and METTL14 dimerize to form an N6-methyladenosine (m6A) RNA methyltransferase with established roles in cancer progression. However, new mouse models indicate that METTL3/METTL14 are also important for embryonic stem cell (ESC) development and postnatal hematopoietic and neural stem cell self-renewal and differentiation. METTL1, METTL5, METTL6, METTL8, and METTL17 also have recently identified roles in ESC pluripotency and differentiation, while METTL11A/11B, METTL4, METTL7A, and METTL22 have been shown to play roles in neural, mesenchymal, bone, and hematopoietic stem cell development, respectively. Additionally, a variety of other METTL family members are translational regulators, a role that could place them as important players in the transition from stem cell quiescence to differentiation. Here we will summarize what is known about the role of METTL proteins in stem cell differentiation and highlight the connection between their growing importance in development and their established roles in oncogenesis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
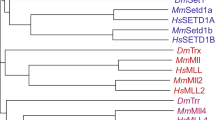
The methyltransferase-like (METTL) family of proteins is a sub-family of seven-beta-strand (7BS) methyltransferases. They, along with the SET-domain (Su(var)3–9, Enhancer-of-zeste, Trithorax) protein methyltransferases, are one of the two major families of methyltransferases [1]. Members of the METTL family are characterized by a conserved S-adenosyl methionine (SAM) binding domain that resides in part of the overall 7BS structure. While members of the SET-domain family only methylate lysine residues in proteins, members of the METTL family are capable of methylating proteins, nucleic acids, and other small molecule metabolites [2]. There are 33 members of the vertebrate METTL family [3]. Fourteen of these methylate DNA or RNA, thirteen methylate protein residues, one methylates alkyl thiol groups, and five remain of unknown function (Fig. 1) [3,4,5,6,7,8]. While enzymes modifying similar biomolecules did not evolve from a common ancestor, they do largely group together phylogenetically (Fig. 1).
Evolutionary analysis of the METTL family by Maximum Likelihood method: The evolutionary history was inferred by using the maximum likelihood method and Le/Gascuel model [9]. The tree with the highest log likelihood (-14,505.17) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 33 amino acid sequences. All positions with less than 90% coverage were eliminated, i.e., fewer than 10% alignment gaps, missing data, and ambiguous bases were allowed at any position (partial deletion option). There were a total of 175 positions in the final dataset. Evolutionary analyses were conducted in MEGA X [10]
METTL proteins have a broad substrate range and have been implicated in a wide range of biological processes, including oncogenesis [11], cardiovascular disease [12], and viral replication [13] (Table 1). While their role in cancer progression is currently the best characterized, and seventeen METTL family members already have documented roles as oncogenes or tumor suppressors, eleven METTL family members have also been implicated in regulating stem cell development (Fig. 2, Table 2). Many of these studies are very recent, suggesting that additional METTL family members will be associated with stem cell regulation in the years to come. Additionally, a strong link is emerging between METTL proteins and the regulation of protein translation, a key process central to stem cell differentiation. Taken together, a growing body of literature suggests that the METTL proteins will take their place as important regulators of stem cell biology, and the focus of this review will be to summarize this expanding field.
Summary of known oncogenic and stem cell developmental roles of METTL family members: (A) To date, the majority of METTL proteins exhibit oncogenic activity, with some acting as both oncogenes and tumor suppressors, but none exhibiting only tumor suppressor activity. (B) There is a more even divide when it comes to stem cell development, with the majority promoting differentiation or playing dual roles in the maintenance of pluripotency and transition to a differentiated state
METTL Proteins and Embryonic Stem Cells
METTL3/METTL14
METTL3 and its binding partner METTL14 are currently the most extensively studied of the METTL family members. Together, they act as an N6-methyladenosine (m6A) RNA methyltransferase [14]. METTL3 and METTL14 form a stable heterodimer [14], with METTL3 supplying the catalytic activity and METTL14 providing structural support and aiding in substrate recognition [15]. m6A marks placed on mRNA targets by the METTL3/14 complex are bound by two families of reader proteins, YTH domain-containing proteins (YTHDF1-3 and YTHDC1-2) and insulin-like growth factor 2 mRNA-biding proteins (IGF2BP1-3) [16, 17]. As m6A is one of the most prevalent modifications of mRNA and regulates both its stability and translation efficiency [18], it is not surprising that the METTL3/14 complex has many known roles in cancer progression. It acts as an oncogene in bladder, gastric, colorectal, cervical, pancreatic, blood, liver, and breast cancers and is being developed as both a prognostic marker and therapeutic target [19,20,21,22,23,24,25,26]. This complex also acts as a tumor suppressor in thyroid and endometrial cancer [27, 28].
The roles of METTL3/14 in stem cell biology are also beginning to come to light, but these roles are complex and likely dictated by both stem cell stage and cell type [29]. An early report suggested that the METTL3/14 complex was required for mouse embryonic stem cells (mESCs) to maintain their pluripotent state and self-renewal capabilities, likely by maintaining the expression of stemness genes and repressing the expression of developmental regulators [30]. However, this paper utilized an siRNA-based knockdown (KD) approach in cultured cells, and a later paper generated conflicting results using CRISPR-mediated genetic knockout (KO) of Mettl3 [31]. Here, the authors showed that Mettl3 KO in mESCs lead to increased self-renewal capacity and blocked differentiation [31]. These results were supported by subsequent work showing METTL3 methylates transcripts needed to maintain pluripotency, thereby promoting their degradation and entry into differentiation [32]. However, more recent work shows that METLL3 KD promotes both pERK activation and departure from pluripotency and supports the original finding that METTL3/14 is needed for maintenance of pluripotency [33]. These conflicting results may arise from the differences between KD and KO, or from the state the cells existed in (naïve if isolated from the inner cell mass (ICM) of blastocysts or more primed if generated in culture), which would dictate time and degree of METTL3 loss or the predominant transcripts available for modification, respectively [29]. Repetition of these experiments in identical, stage-specific conditions and identification of METTL3/14 targets corresponding to each stage will help resolve these conflicts. The dual roles of METTL3/14 as both an oncogene and tumor suppressor suggest it could be similarly regulating both pluripotency and differentiation in a time/tissue-specific manner.
METTL1
During translation, transfer RNAs (tRNAs) serve as adaptor molecules that bridge amino acids with the corresponding mRNA sequence at specific sites in the ribosome. They are subject to numerous post-transcriptional modifications, including methylation, and disruption of these modifications can often lead to disease [34]. N7-methylguanosine (m7G) is one of the most common of these modifications and introduces a positive charge at the modification site. METTL1 was found to be one of the methyltransferases responsible for promoting translation and placing m7G on tRNA, mRNA, microRNA (miRNA), and ribosomal RNA (rRNA) [35,36,37]. METTL1 forms a heterodimer with WD repeat domain 4 (WDR4), and both are essential for m7G methylation [35]. While loss of the budding yeast METTL1 homolog, Trm8p, has a modest phenotype [38, 39], the human homolog has been implicated in double-strand DNA repair and shown to act as an oncogene in bladder cancer, hepatocellular carcinoma (HCC), acute myeloid leukemia (AML), and intrahepatic cholangiocarcinoma (ICC) and a tumor suppressor in colon cancer [40,41,42,43,44,45].
The METTL1/WDR4 complex has also been implicated in stem cell biology. In cultured mESCs, loss of either METTL1 or WDR4 by CRISPR-mediated KO results in partial spontaneous differentiation, failure to self-renew, slower growth rates, and failure of the ectoderm to differentiate into subsequent neuronal lineages [46]. In Mettl1 KO mESCs, there is reduced mRNA expression of the pluripotency markers KLF4 and Nanog, in addition to Nanog protein, and a reduction in alkaline phosphatase (AP) positive pluripotent cells [46]. This suggests that the mESCs are failing to properly self-renew and are undergoing partial differentiation. A later paper corroborated the lineage-specification defect by showing that KD of METTL1 in human induced pluripotent stem cells (iPSCs) enhances differentiation down the mesoderm lineage pathway, while suppressing differentiation into neuroectoderm [47]. Similar to Mettl1 KO mESCs, these cells proliferated more slowly, had a significantly reduced population of AP-positive cells, and appeared morphologically to have prematurely differentiated when cultured in growth media [47]. These data suggest METTL1 is regulating both maintenance of ESC pluripotency and differentiation of neuronal lineages. Accordingly, disruption of m7G in humans results in brain malformation, facial dimorphism, and severe encephalopathy with seizures [46, 48, 49].
METTL5
METTL5 was recently identified as the rRNA methyltransferase responsible for placing the m6A1832 mark on the eukaryotic 18S ribosomal subunit [50, 51]. Similar to other METTL family members, METTL5 requires a stabilizing co-factor, TRM112, for its stability and function [50, 52, 53]. To date, the m6A1832 site on the 18S subunit is the only identified substrate of METTL5 [50], though disruption of this mark has the potential to affect downstream translation of many proteins. Mechanistically, modification of the m6A1832 site on 18S rRNA brings a conformational change to the decoding center of the ribosome that favors increased RNA binding, and loss of METTL5 decreases polysome number [50, 54]. Loss of METTL5 also promotes cardiomyocyte hypertrophy, apoptosis, and mismatch repair [55, 56].
Shortly following its identification, functions were ascribed to METTL5 in both promoting oncogenic growth and mESC pluripotency [54, 57]. Originally identified as promoting breast cancer growth, recent studies have shown METTL5 also promotes pancreatic cancer progression [54, 58]. mESCs with CRISPR/Cas9-mediated KO of METTL5 displayed decreased mRNA and protein levels of several core pluripotency genes, including Klf4, Nanog, Sox2, and Rex1/Zfp42 [57]. Contrastingly, they also exhibited a reduced differentiation potential, specifically in the neuroectodermal and mesodermal lineages [57]. A later paper supported the differentiation phenotype, showing Mettl5 KO mESCs did not properly respond to differentiation signals [59]. They ascribed this phenotype to a ribosomal defect that reduced translation of the differentiation regulator FBXW7 [59]. In the absence of FBXW7, c-Myc expression remained elevated even when cells were induced to differentiate [59]. These data indicate that, similar to METTL3/14 and METTL1, METTL5 is playing a role in both pluripotency and differentiation.
