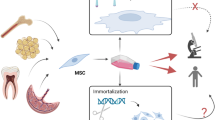
Abstract
Mesenchymal stromal cells (MSCs) are promising candidates for cell-based therapies, mainly due to their unique biological properties such as multipotency, self-renewal and trophic/immunomodulatory effects. However, clinical use has proven complex due to limitations such as high variability of MSCs preparations and high number of cells required for therapies. These challenges could be circumvented with cell immortalization through genetic manipulation, and although many studies show that such approaches are safe, little is known about changes in other biological properties and functions of MSCs. In this study, we evaluated the impact of MSCs immortalization with the TERT gene on the purinergic system, which has emerged as a key modulator in a wide variety of pathophysiological conditions. After cell immortalization, MSCs-TERT displayed similar immunophenotypic profile and differentiation potential to primary MSCs. However, analysis of gene and protein expression exposed important alterations in the purinergic signaling of in vitro cultured MSCs-TERT. Immortalized cells upregulated the CD39/NTPDase1 enzyme and downregulated CD73/NT5E and adenosine deaminase (ADA), which had a direct impact on their nucleotide/nucleoside metabolism profile. Despite these alterations, adenosine did not accumulate in the extracellular space, due to increased uptake. MSCs-TERT cells presented an impaired in vitro immunosuppressive potential, as observed in an assay of co-culture with lymphocytes. Therefore, our data suggest that MSCs-TERT have altered expression of key enzymes of the extracellular nucleotides/nucleoside control, which altered key characteristics of these cells and can potentially change their therapeutic effects in tissue engineering in regenerative medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stromal cells (MSCs) represent a subject of great interest for therapeutic applications in a wide array of pathological conditions, such as tissue injury syndromes, degenerative disorders and immune diseases [1]. MSCs have unique biological properties, such as multipotency, self-renewal capacity and paracrine activity, especially trophic and immunomodulatory effects [2, 3], along with the ability to migrate to injured sites [4]. Altogether, these characteristics make MSCs suitable for cell-based therapies, either to replace dead or defective cells or to act as delivery agents for genes and drugs that need to be carried to the damaged tissues. Our group has addressed some of the major aspects of MSCs biology in previous works, investigating the impact of these cells in tumor cells and in tissue repair [5,6,7,8,9,10,11].
However, despite the enthusiasm based on these potentials, the translation of MSCs to the clinic has proven more complex than previously thought. More than 1000 clinical trials using MSCs have been performed (http://clinicaltrials.gov, search date 4/01/2020), according to data collected by NIH, and most of these trials are still in phases I and II with few conclusive results [1, 12]. One of the main hurdles in the way to the clinic relates to the high variability of MSC preparations, that can be associated to the different sources from which they are obtained, the methods of isolation and culture conditions used to maintain the cells, and even to donor characteristics such as age and gender [12,13,14].
Furthermore, as the number of cells required for therapies is usually high, another challenge in the use of MSCs in clinical applications involves their limited lifespan for ex-vivo expansion. Despite their initial good proliferative rates in culture, MSCs significantly decrease cell proliferation and undergo replicative senescence [15,16,17]. A possible solution to circumvent both MSCs heterogeneity and limited expansion issues is cell immortalization through genetic manipulation. To this end, several methods have been described, of which the most common is the introduction of specific genes to the target cell genome, such as the viral early region genes of simian virus 40 (SV40) [18, 19], the E6/E7 oncogenes of human papilloma-virus (HPV) [20] and the telomerase gene (TERT) [21,22,23].
Most of the reports in the literature show that such approaches are safe (non-tumorigenic) and that immortalized MSCs present high proliferation rates while maintaining their differentiation capacity and expression of the same markers of their non-immortalized counterparts [24,25,26,27,28]. Nevertheless, very few studies run side by side experiments to compare immortalized and primary MSCs, and there is still limited information regarding alterations in biological properties and function of MSCs other than differentiation potential and surface marker expression. It is becoming more clear, however, that the introduction of genes such as TERT into MSCs may exert effects on cells beyond extension of proliferative potential [29]. In this scenario, the present work aimed to immortalize murine MSCs and compare their behavior and biological features to primary MSCs in order to determine whether this approach is worth pursuing in therapeutic applications.
More specifically, here we investigated the purinergic system, which has emerged as a key modulator in a wide variety of pathophysiological functions [30]. This system comprises signaling mediated mainly by extracellular nucleotides / nucleosides - purines (adenosine, AMP, ADP, and ATP) and pyrimidines (uridine, UMP, UDP and UTP) - that are controlled by a wide range of enzymes, including E-NTPDases (ecto-nucleoside triphosphate-diphosphohydrolases), E-NPPs (ecto-nucleotide pyrophosphatase / phosphodiesterases), alkaline phosphatase, NT5E (Ecto-5′-nucleotidase / CD73) and ADA (adenosine deaminase). In the context, CD39/NTPDase1 enzyme, which degrades ATP and ADP, and the CD73/NT5E enzyme, one of the MSCs surface markers that degrades AMP to adenosine, are the main metabolizing enzymes. These enzymes are expressed in different types of MSCs and, through the control of extracellular nucleotides levels, they can interfere with proliferation and migration rates, cell fate, immunosuppressive properties, and other important MSCs functions [31,32,33,34]. For instance, it has already been shown that ATP decreases the proliferation of MSCs through P2Y1 receptors [35] and also downregulates the levels of genes involved in proliferation, while upregulates genes involved in cell migration and potentiates their chemotactic response to CXCL12 [34]. In addition, ATP appears to stimulate adipogenic differentiation of MSCs through P2Y1 and P2Y4 receptors, while adenosine contributes to osteogenic differentiation by engaging A2B receptors [36].