Interestingly, Mettl5-deficient mice are subviable, as they exhibit non-Mendelian birth rates [53, 57]. They also exhibit reduced body weight, eye and craniofacial abnormalities, male infertility, intellectual disabilities, and hyperactivity [57, 60]. It is thought the intellectual disabilities result from abnormal myelination [60]. In zebrafish, knockdown of METTL5 results in reduced brain size [61], and human patients with frameshift variants of METTL5 have been shown to have intellectual disabilities, microcephaly, and facial dimorphisms [61].
METTL6, METTL17, and METTL8
METTL6 is an N3-methylcytidine (m3C) methyltransferase that methylates tRNA and regulates tumor growth and pluripotency [62,63,64]. In HCC cells, METTL6 promotes oncogenic phenotypes by increasing expression of cell adhesion-related genes [65]. In mESCs, deletion of Mettl6 limits pluripotency, resulting in flat, differentiated-like cells and a decrease over time of AP-positive cells [62]. Not surprisingly, RNA-seq analysis of these cells demonstrated a decrease in pluripotency gene expression (Rex1, Klf4, Esrrb, Tbx3, Dppa3) [62]. Concomitantly, there was an increase in gastrulation (Fgf5, Lefty1, Nodal), mesoderm (Brachyury), and ectoderm (Nestin) markers [62]. Mettl6 KO mice, while found to have decreased liver weight, altered glucose homeostasis and metabolic imbalance, were viable and exhibited no gross morphological phenotypes [62], indicating the role of METTL6 in stem cell development may ultimately be tissue-specific or redundant with that of other family members.
METTL17 is an N4-methylcytidine (m4C) methyltransferase in the mitochondria that methylates C840 and C842 of 12S mt-rRNA to promote its stability [66]. Loss of METTL17 results in defective mitoribosome assembly and decreased protein translation [66]. A recent study has shown METTL17 is required for mESC differentiation [66]. Mettl17 KO in mESCs negatively affects their ability for oxidative phosphorylation (oxphos) and inhibits their differentiation [66]. Specifically, Mettl17 KO mESCs have a significant decrease in basal oxygen consumption rate (OCR) and reduced levels of the TCA intermediate metabolites citrate and isocitrate [66]. These cells also have a dramatic increase in the ratio of reduced to oxidized forms of γ-glutamyl-cysteinyl-glycine (GSH) [66]. It is hypothesized that the protein translation defects seen in Mettl17 KO mESCs lead to the disruption of oxphos because components of the electron transport chain (ETC) are translated by the mitochondrial ribosome [66]. Genes that control cellular metabolism are commonly found to regulate stem cell development, as ESCs rely heavily on anaerobic glycolysis and somatic cells favor aerobic metabolism, indicating the transition from glycolysis to oxphos is imperative for the transition from ESC to differentiated cell [67]. Mettl17 was also identified as a gene essential for oxphos in human myelogenous leukemia K562 cells [68], and its knockdown in breast cancer cells reduces proliferation [69].
METTL8 was originally identified as the first methyltransferase to place the m3C modification on mRNA in mammalian systems [63]. However, subsequent studies have shown it can also act as a mitochondrial tRNA methyltransferase that performs m3C32 methylation of mt-tRNAThr and mt-tRNASer(UCN) [70,71,72]. Through its mtRNA m3C32 methylation activity, METTL8 promotes mitochondrial translation and respiratory chain activity similar to METTL17, and as such, acts as an oncogene [71, 73]. METTL8 has high expression in multiple lung cancer cell lines and lung squamous cell carcinomas and promotes proliferation [73]. In contrast, during mESC development, METTL8 promotes pluripotency, and this seems dependent on its mRNA methylation activity [74]. Mettl8 transcripts are positively regulated by STAT3, a transcription factor that has been shown to be crucial for maintaining the pluripotency of ES cells [75,76,77,78]. METTL8 promotes pluripotency by negatively regulating the JNK signaling pathway and impeding differentiation [74]. Specifically, METTL8 binds the mRNA of MAPKBP1, a key component of the JNK signaling cascade, and prevents its translation [74]. Levels of Mapkbp1 mRNA translation and lineage marker expression are increased following METTL8 loss, though whether this results directly from methylation of Makpbp1 mRNA by METTL8 remains to be determined [74]. Increased expression of METTL8 alone is not strong enough to promote pluripotency, indicating it too may be acting as part of a larger complex [74].
METTL Proteins and Tissue-specific Stem Cells
METTL3/METTL14
In addition to its role in ESC regulation, the METTL3/14 complex has also been shown to be critical in later postnatal stem cell development. One of the best-studied examples is in the hematopoietic system. Arising from the endothelial lineage, hematopoietic stem cells (HSCs) are lineage-committed and give rise to all cell types of the blood. Conditional KO of Mettl3 in mouse adult bone marrow causes HSCs to prematurely exit the quiescent state, indicating METTL3 is needed to retain quiescence [79]. Similarly, METTL14 loss in primed hematopoietic stem and progenitor cells (HSPCs) promotes their terminal differentiation [80]. Mechanistically, it is hypothesized that m6A methylation by METTL3/14 promotes degradation of transcripts needed for differentiation, and it has been recently shown that long-term HSCs have a subset of mRNAs involved in pluripotency (Kit, Hoxa5, Gata2, Ctnnb1, Ldb1, Hoxb4) with nearly undetectable levels of m6A modification and high mRNA expression [81]. Conversely, they also have a subset of mRNAs involved in differentiation (Eomes, Bcl6, Prmt6) with high levels of m6A modification and low levels of mRNA expression [81], indicating METTL3/14 helps retain HSC quiescence by preventing transcription of differentiation factors.
The METTL3/14 complex is also strongly implicated in neural stem cell (NSC) biology, as the brain contains the highest abundance of m6A RNA among human tissues [82,83,84]. Similar to HSCs, transcripts involved in NSC differentiation and highly expressed in late intermediate progenitor cells (IPCs) and post-mitotic neurons have high m6A methylation [85]. However, in contrast to HSCs, many transcripts involved in NSC pluripotency are also subject to m6A methylation [85]. Accordingly, conditional KO of Mettl14 during embryogenesis using the Nestin-Cre promoter (Mettl14flox/flox; Nestin-Cre, referred to as Mettl14 cKO) results in prolonged retention of a pluripotent NSC pool that abnormally expresses neuronal proteins [85]. These data indicate both NSC pluripotency and differentiation are affected by loss of m6A. In addition, Mettl14 cKO animals are much smaller than their control littermates and have enlarged lateral ventricles [85]. Mettl3 conditional KO mice (Mettl3flox/flox;Nestin-Cre, referred to as Mettl3 cKO) have similar phenotypes, including significantly smaller brains, and ventricle enlargement [84]. These animals also exhibit tremors, balance problems, uneven gait, feet and hand clasping in tail hold test, and decreased foliation and disorganization of the layers contained within the cerebellum [84].
New evidence indicates METTL3/14 is also important for retaining quiescence in skeletal muscle myoblasts, dental pulp stem cells, and human mesenchymal stem cells (hMSCs) [86,87,88]. In myoblasts, levels of METTL3 have been shown to markedly decline as they enter the differentiation process (both in C2C12 cultured myoblasts and primary myoblasts) [86]. Functionally, the loss of METTL3 causes premature differentiation in C2C12 myoblasts, with increases in myogenic differentiation 1 (MyoD) (~ twofold) and myogenin (~ 18-fold) mRNA in cells grown in high serum media [86]. In pulp cells, KD of METTL3 results in an increase in senescence and apoptosis [88]. Similarly, in hMSCs, KO of Mettl3 causes an acceleration of senescence and premature aging phenotypes [89]. This is due, at least in part, to loss of the m6A mark on the cell cycle regulator Mis12 [89]. Taken together, these data indicate m6A methylation by METTL3/14 generally protects the quiescent state of tissue-specific stem cells but may promote differentiation in a subset of tissues as well. This is likely controlled by the availability of substrates in each tissue at each developmental stage.
METTL11A/METTL11B
METTL11A, also known as N-terminal RCC1 methyltransferase 1 (NRMT1), is the first identified eukaryotic N-terminal methyltransferase [90]. It is a ubiquitously expressed trimethylase that acts on the N-alpha (Nα) amino group of proteins with a defined consensus sequence [91]. Based on this consensus sequence, it is predicted that METTL11A methylates more than 300 targets [91]. Nα-methylation has been shown to regulate protein/DNA interactions, and many of its targets are involved in chromatin assembly, transcription, or DNA damage repair [90, 92,93,94]. Accordingly, loss of METTL11A results in a variety of oncogenic and developmental phenotypes [95, 96]. METTL11A acts as a tumor suppressor in breast cancer cells, as its KD results in increased cell proliferation, migration, and xenograft growth [96]. It can also serve as an oncogene, promoting migration of cervical cancer cells and growth of colorectal cancer cells [97, 98]. Developmentally, Mettl11A KO mice demonstrate premature aging phenotypes, including early graying, kyphosis, neurodegeneration, and impaired mitochondrial function [95, 99].
To begin to understand the molecular mechanism behind the neurodegeneration seen in Mettl11A KO mice, molecular analysis of the subventricular zone (SVZ) NSC niche was performed [99]. It was found that, at birth, Mettl11A KO mice had significant expansion of the quiescent NSC pool in the SVZ [99]. However, by postnatal day 14 (P14), this NSC pool became depleted due to premature differentiation into intermediate progenitor cells (IPCs) and neuroblasts [99]. The resulting neuroblasts differentiated into mature neurons, but many of these neurons were unable to completely exit the cell cycle and ultimately underwent apoptosis [99]. The observed NSC differentiation phenotypes were reminiscent of those seen with loss of the METTL11A target retinoblastoma protein (RB) [100, 101], and it was shown that RB phosphorylation and function are impaired in Mettl11A KO mice [99]. These data indicate that, in neuronal cells, METTL11A is necessary for inhibiting the cell cycle during both quiescence and terminal differentiation.
A separate study identified cAMP-response element binding protein 1 (CREB1) as one of the primary transcription factors driving Mettl11A expression [102]. Screening for conditions of CREB1-induced Mettl11A expression identified serum starvation and C2C12 mouse myoblast differentiation [102]. Accordingly, CRISPR/Cas9-mediated Mettl11A KO in C2C12 cells inhibited myoblast differentiation. In growth media, the Mettl11A KO cells failed to upregulate expression of the master regulator of postnatal myogenesis, Pax7. After three days of culturing in low-serum differentiation media, they also failed to upregulate the downstream myogenesis markers MyoD and myogenin [102]. As myoblasts are derived from a mesenchymal stem cell progenitor pool, and could potentially transdifferentiate into osteoblasts, adipocytes, or chondrocytes, markers of these lineages were assayed. While expression of Sox9 (chondrocytes) and Pparγ (adipocytes) was unchanged in the KO cells, the osteoblastic markers alkaline phosphatase, Runx2, osteocalcin, osterix, and type 1 collagen were significantly increased [102]. These data indicate that, in myoblasts, METTL11A is necessary for responding to differentiation cues and undergoing myogenesis. The closest METTL11A homolog, METTL11B, is a tissue-specific Nα-monomethylase that is upregulated during osteocytic and myogenic differentiation [103]. Similar to METTL3/METTL14, METTL11A and METTL11B complex together and METTL11B activates METTL11A activity [104], indicating METTL11A and METTL11B could be working synergistically to regulate mesenchymal stem cell differentiation. Interestingly, loss of METTL15 and METTL21C produce similar muscle phenotypes to loss of METTL11A [105,106,107,108], indicating they too could be METTL11A interactors.
Similar to METTL3/14, METTL11A appears to regulate pluripotency or differentiation differently in different tissues, and this could also be due to substrate availability. Though studies in NSCs indicate that loss of methylation of the METTL11A target RB is contributing to the observed phenotypes, METTL11A has numerous other targets that may predominate in other tissues. Many of these are transcription factors, but interestingly, the budding yeast homolog of METTL11A, TAE1, was initially identified in a screen for genes that alter protein synthesis rates [109]. Deletion of Tae1 in yeast results in decreased polysome formation and downstream impairment of translation efficiency [109], and METTL11A is known or predicted to methylate numerous ribosomal proteins, including RPL23a, RPS25, and RPL12 [90, 110]. These data indicate METTL11A and METTL11B could also be regulating stem cell development through translational regulation, like many of the above-mentioned METTL family members.
METTL4, METTL7A, and METTL22
METTL4 is an additional m6A methyltransferase that is conserved from yeast to humans [111]. Though, to date, human METTL4 has only been shown to methylate small nuclear RNA (snRNA), miRNA, and mitochondrial DNA (mtDNA) [112,113,114], the C. elegans and M. musculus homologs of METTL4 also have genomic DNA m6A activity [115, 116]. In humans, m6A methylation of snRNA by METTL4 is important for regulation of RNA splicing, m6A methylation of miRNA affects mRNA repression efficacy, and m6A methylation of mtDNA promotes mitochondrial function [111, 113, 114]. METTL4 promotes melanoma cell growth, but is predicted to act as a tumor suppressor in soft tissue sarcomas [117, 118]. In mice, m6A DNA methylation by METTL4 has been shown to be important for adipogenesis [116]. Loss of METTL4 in 3T3-L1 mouse preadipocytes inhibits their ability to properly differentiate, as they have lowered expression of the major adipogenic factors and decreased lipid production [116]. It also resulted in decreased glucose uptake and consumption, due to decreased expression of the insulin receptor gene (Insr) [116]. It is hypothesized that m6A DNA methylation of promoter regions is necessary to promote transcription of genes that drive differentiation [116].
METTL7A is an m6A methyltransferase known to methylate both DNA and long non-coding RNAs (lncRNAs) [119, 120]. It was identified in an RNA-seq screen as a gene associated with enhanced osteogenesis and cell survival in human bone marrow stem cells (hBMSCs) [120]. Knockdown of METTL7A reduced osteogenic differentiation as determined by Alizarin Red staining, while overexpression of METTL7A could reverse the differentiation defects seen in glucose-free conditions [120]. Similarly, METTL7A knockdown in human dental pulp stem cells (DPSCs) inhibited their differentiation, while overexpression enhanced odontogenic differentiation [121]. It is hypothesized that DNA methylation by METTL7A promotes osteogenic and odontogenic differentiation by upregulating expression of genes that promote differentiation and survival [120]. METTL7A has also been shown to act as an oncogene in multiple myeloma and choriocarcinoma cells and a tumor suppressor in breast cancer cells [119, 122, 123].
METTL22 is a lysine methyltransferase with one known target, the DNA-binding protein Kin17 [124]. Methylation of lysine 135 in Kin17 by METTL22 impairs the binding of Kin17 to chromatin and promotes its cytoplasmic localization [124]. Though little is known of its targets and biological function, METTL22 emerged as one of the top candidates in a genome-wide RNAi screen for genes required for the differentiation of ESCs into HSPCs and is involved in later steps of HSPC specification [125]. Knockdown of METTL22 in zebrafish reduces the expression of Runx1 and c-myb during embryonic HSC development, while METTL22 knockdown in human cord blood CD34 + cells led to a marked reduction in HSPCs [125]. It remains uncertain whether the role of METTL22 in HSPC specification is through its methylation of Kin17 or additional unknown protein targets [125].
METTL Proteins and Translation in Stem Cells
In recent years there has been a growing appreciation of the role translational control plays in stem cell biology [126, 127]. Differentiation of pluripotent stem cells represents a state change for the cell and often requires a rapid response. As transcriptional change is not immediate, cells often rely on post-transcriptional (translational) control of protein levels to quickly respond to differentiation cues [128]. As far back as 2008, it was appreciated that mESCs, in addition to upregulating transcription of genes during differentiation, also greatly upregulated translation [129]. Protein levels remain low in undifferentiated, pluripotent mESCs, but upon removal of LIF many notable changes were observed [130]. The majority of genes studied (76%) showed an increase in both transcriptional and translational upregulation, and a small subset (~ 2%) that showed no increase in transcript abundance did show upregulated translation [130]. Moreover, very few transcripts showed translational downregulation during differentiation [130]. Taken together, this study indicated that ESCs seem to inhibit translation of nascent transcripts as a way to remain poised to rapidly respond to different differentiation cues.
Many METTL proteins play a role in translational regulation, and this could contribute to their role in stem cell development. m6A methylation is one of the most prominent and well-studied modifications made on RNA. It occurs on RRACH motifs and in mRNA is mostly enriched in the coding sequences (CDS) of long internal exons, 3’ untranslated regions (UTRs), and near stop codons [82, 131, 132]. It is thought to promote mRNA degradation when in non-coding regions, and alternatively, promote translation when in coding regions [18, 82]. m6A methylation promotes translation by facilitating the recruitment of translation initiation factors through its YTHD readers [18, 133]. In addition to those discussed already (METTL3/14, METTL4, METTL5 and METTL7A), METTL16 is also an m6A methyltransferase that methylates mRNA, lncRNA, and snRNA [134]. It is essential for embryonic development in mice [135], and its expression is induced as mESCs are differentiated into cardiomyocytes [136]. It also works as an oncogene in gastric cancer and hepatocellular carcinoma [137, 138].
m3C modification has also been implemented in translational control, hypothesized to be one of the modifications promoting translation efficiency by maintaining the conformation of the anticodon loop, enhancing codon interactions, and preventing frameshifts [139]. Mettl8 KO in HCT116 cells alters the polysome to monosome ratio, which suggests increased ribosomal stalling with loss of METTL8, and loss of METTL6 also affects translation [63, 139]. In addition to METTL8 and METTL6, METTL2A and METTL2B are also m3C methyltransferases [64]. Little is known about the biological functions of METTL2A and METTL2B, though METTL2B is a predicted prognostic marker in gastric cancer [140]. Given their similar enzymatic functions to METTL6 and METTL8, it is likely their part in translational regulation and stem cell development will eventually be uncovered.
It is also interesting to note that three of the METTL family proteins (METTL10, METTL13, METTL21B) have now been identified as enzymes that methylate human eukaryotic elongation factor 1 alpha (eEF1A), the second most abundant protein in the cell and a critical player in translational control [141,142,143]. METTL10 trimethylates eEF1A on lysine 318 [143, 144], and METTL21B methylates lysine 165 [141, 145]. METTL13 methylates both the Nα-amino group and lysine 55 (K55) of eEF1A [142]. While the role of eEF1A in stem cells is largely unstudied, it has been shown to regulate primordial germ cells (PGCs) in the sea urchin embryo [146]. Here, eEF1A mRNA is regulated in its 3’UTR by the translational repressor Nanos, in a manner that excludes it from the PGCs early in development and keeps translation rates low [146]. Moreover, cancer cells are often thought to hijack the machinery used to maintain stem cell populations, and both METTL13 and METTL21B are overexpressed in certain cancer types [147, 148]. It has been shown that methylation of eEF1A by METTL13 upregulates protein translation to sustain the needs of rapidly proliferating cancer cells, but METTL13 has also been shown to act as a tumor suppressor [149,150,151]. It is possible METTL13 will also play dual roles during stem cell development.
Conclusions
Given the extensive roles of METTL family members in cancer progression, it is not surprising that corresponding roles in stem cell biology are beginning to emerge (Table 2). Both cancer cells and stem cells are unique in being able to retain the ability to re-enter the cell cycle from a quiescent state and undergo proliferation [152]. Therefore, proteins that regulate cellular process that support and/or repress proliferation are strong candidates for playing a role in both development and oncogenesis. One obvious prerequisite for altering proliferative growth is transcriptional regulation of genes that control the cell cycle, and a variety of METTL proteins target transcription factors or DNA directly. METTL11A methylates RB and inhibits cell cycle progression [99]. METTL22 promotes the DNA-binding of KIN17, and KIN17 has recently been found to activate p38 MAPK signaling, a pathway known to regulate cell cycle [124, 153, 154]. METTL9, a histidine methyltransferase overexpressed in HCC [155], methylates the zinc transporter SLC39A7, and its loss impairs zinc homeostasis and alters gene expression [156], indicating diminished function of zinc-responsive transcription factors. DNA methylation by METTL7A is thought to regulate the transcription of genes involved in pluripotency, and in mice, DNA methylation by METTL4 is thought to regulate transcription of differentiation factors [116, 120].
Another prerequisite for proliferative growth is increased uptake and catabolism of nutrients [152], and there are also METTL genes that regulate metabolism. Loss of METTL8, METTL17, METTL15, METTL20, METTL11A, and METTL9 all decrease mitochondrial respiration. METTL8 and METTL17 regulate respiration by promoting translation of ETC complex members [66, 71]. METTL15 methylates 12S mt-rRNA, promoting both mitochondrial ribosome assembly and respiration [157, 158]. METTL20 methylates the β-subunit of the electron transfer flavoprotein (ETFβ) and promotes oxygen consumption, presumably by regulating the interaction of ETFβ with acyl-CoA dehydrogenase [159]. METTL11A regulates transcription of liver metabolic genes [95, 160], and methylation by METTL9 activates NDUFB3 activity, a subunit of mitochondrial respiratory Complex I [161]. METTL12 methylates K395 of citrate synthase [162], and METTL7B is an alkyl thiol methyltransferase [4]. METTL7B is upregulated in many cancers and involved in hydrogen sulfide metabolism, which regulates both EGFR/ERK/MMP-2 and PTEN/AKT signaling [4, 163,164,165,166,167,168]. These pathways also regulate stem cell development, providing a potential role for METTL7B in this area as well [169,170,171].
Finally, as mentioned above, translational upregulation is necessary for both oncogenic growth and the onset of differentiation, and many METTL family proteins have roles in the regulation of translation. The m6A activity of METTL3/14, METTL4, METTL5, and METTL16 is necessary for many aspects of RNA metabolism, including translation, degradation, splicing, export, and folding [172], and all have been found to be misregulated in human cancers (Table 2) [20, 54, 118, 122, 138, 173]. METTL1, METTL6, METTL8, METTL11A/11B, METTL13, METTL15, METTL17, METTL18, and METTL21B are all also involved in protein translation and play a role in oncogenic growth (Table 2) [65, 73, 96, 148, 149, 158, 174,175,176,177]. While the primary role of many METTL proteins is translational regulation, some also have overlapping roles in transcriptional and/or metabolic regulation (Fig. 3). These roles appear to be substrate-specific and are likely to expand as additional targets are identified. Taken together, it is not surprising that the diverse family of METTL proteins is playing roles in both stem cell development and cancer growth, but it is important to recognize and more comprehensively understand these dual roles to better understand the mechanism, efficacy, and safety of METTL-targeted therapeutics.
Mechanisms shared by cancer and stem cells to exit quiescence and begin proliferation: To re-enter the cell cycle both cancer and stem cells have to alter their transcriptional programs, activate metabolism, and increase protein translation. METTL family members play roles in all three of these processes, and some have overlapping roles in more than one. These roles are substrate-specific and are likely to expand as additional METTL family targets are identified
Future Directions
Until recently, focus in the METTL field has been directed at assigning specific activities to each methyltransferase. Now that only five members remain unclassified, focus will begin to shift to a more comprehensive analysis of substrate identity, and this will help better clarify the functions of the METTL family members in retaining pluripotency and promoting differentiation. Many METTL methyltransferases still have only one known target, which limits understanding of how they affect these processes. It is likely that over time the substrate list of each enzyme will greatly expand, and the challenge in the field will be to discern which substrate is most important at each developmental stage and in each tissue type. For example, we have shown that loss of METTL11A in the brain results in depletion of the neural stem cell pool and this correlates with misregulation of the cell cycle and the NRMT1 target RB [178]. However in the liver, loss of NRMT1 impairs the function of the transcription factor ZHX2 and increases Gpc3 expression, which could promote expansion of the hepatic stem cell pool [179]. Therefore, the effects of METTL11A loss could differ and produce opposing results due to the specific substrate driving each phenotype. It is also likely that additional METTL11A complex members could be tissue-specific and the exchange of interactors, regulators, and readers could fine-tune downstream signaling targets to result in different phenotypic outcomes.
Finally, in addition to tissue-specific functions, there is new evidence that METTL proteins also have cell compartment-specific functions. METTL16, which was initially identified as a nuclear m6A writer with hundreds of mRNA targets, is now known to have an additional cytoplasmic role promoting translation by facilitating the assembly of the translation-initiation complex [138]. This function is independent of its methylation activity [138]. The dual substrate specificity of METTL8 has been explained by isoforms that are specific to either the mitochondria or the nucleolus [180], and we have recently found that METTL11A, previously thought to be primarily a nuclear protein, interacts with and regulates METTL13 in the cytoplasm (unpublished data). We are currently working to better understand the phenotypic consequences of this new cytoplasmic role of METTL11A. In summary, production of a more comprehensive and detailed “road map” of METTL substrates, interactions, and localization will provide the bigger context in which METTL proteins are acting and should help resolve current data conflicts in the field.
Data Availability
Not applicable.
Code availability
Not applicable.
References
Petrossian, T. C., & Clarke, S. G. (2011). Uncovering the human methyltransferasome. Mol Cell Proteomics, 10(1), M110.000976.
Schubert, H. L., Blumenthal, R. M., & Cheng, X. (2003). Many paths to methyltransfer: A chronicle of convergence. Trends in Biochemical Sciences, 28(6), 329–335.
Wong, J. M., & Eirin-Lopez, J. M. (2021). Evolution of Methyltransferase-Like (METTL) Proteins in Metazoa: A Complex Gene Family Involved in Epitranscriptomic Regulation and Other Epigenetic Processes. Molecular biology and evolution, 38(12), 5309–5327.
Maldonato, B. J., Russell, D. A., & Totah, R. A. (2021). Human METTL7B is an alkyl thiol methyltransferase that metabolizes hydrogen sulfide and captopril. Scientific reports, 11(1), 4857.
Towns, W. L., & Begley, T. J. (2012). Transfer RNA methytransferases and their corresponding modifications in budding yeast and humans: Activities, predications, and potential roles in human health. DNA and cell biology, 31(4), 434–454.
Sieber, J., Wieder, N., Ostrosky-Frid, M., Dvela-Levitt, M., Aygün, O., Udeshi, N. D., et al. (2017). Lysine trimethylation regulates 78-kDa glucose-regulated protein proteostasis during endoplasmic reticulum stress. Journal of Biological Chemistry, 292(46), 18878–18885.
Kernstock, S., Davydova, E., Jakobsson, M., Moen, A., Pettersen, S., Mælandsmo, G. M., et al. (2012). Lysine methylation of VCP by a member of a novel human protein methyltransferase family. Nature Communications, 3, 1038.
Zoabi, M., Zhang, L., Li, T. M., Elias, J. E., Carlson, S. M., & Gozani, O. (2020). Methyltransferase-like 21C (METTL21C) methylates alanine tRNA synthetase at Lys-943 in muscle tissue. Journal of Biological Chemistry, 295(33), 11822–11832.
Le, S. Q., & Gascuel, O. (2008). An improved general amino acid replacement matrix. Molecular biology and evolution, 25(7), 1307–1320.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular biology and evolution, 35(6), 1547–1549.
Campeanu, I. J., Jiang, Y., Liu, L., Pilecki, M., Najor, A., Cobani, E., et al. (2021). Multi-omics integration of methyltransferase-like protein family reveals clinical outcomes and functional signatures in human cancer. Science and Reports, 11(1), 14784.
Chen, J., Wei, X., Yi, X., & Jiang, D. S. (2021). RNA Modification by m(6)A Methylation in Cardiovascular Disease. Oxidative Medicine and Cellular Longevity, 2021, 8813909.
Hao, H., Hao, S., Chen, H., Chen, Z., Zhang, Y., Wang, J., et al. (2019). N6-methyladenosine modification and METTL3 modulate enterovirus 71 replication. Nucleic Acids Research, 47(1), 362–374.
Liu, J., Yue, Y., Han, D., Wang, X., Fu, Y., Zhang, L., et al. (2014). A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nature chemical biology, 10(2), 93–95.
Wang, P., Doxtader, K. A., & Nam, Y. (2016). Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Molecular cell, 63(2), 306–317.
Wang, X., Lu, Z., Gomez, A., Hon, G. C., Yue, Y., Han, D., et al. (2014). N6-methyladenosine-dependent regulation of messenger RNA stability. Nature, 505(7481), 117–120.
Huang, H., Weng, H., Sun, W., Qin, X., Shi, H., Wu, H., et al. (2018). Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nature cell biology, 20(3), 285–295.
Wang, X., Zhao, B. S., Roundtree, I. A., Lu, Z., Han, D., Ma, H., et al. (2015). N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell, 161(6), 1388–1399.
Wang, Q., Guo, X., Li, L., Gao, Z., Su, X., Ji, M., et al. (2020). N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death & Disease, 11(10), 911.
Han, J., Wang, J. Z., Yang, X., Yu, H., Zhou, R., Lu, H. C., et al. (2019). METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Molecular cancer, 18(1), 110.
Wang, Q., Chen, C., Ding, Q., Zhao, Y., Wang, Z., Chen, J., et al. (2020). METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut, 69(7), 1193–1205.
Chen, H., Gao, S., Liu, W., Wong, C. C., Wu, J., Wu, J., et al. (2021). RNA N(6)-Methyladenosine Methyltransferase METTL3 Facilitates Colorectal Cancer by Activating the m(6)A-GLUT1-mTORC1 Axis and Is a Therapeutic Target. Gastroenterology, 160(4), 1284–300.e16.
Cheng, L., Zhang, X., Huang, Y. Z., Zhu, Y. L., Xu, L. Y., Li, Z., et al. (2021). Metformin exhibits antiproliferation activity in breast cancer via miR-483-3p/METTL3/m(6)A/p21 pathway. Oncogenesis, 10(1), 7.
Xia, T., Wu, X., Cao, M., Zhang, P., Shi, G., Zhang, J., et al. (2019). The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathology, research and practice, 215(11), 152666.
Yankova, E., Blackaby, W., Albertella, M., Rak, J., De Braekeleer, E., Tsagkogeorga, G., et al. (2021). Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature, 593(7860), 597–601.
Li, L., Zheng, Y. L., Jiang, C., Fang, S., Zeng, T. T., Zhu, Y. H., et al. (2019). HN1L-mediated transcriptional axis AP-2γ/METTL13/TCF3-ZEB1 drives tumor growth and metastasis in hepatocellular carcinoma. Cell death and differentiation, 26(11), 2268–2283.
He, J., Zhou, M., Yin, J., Wan, J., Chu, J., Jia, J., et al. (2021). METTL3 restrains papillary thyroid cancer progression via m(6)A/c-Rel/IL-8-mediated neutrophil infiltration. Molecular therapy : The journal of the American Society of Gene Therapy, 29(5), 1821–1837.
Liu, J., Eckert, M. A., Harada, B. T., Liu, S. M., Lu, Z., Yu, K., et al. (2018). m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nature cell biology, 20(9), 1074–1083.
Malla, S., Melguizo-Sanchis, D., & Aguilo, F. (2019). Steering pluripotency and differentiation with N(6)-methyladenosine RNA modification. Biochimica et Biophysica Acta, Gene Regulatory Mechanisms, 1862(3), 394–402.
Wang, Y., Li, Y., Toth, J. I., Petroski, M. D., Zhang, Z., & Zhao, J. C. (2014). N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nature cell biology, 16(2), 191–198.
Batista, P. J., Molinie, B., Wang, J., Qu, K., Zhang, J., Li, L., et al. (2014). m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell, 15(6), 707–719.
Aguilo, F., Zhang, F., Sancho, A., Fidalgo, M., Di Cecilia, S., Vashisht, A., et al. (2015). Coordination of m(6)A mRNA Methylation and Gene Transcription by ZFP217 Regulates Pluripotency and Reprogramming. Cell Stem Cell, 17(6), 689–704.
Jin, K. X., Zuo, R., Anastassiadis, K., Klungland, A., Marr, C., Filipczyk, A. (2021). N6-methyladenosine (m(6)A) depletion regulates pluripotency exit by activating signaling pathways in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 118(51), e2105192118.
Kirchner, S., & Ignatova, Z. (2015). Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nature Reviews Genetics, 16(2), 98–112.
Alexandrov, A., Martzen, M. R., & Phizicky, E. M. (2002). Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA (New York, NY), 8(10), 1253–1266.
Cheng, W., Gao, A., Lin, H., & Zhang, W. (2022). Novel roles of METTL1/WDR4 in tumor via m(7)G methylation. Molecular therapy oncolytics, 26, 27–34.
Zhao, Y., Kong, L., Pei, Z., Li, F., Li, C., Sun, X., et al. (2021). m7G Methyltransferase METTL1 Promotes Post-ischemic Angiogenesis via Promoting VEGFA mRNA Translation. Frontiers in cell and developmental biology, 9, 642080.
Alexandrov, A., Grayhack, E. J., & Phizicky, E. M. (2005). tRNA m7G methyltransferase Trm8p/Trm82p: Evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA, 11(5), 821–830.
Alexandrov, A., Chernyakov, I., Gu, W., Hiley, S. L., Hughes, T. R., Grayhack, E. J., et al. (2006). Rapid tRNA decay can result from lack of nonessential modifications. Molecular Cell, 21(1), 87–96.
Ying, X., Liu, B., Yuan, Z., Huang, Y., Chen, C., Jiang, X., et al. (2021). METTL1-m(7) G-EGFR/EFEMP1 axis promotes the bladder cancer development. Clinical and translational medicine, 11(12), e675.
Chen, Z., Zhu, W., Zhu, S., Sun, K., Liao, J., Liu, H., et al. (2021). METTL1 promotes hepatocarcinogenesis via m(7) G tRNA modification-dependent translation control. Clinical and translational medicine, 11(12), e661.
Liu, Y., Zhang, Y., Chi, Q., Wang, Z., & Sun, B. (2020). Methyltransferase-like 1 (METTL1) served as a tumor suppressor in colon cancer by activating 7-methyguanosine (m7G) regulated let-7e miRNA/HMGA2 axis. Life sciences, 249, 117480.
Orellana, E. A., Liu, Q., Yankova, E., Pirouz, M., De Braekeleer, E., Zhang, W., et al. (2021). METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Molecular cell, 81(16), 3323–38.e14.
Dai, Z., Liu, H., Liao, J., Huang, C., Ren, X., Zhu, W., et al. (2021). N(7)-Methylguanosine tRNA modification enhances oncogenic mRNA translation and promotes intrahepatic cholangiocarcinoma progression. Molecular cell, 81(16), 3339–55.e8.
Liao, J., Yi, Y., Yue, X., Wu, X., Zhu, M., Chen, Y., et al. (2022). Methyltransferase 1 is required for nonhomologous end-joining repair and renders hepatocellular carcinoma resistant to radiotherapy. Hepatology (Baltimore, Md). https://doi.org/10.1002/hep.32615
Lin, S., Liu, Q., Lelyveld, V. S., Choe, J., Szostak, J. W., & Gregory, R. I. (2018). Mettl1/Wdr4-Mediated m(7)G tRNA Methylome Is Required for Normal mRNA Translation and Embryonic Stem Cell Self-Renewal and Differentiation. Molecular cell, 71(2), 244–55.e5.
Deng, Y., Zhou, Z., Ji, W., Lin, S., & Wang, M. (2020). METTL1-mediated m(7)G methylation maintains pluripotency in human stem cells and limits mesoderm differentiation and vascular development. Stem cell research & therapy, 11(1), 306.
Trimouille, A., Lasseaux, E., Barat, P., Deiller, C., Drunat, S., Rooryck, C., et al. (2018). Further delineation of the phenotype caused by biallelic variants in the WDR4 gene. Clinical Genetics, 93(2), 374–377.
Shaheen, R., Abdel-Salam, G. M., Guy, M. P., Alomar, R., Abdel-Hamid, M. S., Afifi, H. H., et al. (2015). Mutation in WDR4 impairs tRNA m(7)G46 methylation and causes a distinct form of microcephalic primordial dwarfism. Genome Biology, 16, 210.
van Tran, N., Ernst, F. G. M., Hawley, B. R., Zorbas, C., Ulryck, N., Hackert, P., et al. (2019). The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Research, 47(15), 7719–7733.
Maden, B. E. (1986). Identification of the locations of the methyl groups in 18 S ribosomal RNA from Xenopus laevis and man. Journal of Molecular Biology, 189(4), 681–699.
van Tran, N., Muller, L., Ross, R. L., Lestini, R., Létoquart, J., Ulryck, N., et al. (2018). Evolutionary insights into Trm112-methyltransferase holoenzymes involved in translation between archaea and eukaryotes. Nucleic Acids Research, 46(16), 8483–8499.
Sepich-Poore, C., Zheng, Z., Schmitt, E., Wen, K., Zhang, Z. S., Cui, X. L., et al. (2022). The METTL5-TRMT112 N(6)-methyladenosine methyltransferase complex regulates mRNA translation via 18S rRNA methylation. Journal of Biological Chemistry, 298(3), 101590.
Rong, B., Zhang, Q., Wan, J., Xing, S., Dai, R., Li, Y., et al. (2020). Ribosome 18S m(6)A Methyltransferase METTL5 Promotes Translation Initiation and Breast Cancer Cell Growth. Cell reports, 33(12), 108544.
Han, Y., Du, T., Guo, S., Wang, L., Dai, G., Long, T., et al. (2022). Loss of m(6)A Methyltransferase METTL5 Promotes Cardiac Hypertrophy Through Epitranscriptomic Control of SUZ12 Expression. Frontiers in cardiovascular medicine, 9, 852775.
Liu, X., Ma, H., Ma, L., Li, K., & Kang, Y. (2022). The potential role of methyltransferase-like 5 in deficient mismatch repair of uterine corpus endometrial carcinoma. Bioengineered, 13(3), 5525–5536.
Ignatova, V. V., Stolz, P., Kaiser, S., Gustafsson, T. H., Lastres, P. R., Sanz-Moreno, A., et al. (2020). The rRNA m(6)A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes & Development, 34(9–10), 715–729.
Huang, H., Li, H., Pan, R., Wang, S., Khan, A. A., Zhao, Y., et al. (2022). Ribosome 18S m(6)A methyltransferase METTL5 promotes pancreatic cancer progression by modulating c-Myc translation. International Journal of Oncology, 60(1), 9.
Xing, M., Liu, Q., Mao, C., Zeng, H., Zhang, X., Zhao, S., et al. (2020). The 18S rRNA m(6) A methyltransferase METTL5 promotes mouse embryonic stem cell differentiation. EMBO reports, 21(10), e49863.
Wang, L., Liang, Y., Lin, R., Xiong, Q., Yu, P., Ma, J., et al. (2022). Mettl5 mediated 18S rRNA N6-methyladenosine (m(6)A) modification controls stem cell fate determination and neural function. Genes & diseases, 9(1), 268–274.
Richard, E. M., Polla, D. L., Assir, M. Z., Contreras, M., Shahzad, M., Khan, A. A., et al. (2019). Bi-allelic Variants in METTL5 Cause Autosomal-Recessive Intellectual Disability and Microcephaly. American journal of human genetics, 105(4), 869–878.
Ignatova, V. V., Kaiser, S., Ho, J. S. Y., Bing, X., Stolz, P., Tan, Y. X., et al. (2020). METTL6 is a tRNA m(3)C methyltransferase that regulates pluripotency and tumor cell growth. Science advances, 6(35), eaaz4551.
Xu, L., Liu, X., Sheng, N., Oo, K. S., Liang, J., Chionh, Y. H., et al. (2017). Three distinct 3-methylcytidine (m(3)C) methyltransferases modify tRNA and mRNA in mice and humans. Journal of Biological Chemistry, 292(35), 14695–14703.
Mao, X. L., Li, Z. H., Huang, M. H., Wang, J. T., Zhou, J. B., Li, Q. R., et al. (2021). Mutually exclusive substrate selection strategy by human m3C RNA transferases METTL2A and METTL6. Nucleic Acids Research, 49(14), 8309–8323.
Bolatkan, A., Asada, K., Kaneko, S., Suvarna, K., Ikawa, N., Machino, H., et al. (2022). Downregulation of METTL6 mitigates cell progression, migration, invasion and adhesion in hepatocellular carcinoma by inhibiting cell adhesion molecules. International Journal of Oncology, 60(1), 4.
Shi, Z., Xu, S., Xing, S., Yao, K., Zhang, L., Xue, L., et al. (2019). Mettl17, a regulator of mitochondrial ribosomal RNA modifications, is required for the translation of mitochondrial coding genes. FASEB journal : Official publication of the Federation of American Societies for Experimental Biology, 33(11), 13040–13050.
Xu, X., Duan, S., Yi, F., Ocampo, A., Liu, G. H., & Izpisua Belmonte, J. C. (2013). Mitochondrial regulation in pluripotent stem cells. Cell metabolism, 18(3), 325–332.
Arroyo, J. D., Jourdain, A. A., Calvo, S. E., Ballarano, C. A., Doench, J. G., Root, D. E., et al. (2016). A Genome-wide CRISPR Death Screen Identifies Genes Essential for Oxidative Phosphorylation. Cell metabolism, 24(6), 875–885.
Du, P., Yuan, B., Cao, J., Zhao, J., Ding, L., Chen, L., et al. (2015). Methyltransferase-like 17 physically and functionally interacts with estrogen receptors. IUBMB Life, 67(11), 861–868.
Lentini, J. M., Bargabos, R., Chen, C., Fu, D. (2022). Methyltransferase METTL8 is required for 3-methylcytosine modification in human mitochondrial tRNAs. Journal of Biological Chemistry, 298(4), 101788.
Schöller, E., Marks, J., Marchand, V., Bruckmann, A., Powell, C. A., Reichold, M., et al. (2021). Balancing of mitochondrial translation through METTL8-mediated m(3)C modification of mitochondrial tRNAs. Molecular cell, 81(23), 4810–25.e12.
Kleiber, N., Lemus-Diaz, N., Stiller, C., Heinrichs, M., Mai, M. M., Hackert, P., et al. (2022). The RNA methyltransferase METTL8 installs m(3)C(32) in mitochondrial tRNAs(Thr/Ser(UCN)) to optimise tRNA structure and mitochondrial translation. Nature Communications, 13(1), 209.
Tang, M., Li, Y., Luo, X., Xiao, J., Wang, J., Zeng, X., et al. (2021). Identification of Biomarkers Related to CD8(+) T Cell Infiltration With Gene Co-expression Network in Lung Squamous Cell Carcinoma. Frontiers in cell and developmental biology, 9, 606106.
Gu, H., Do, D. V., Liu, X., Xu, L., Su, Y., Nah, J. M., et al. (2018). The STAT3 Target Mettl8 Regulates Mouse ESC Differentiation via Inhibiting the JNK Pathway. Stem cell reports, 10(6), 1807–1820.
Boyer, L. A., Lee, T. I., Cole, M. F., Johnstone, S. E., Levine, S. S., Zucker, J. P., et al. (2005). Core transcriptional regulatory circuitry in human embryonic stem cells. Cell, 122(6), 947–956.
Chambers, I. (2004). The molecular basis of pluripotency in mouse embryonic stem cells. Cloning and Stem Cells, 6(4), 386–391.
Boeuf, H., Hauss, C., Graeve, F. D., Baran, N., & Kedinger, C. (1997). Leukemia inhibitory factor-dependent transcriptional activation in embryonic stem cells. Journal of Cell Biology, 138(6), 1207–1217.
Niwa, H., Burdon, T., Chambers, I., & Smith, A. (1998). Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes & Development, 12(13), 2048–2060.
Yao, Q. J., Sang, L., Lin, M., Yin, X., Dong, W., Gong, Y., et al. (2018). Mettl3-Mettl14 methyltransferase complex regulates the quiescence of adult hematopoietic stem cells. Cell research, 28(9), 952–954.
Weng, H., Huang, H., Wu, H., Qin, X., Zhao, B. S., Dong, L., et al. (2018). METTL14 Inhibits Hematopoietic Stem/Progenitor Differentiation and Promotes Leukemogenesis via mRNA m(6)A Modification. Cell Stem Cell, 22(2), 191-205.e9.
Yin, R., Chang, J., Li, Y., Gao, Z., Qiu, Q., Wang, Q., et al. (2022). Differential m(6)A RNA landscapes across hematopoiesis reveal a role for IGF2BP2 in preserving hematopoietic stem cell function. Cell Stem Cell, 29(1), 149–59.e7.
Meyer, K. D., Saletore, Y., Zumbo, P., Elemento, O., Mason, C. E., & Jaffrey, S. R. (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell, 149(7), 1635–1646.
Wang, Y., Li, Y., Yue, M., Wang, J., Kumar, S., Wechsler-Reya, R. J., et al. (2018). N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nature neuroscience, 21(2), 195–206.
Wang, C. X., Cui, G. S., Liu, X., Xu, K., Wang, M., Zhang, X. X., et al. (2018). METTL3-mediated m6A modification is required for cerebellar development. PLoS biology, 16(6), e2004880.
Yoon, K. J., Ringeling, F. R., Vissers, C., Jacob, F., Pokrass, M., Jimenez-Cyrus, D., et al. (2017). Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell, 171(4), 877–89.e17.
Gheller, B. J., Blum, J. E., Fong, E. H. H., Malysheva, O. V., Cosgrove, B. D., & Thalacker-Mercer, A. E. (2020). A defined N6-methyladenosine (m(6)A) profile conferred by METTL3 regulates muscle stem cell/myoblast state transitions. Cell death discovery, 6(1), 95.
Wu, Y., Xie, L., Wang, M., Xiong, Q., Guo, Y., Liang, Y., et al. (2018). Mettl3-mediated m(6)A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nature Communications, 9(1), 4772.
Luo, H., Liu, W., Zhang, Y., Yang, Y., Jiang, X., Wu, S., et al. (2021). METTL3-mediated m(6)A modification regulates cell cycle progression of dental pulp stem cells. Stem cell research & therapy, 12(1), 159.
Wu, Z., Shi, Y., Lu, M., Song, M., Yu, Z., Wang, J., et al. (2020). METTL3 counteracts premature aging via m6A-dependent stabilization of MIS12 mRNA. Nucleic Acids Research, 48(19), 11083–11096.
Tooley, C. E., Petkowski, J. J., Muratore-Schroeder, T. L., Balsbaugh, J. L., Shabanowitz, J., Sabat, M., et al. (2010). NRMT is an alpha-N-methyltransferase that methylates RCC1 and retinoblastoma protein. Nature, 466(7310), 1125–1128.
Petkowski, J. J., Schaner Tooley, C. E., Anderson, L. C., Shumilin, I. A., Balsbaugh, J. L., Shabanowitz, J., et al. (2012). Substrate specificity of mammalian N-terminal alpha-amino methyltransferase NRMT. Biochemistry, 51(30), 5942–5950.
Chen, T., Muratore, T. L., Schaner-Tooley, C. E., Shabanowitz, J., Hunt, D. F., & Macara, I. G. (2007). N-terminal alpha-methylation of RCC1 is necessary for stable chromatin association and normal mitosis. Nature cell biology, 9(5), 596–603.
Cai, Q., Fu, L., Wang, Z., Gan, N., Dai, X., & Wang, Y. (2014). alpha-N-methylation of damaged DNA-binding protein 2 (DDB2) and its function in nucleotide excision repair. Journal of Biological Chemistry, 289(23), 16046–16056.
Dai, X., Otake, K., You, C., Cai, Q., Wang, Z., Masumoto, H., et al. (2013). Identification of novel alpha-n-methylation of CENP-B that regulates its binding to the centromeric DNA. Journal of proteome research, 12(9), 4167–4175.
Bonsignore, L. A., Tooley, J. G., Van Hoose, P. M., Wang, E., Cheng, A., Cole, M. P., et al. (2015). NRMT1 knockout mice exhibit phenotypes associated with impaired DNA repair and premature aging. Mechanisms of Ageing and Development, 146–148, 42–52.
Bonsignore, L. A., Butler, J. S., Klinge, C. M., & Schaner Tooley, C. E. (2015). Loss of the N-terminal methyltransferase NRMT1 increases sensitivity to DNA damage and promotes mammary oncogenesis. Oncotarget, 6(14), 12248–12263.
Zhang, J., Song, H., Chen, C., Chen, L., Dai, Y., Sun, P. H., et al. (2021). Methyltransferase-like protein 11A promotes migration of cervical cancer cells via up-regulating ELK3. Pharmacological research, 172, 105814.
Shields, K. M., Tooley, J. G., Petkowski, J. J., Wilkey, D. W., Garbett, N. C., Merchant, M. L., et al. (2017). Select human cancer mutants of NRMT1 alter its catalytic activity and decrease N-terminal trimethylation. Protein science : A publication of the Protein Society, 26(8), 1639–1652.
Catlin, J. P., Marziali, L. N., Rein, B., Yan, Z., Feltri, M. L., & Schaner Tooley, C. E. (2021). Age-related neurodegeneration and cognitive impairments of NRMT1 knockout mice are preceded by misregulation of RB and abnormal neural stem cell development. Cell Death & Disease, 12(11), 1014.
Andrusiak, M. G., Vandenbosch, R., Park, D. S., & Slack, R. S. (2012). The retinoblastoma protein is essential for survival of postmitotic neurons. The Journal of neuroscience : The official journal of the Society for Neuroscience, 32(42), 14809–14814.
Naser, R., Vandenbosch, R., Omais, S., Hayek, D., Jaafar, C., Al Lafi, S., et al. (2016). Role of the Retinoblastoma protein, Rb, during adult neurogenesis in the olfactory bulb. Scientific reports, 6, 20230.
Tooley, J. G., Catlin, J. P., & Schaner Tooley, C. E. (2021). CREB-mediated transcriptional activation of NRMT1 drives muscle differentiation. Transcription, 12(2–3), 72–88.
Hong, A. R., Kim, K., Lee, J. Y., Yang, J. Y., Kim, J. H., Shin, C. S., et al. (2020). Transformation of Mature Osteoblasts into Bone Lining Cells and RNA Sequencing-Based Transcriptome Profiling of Mouse Bone during Mechanical Unloading. Endocrinology and metabolism (Seoul, Korea), 35(2), 456–469.
Faughn, J. D., Dean, W. L., & Schaner Tooley, C. E. (2018). The N-terminal methyltransferase homologs NRMT1 and NRMT2 exhibit novel regulation of activity through heterotrimer formation. Protein science : A publication of the Protein Society, 27(9), 1585–1599.
Averina, O. A., Laptev, I. G., Emelianova, M. A., Permyakov, O. A., Mariasina, S. S., Nikiforova, A. I., et al. (2022). Mitochondrial rRNA Methylation by Mettl15 Contributes to the Exercise and Learning Capability in Mice. International Journal of Molecular Sciences, 23(11), 6056.
Wang, C., Arrington, J., Ratliff, A. C., Chen, J., Horton, H. E., Nie, Y., et al. (2019). Methyltransferase-like 21c methylates and stabilizes the heat shock protein Hspa8 in type I myofibers in mice. Journal of Biological Chemistry, 294(37), 13718–13728.
Wiederstein, J. L., Nolte, H., Günther, S., Piller, T., Baraldo, M., Kostin, S., et al. (2018). Skeletal Muscle-Specific Methyltransferase METTL21C Trimethylates p97 and Regulates Autophagy-Associated Protein Breakdown. Cell reports, 23(5), 1342–1356.
Huang, J., Hsu, Y. H., Mo, C., Abreu, E., Kiel, D. P., Bonewald, L. F., et al. (2014). METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-κB signaling pathway. Journal of bone and mineral research : The official journal of the American Society for Bone and Mineral Research, 29(7), 1531–1540.
Alamgir, M., Eroukova, V., Jessulat, M., Xu, J., & Golshani, A. (2008). Chemical-genetic profile analysis in yeast suggests that a previously uncharacterized open reading frame, YBR261C, affects protein synthesis. BMC Genomics, 9, 583.
Webb, K. J., Lipson, R. S., Al-Hadid, Q., Whitelegge, J. P., & Clarke, S. G. (2010). Identification of protein N-terminal methyltransferases in yeast and humans. Biochemistry, 49(25), 5225–5235.
Chen, H., Gu, L., Orellana, E. A., Wang, Y., Guo, J., Liu, Q., et al. (2020). METTL4 is an snRNA m(6)Am methyltransferase that regulates RNA splicing. Cell research, 30(6), 544–547.
Goh, Y. T., Koh, C. W. Q., Sim, D. Y., Roca, X., & Goh, W. S. S. (2020). METTL4 catalyzes m6Am methylation in U2 snRNA to regulate pre-mRNA splicing. Nucleic Acids Research, 48(16), 9250–9261.
van den Homberg, D. A. L., van der Kwast, R., Quax, P. H. A., Nossent, A. Y. (2022). N-6-Methyladenosine in Vasoactive microRNAs during Hypoxia; A Novel Role for METTL4. International Journal of Molecular Sciences, 23(3), 1057.
Hao, Z., Wu, T., Cui, X., Zhu, P., Tan, C., Dou, X., et al. (2020). N(6)-Deoxyadenosine Methylation in Mammalian Mitochondrial DNA. Molecular cell, 78(3), 382–95.e8.
Greer, E. L., Blanco, M. A., Gu, L., Sendinc, E., Liu, J., Aristizábal-Corrales, D., et al. (2015). DNA Methylation on N6-Adenine in C. elegans. Cell, 161(4), 868–78.
Zhang, Z., Hou, Y., Wang, Y., Gao, T., Ma, Z., Yang, Y., et al. (2020). Regulation of Adipocyte Differentiation by METTL4, a 6 mA Methylase. Scientific reports, 10(1), 8285.
Wang, X., Han, Y., Li, J., Hong, D., Xue, Z., Huang, H., et al. (2021). Multi-omics analysis of copy number variations of RNA regulatory genes in soft tissue sarcoma. Life sciences, 265, 118734.
Malvi, P., Wang, B., Shah, S., & Gupta, R. (2019). Dissecting the role of RNA modification regulatory proteins in melanoma. Oncotarget, 10(38), 3745–3759.
Wang, Z., He, J., Bach, D. H., Huang, Y. H., Li, Z., Liu, H., et al. (2022). Induction of m(6)A methylation in adipocyte exosomal LncRNAs mediates myeloma drug resistance. Journal of experimental & clinical cancer research : CR, 41(1), 4.
Lee, E., Kim, J. Y., Kim, T. K., Park, S. Y., & Im, G. I. (2021). Methyltransferase-like protein 7A (METTL7A) promotes cell survival and osteogenic differentiation under metabolic stress. Cell death discovery, 7(1), 154.
Wang, N., Han, X., Yang, H., Xia, D., & Fan, Z. (2021). miR-6807-5p Inhibited the Odontogenic Differentiation of Human Dental Pulp Stem Cells Through Directly Targeting METTL7A. Frontiers in cell and developmental biology, 9, 759192.
Jun, F., Peng, Z., Zhang, Y., & Shi, D. (2020). Quantitative proteomic analysis identifies novel regulators of methotrexate resistance in choriocarcinoma. Gynecologic Oncology, 157(1), 268–279.
Briata, P., Caputo, L., Zapparoli, E., Marcaccini, E., Passalacqua, M., Brondolo, L., et al. (2022). LncRNA EPR-induced METTL7A1 modulates target gene translation. Nucleic Acids Research, 50(13), 7608–7622.
Cloutier, P., Lavallée-Adam, M., Faubert, D., Blanchette, M., & Coulombe, B. (2014). Methylation of the DNA/RNA-binding protein Kin17 by METTL22 affects its association with chromatin. Journal of proteomics, 100, 115–124.
Han, T., Yang, C. S., Chang, K. Y., Zhang, D., Imam, F. B., & Rana, T. M. (2016). Identification of novel genes and networks governing hematopoietic stem cell development. EMBO reports, 17(12), 1814–1828.
Tahmasebi, S., Amiri, M., & Sonenberg, N. (2018). Translational Control in Stem Cells. Frontiers in Genetics, 9, 709.
Saba, J. A., Liakath-Ali, K., Green, R., & Watt, F. M. (2021). Translational control of stem cell function. Nature reviews Molecular cell biology, 22(10), 671–690.
Liu, Y., Beyer, A., & Aebersold, R. (2016). On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell, 165(3), 535–550.
Sampath, P., Pritchard, D. K., Pabon, L., Reinecke, H., Schwartz, S. M., Morris, D. R., et al. (2008). A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell, 2(5), 448–460.
Bain, G., Kitchens, D., Yao, M., Huettner, J. E., & Gottlieb, D. I. (1995). Embryonic stem cells express neuronal properties in vitro. Developmental biology, 168(2), 342–357.
Dominissini, D., Moshitch-Moshkovitz, S., Schwartz, S., Salmon-Divon, M., Ungar, L., Osenberg, S., et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature, 485(7397), 201–206.
Zhang, C., Chen, Y., Sun, B., Wang, L., Yang, Y., Ma, D., et al. (2017). m(6)A modulates haematopoietic stem and progenitor cell specification. Nature, 549(7671), 273–276.
Mao, Y., Dong, L., Liu, X. M., Guo, J., Ma, H., Shen, B., et al. (2019). m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nature Communications, 10(1), 5332.
Warda, A. S., Kretschmer, J., Hackert, P., Lenz, C., Urlaub, H., Höbartner, C., et al. (2017). Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO reports, 18(11), 2004–2014.
Mendel, M., Chen, K. M., Homolka, D., Gos, P., Pandey, R. R., McCarthy, A. A., et al. (2018). Methylation of Structured RNA by the m(6)A Writer METTL16 Is Essential for Mouse Embryonic Development. Molecular cell, 71(6), 986-1000.e11.
Arcidiacono, O. A., Krejčí, J., Bártová, E. (2020). The Distinct Function and Localization of METTL3/METTL14 and METTL16 Enzymes in Cardiomyocytes. International Journal of Molecular Sciences, 21(21), 8139.
Wang, X. K., Zhang, Y. W., Wang, C. M., Li, B., Zhang, T. Z., Zhou, W. J., et al. (2021). METTL16 promotes cell proliferation by up-regulating cyclin D1 expression in gastric cancer. Journal of cellular and molecular medicine, 25(14), 6602–6617.
Su, R., Dong, L., Li, Y., Gao, M., He, P. C., Liu, W., et al. (2022). METTL16 exerts an m(6)A-independent function to facilitate translation and tumorigenesis. Nature cell biology, 24(2), 205–216.
Chen, R., Zhou, J., Liu, L., Mao, X. L., Zhou, X., & Xie, W. (2021). Crystal structure of human METTL6, the m(3)C methyltransferase. Communications biology, 4(1), 1361.
Lou, S., Meng, F., Yin, X., Zhang, Y., Han, B., & Xue, Y. (2021). Comprehensive Characterization of RNA Processing Factors in Gastric Cancer Identifies a Prognostic Signature for Predicting Clinical Outcomes and Therapeutic Responses. Frontiers in immunology, 12, 719628.
Malecki, J., Aileni, V. K., Ho, A. Y. Y., Schwarz, J., Moen, A., Sørensen, V., et al. (2017). The novel lysine specific methyltransferase METTL21B affects mRNA translation through inducible and dynamic methylation of Lys-165 in human eukaryotic elongation factor 1 alpha (eEF1A). Nucleic Acids Research, 45(8), 4370–4389.
Jakobsson, M. E., Małecki, J. M., Halabelian, L., Nilges, B. S., Pinto, R., Kudithipudi, S., et al. (2018). The dual methyltransferase METTL13 targets N terminus and Lys55 of eEF1A and modulates codon-specific translation rates. Nature Communications, 9(1), 3411.
Shimazu, T., Barjau, J., Sohtome, Y., Sodeoka, M., & Shinkai, Y. (2014). Selenium-based S-adenosylmethionine analog reveals the mammalian seven-beta-strand methyltransferase METTL10 to be an EF1A1 lysine methyltransferase. PLoS One 9(8), e105394.
Li, Z., Gonzalez, P. A., Sasvari, Z., Kinzy, T. G., & Nagy, P. D. (2014). Methylation of translation elongation factor 1A by the METTL10-like See1 methyltransferase facilitates tombusvirus replication in yeast and plants. Virology, 448, 43–54.
Hamey, J. J., Wienert, B., Quinlan, K. G. R., & Wilkins, M. R. (2017). METTL21B Is a Novel Human Lysine Methyltransferase of Translation Elongation Factor 1A: Discovery by CRISPR/Cas9 Knockout. Molecular & cellular proteomics : MCP, 16(12), 2229–2242.
Oulhen, N., Swartz, S. Z., Laird, J., Mascaro, A., & Wessel, G. M. (2017). Transient translational quiescence in primordial germ cells. Development (Cambridge, England), 144(7), 1201–1210.
Wang, X., Li, K., Wan, Y., Chen, F., Cheng, M., Xiong, G., et al. (2021). Methyltransferase like 13 mediates the translation of Snail in head and neck squamous cell carcinoma. International journal of oral science, 13(1), 26.
Shu, X., Li, X., Xiang, X., Wang, Q., & Wu, Q. (2021). METTL21B is a prognostic biomarker and potential therapeutic target in low-grade gliomas. Aging (Albany NY), 13(16), 20661–20683.
Liu, S., Hausmann, S., Carlson, S. M., Fuentes, M. E., Francis, J. W., Pillai, R., et al. (2019). METTL13 Methylation of eEF1A Increases Translational Output to Promote Tumorigenesis. Cell, 176(3), 491-504.e21.
Liu, Z., Sun, T., Piao, C., Zhang, Z., & Kong, C. (2021). METTL13 inhibits progression of clear cell renal cell carcinoma with repression on PI3K/AKT/mTOR/HIF-1α pathway and c-Myc expression. Journal of translational medicine, 19(1), 209.
Zhang, Z., Zhang, G., Kong, C., Zhan, B., Dong, X., & Man, X. (2016). METTL13 is downregulated in bladder carcinoma and suppresses cell proliferation, migration and invasion. Scientific reports, 6, 19261.
Intlekofer, A. M., & Finley, L. W. S. (2019). Metabolic signatures of cancer cells and stem cells. Nature metabolism, 1(2), 177–188.
Zhang, W., & Liu, H. T. (2002). MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell research, 12(1), 9–18.
Jiang, Q. G., Xiong, C. F., & Lv, Y. X. (2021). Kin17 facilitates thyroid cancer cell proliferation, migration, and invasion by activating p38 MAPK signaling pathway. Molecular and cellular biochemistry, 476(2), 727–739.
Zheng, Q., Yang, Q., Zhou, J., Gu, X., Zhou, H., Dong, X., et al. (2021). Immune signature-based hepatocellular carcinoma subtypes may provide novel insights into therapy and prognosis predictions. Cancer cell international, 21(1), 330.
Lv, M., Cao, D., Zhang, L., Hu, C., Li, S., Zhang, P., et al. (2021). METTL9 mediated N1-histidine methylation of zinc transporters is required for tumor growth. Protein & cell, 12(12), 965–970.
Chen, H., Shi, Z., Guo, J., Chang, K. J., Chen, Q., Yao, C. H., et al. (2020). The human mitochondrial 12S rRNA m(4)C methyltransferase METTL15 is required for mitochondrial function. Journal of Biological Chemistry, 295(25), 8505–8513.
Van Haute, L., Hendrick, A. G., D’Souza, A. R., Powell, C. A., Rebelo-Guiomar, P., Harbour, M. E., et al. (2019). METTL15 introduces N4-methylcytidine into human mitochondrial 12S rRNA and is required for mitoribosome biogenesis. Nucleic Acids Research, 47(19), 10267–10281.
Rhein, V. F., Carroll, J., He, J., Ding, S., Fearnley, I. M., & Walker, J. E. (2014). Human METTL20 methylates lysine residues adjacent to the recognition loop of the electron transfer flavoprotein in mitochondria. Journal of Biological Chemistry, 289(35), 24640–24651.
Conner, M. M., Parker, H. V., Falcone, D. R., Chung, G., Schaner Tooley, C. (2022). Novel regulation of the transcription factor ZHX2 by N-terminal methylation. Transcription, 13(1–3), 1–15.
Davydova, E., Shimazu, T., Schuhmacher, M. K., Jakobsson, M. E., Willemen, H., Liu, T., et al. (2021). The methyltransferase METTL9 mediates pervasive 1-methylhistidine modification in mammalian proteomes. Nature Communications, 12(1), 891.
Małecki, J., Jakobsson, M. E., Ho, A. Y. Y., Moen, A., Rustan, A. C., & Falnes, P. (2017). Uncovering human METTL12 as a mitochondrial methyltransferase that modulates citrate synthase activity through metabolite-sensitive lysine methylation. Journal of Biological Chemistry, 292(43), 17950–17962.
Wu, D., Li, M., Tian, W., Wang, S., Cui, L., Li, H., et al. (2017). Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Scientific reports, 7(1), 5134.
Fu, R., Luo, X., Ding, Y., Guo, S. (2022). Prognostic Potential of METTL7B in Glioma. Neuroimmunomodulation, 29(3), 186–201.
Ye, D., Jiang, Y., Sun, Y., Li, Y., Cai, Y., Wang, Q., et al. (2019). METTL7B promotes migration and invasion in thyroid cancer through epithelial-mesenchymal transition. Journal of molecular endocrinology, 63(1), 51–61.
Li, W., Xu, S., Peng, N., Zhang, Z., He, H., Chen, R., et al. (2021). Downregulation of METTL7B Inhibits Proliferation of Human Clear Cell Renal Cancer Cells In Vivo and In Vitro. Frontiers in oncology, 11, 634542.
McKinnon, C. M., & Mellor, H. (2017). The tumor suppressor RhoBTB1 controls Golgi integrity and breast cancer cell invasion through METTL7B. BMC Cancer, 17(1), 145.
Liu, D., Li, W., Zhong, F., Yin, J., Zhou, W., Li, S., et al. (2020). METTL7B Is Required for Cancer Cell Proliferation and Tumorigenesis in Non-Small Cell Lung Cancer. Frontiers in pharmacology, 11, 178.
Kessenbrock, K., Wang, C. Y., & Werb, Z. (2015). Matrix metalloproteinases in stem cell regulation and cancer. Matrix biology : Journal of the International Society for Matrix Biology, 44–46, 184–190.
Voskas, D., Ling, L. S., & Woodgett, J. R. (2014). Signals controlling un-differentiated states in embryonic stem and cancer cells: Role of the phosphatidylinositol 3’ kinase pathway. Journal of cellular physiology, 229(10), 1312–1322.
de Laat, S. W., Boonstra, J., Defize, L. H., Kruijer, W., van der Saag, P. T., Tertoolen, L. G., et al. (1999). Growth factor signalling. The International journal of developmental biology, 43(7), 681–691.
He, L., Li, H., Wu, A., Peng, Y., Shu, G., & Yin, G. (2019). Functions of N6-methyladenosine and its role in cancer. Molecular cancer, 18(1), 176.
Ma, C., Ma, R. J., Hu, K., Zheng, Q. M., Wang, Y. P., Zhang, N., et al. (2022). The molecular mechanism of METTL3 promoting the malignant progression of lung cancer. Cancer cell international, 22(1), 133.
Li, T. H., Qin, C., Zhao, B. B., Cao, H. T., Yang, X. Y., Wang, Y. Y., et al. (2021). Identification METTL18 as a Potential Prognosis Biomarker and Associated With Immune Infiltrates in Hepatocellular Carcinoma. Frontiers in oncology, 11, 665192.
Zhang, R., Shang, L., Nan, J., Niu, K., Dai, J., Jin, X., et al. (2022). Circ-METTL15 contributes to the proliferation, metastasis, immune escape and restrains apoptosis in lung cancer by regulating miR-1299/PDL1 axis. Autoimmunity, 55(1), 8–20.
Małecki, J. M., Odonohue, M. F., Kim, Y., Jakobsson, M. E., Gessa, L., Pinto, R., et al. (2021). Human METTL18 is a histidine-specific methyltransferase that targets RPL3 and affects ribosome biogenesis and function. Nucleic Acids Research, 49(6), 3185–3203.
Hong, Y. H., Aziz, N., Park, J. G., Lee, D., Kim, J. K., Kim, S. A., et al. (2022). The EEF1AKMT3/MAP2K7/TP53 axis suppresses tumor invasiveness and metastasis in gastric cancer. Cancer letters, 544, 215803.
Catlin, J. P., Marziali, L. N., Rein, B., Yan, Z., Feltri, M. L., & Schaner Tooley, C. E. (2021). Age-related neurodegeneration and cognitive impairments of NRMT1 knockout mice are preceded by misregulation of RB and abnormal neural stem cell development. Cell death & disease, 12(11), 1014.
Grozdanov, P. N., Yovchev, M. I., & Dabeva, M. D. (2006). The oncofetal protein glypican-3 is a novel marker of hepatic progenitor/oval cells. Laboratory investigation; a journal of technical methods and pathology, 86(12), 1272–84.
Huang, M. H., Peng, G. X., Mao, X. L., Wang, J. T., Zhou, J. B., Zhang, J. H., et al. (2022). Molecular basis for human mitochondrial tRNA m3C modification by alternatively spliced METTL8. Nucleic Acids Research, 50(7), 4012–4028.
Acknowledgements
The authors thank Meghan Conner and Haley Parker for critical reading of the manuscript.
Funding
This work was supported by a research grant from the National Institutes of Health, USA, to C.E.S.T [R35GM144111].
Author information
Authors and Affiliations
Contributions
JGT and CST co-wrote the majority of the manuscript. JC wrote the sections on neural stem cells. CST created the figures.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tooley, J.G., Catlin, J.P. & Tooley, C.E.S. METTLing in Stem Cell and Cancer Biology. Stem Cell Rev and Rep 19, 76–91 (2023). https://doi.org/10.1007/s12015-022-10444-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10444-7