Our results demonstrate that although TERT-immortalized MSCs are able to maintain their differentiation capacity as well as the expression of MSC-related cell surface markers, the in vitro nucleotide metabolism profile is altered. Changes in the expression of purinergic enzymes, and in adenosine uptake, resulted in less nucleoside accumulation in the extracellular space, which may have influenced the reduction of the MSCs-TERT immunosuppressive potential. In this way, these data have important implications for the use of immortalized MSCs in experimental research and on its way to clinical settings.
Materials and Methods
Isolation and Culture of Adipose-Derived Mesenchymal Stromal Cells
Adipose-derived mesenchymal stromal cells (MSCs) were extracted from adipose tissue of Wistar rats (6–8 weeks). Briefly, the abdominal fat tissue was washed with phosphate-buffered saline (PBS) and fragments were digested with collagenase type I (2 mg/mL) in a water bath at 37 °C for 45 min. Next, the sample was diluted by addition of an equal volume of Dulbecco’s Modified Eagle Medium Low Glucose (DMEM-LG) (Sigma-Aldrich, MO, USA) supplemented with 10% of fetal bovine serum (FBS), centrifuged for 5 min at 500 g and the supernatant was discarded. Cells were resuspended in complete medium (DMEM-LG + 10% FBS supplemented with 1.0% penicillin / streptomycin and 0.1% amphotericin B) (Sigma-Aldrich, MO, USA), seeded in six-well dishes and kept at a temperature of 37 °C with humidity of 95%/5% CO2 in air. All the experimental procedures were performed according to institutional guidelines and were approved by the Animal Use Ethics committee (CEUA) from UFCSPA at number 166/15.
Retroviral-Mediated Transduction of MSCs with TERT Vector
Retroviruses were generated by co-transfecting the pBABE-neo-hTERT (Addgene plasmid, #1774) [37] together with the helper plasmids pRSVREV, pVSVG and pMDLgRRE in sub-confluent HEK293, as described previously [38]. The media containing recombinant retroviruses were collected, filtered through 0.45 μm membranes, mixed with polybrene up to 9 μg/mL (Sigma-Aldrich, MO, USA) and used to gene transfer on MSCs. Twenty-four hours posttransduction, transfected MSCs (MSCs-TERT) were selected using 1 mg/mL Geneticin Sulfate (G418) (Sigma-Aldrich, MO, USA) for two weeks and cultured as described above.
RNA Isolation, cDNA Synthesis and RT-qPCR
Total RNA was extracted with Trizol® Reagent (Invitrogen, Carlsbad, CA) and reverse transcribed with the M-MLV reverse transcriptase (Promega, Madison, WI) and using random and oligo-dT primers. mRNA levels for target genes were detected by real-time quantitative PCR (RT-qPCR) using the primer sets detailed in Table S1 and the Actb gene was used as reference gene. Reactions were performed in 12.50 μl final volume containing 6.25 μl SYBR™ Select Master Mix (Applied Biosystems). RT-qPCR was performed on a StepOne Plus System (Applied Biosystems) and the amplification program consisted of an initial denaturation step at 50 °C for 2 min, initial denaturation 94 °C for 5 min, then 40 cycles at 94 °C for 30 s, 60 °C for 30 s, 72 °C for 45 s; and 72 °C for 15 min, and the melting curves were performed at incrementing temperatures of 0.3 °C, from 70 °C to 95 °C to generate a denaturation curve of amplified products. Relative quantification of mRNA expression was performed based on amplification of a standard curve method with five successive ten-fold dilution points of a pool of cDNA samples. In Fig. 1, the relative gene expression was determined using the formula 10,000/2-ΔCt, as described previously [39].
Characterization of the TERT-immortalized MSCs. a Rat primary MSCs were stably transfected with the TERT gene (MSCs-TERT) and its insertion confirmed by RT-qPCR, as demonstrated by representative agarose gel and melting curve peak chart collected using the StepOnePlusTM (Applied Biosystems) (n = 3). The TERT gene was not detected in non-immortalized MSCs (non-detected = ND). mRNA levels were normalized by Actb gene expression. Results are expressed as mean ± SD. b MSCs-TERT and primary MSCs cell proliferation were evaluated by cumulative population doubling (cPD). Cells were expanded for 84 days and a significant increase was detected in MSCs-TERT proliferation potential. The experiment was carried out in triplicate (* P < 0.05). c At the end of cPD, both cells were evaluated to senescence signals. Representative photomicrographs performed after staining with β-galactosidase solution demonstrate blue color, which is characteristic of cellular senescence, only in non-immortalized MSCs (×40 magnification)
Proliferation and Cellular Senescence Assay
To measure proliferation rates in a long-term culture, cells were evaluated by the cumulative population doublings (cPD) method. Cells were plated in 6 well-microplates at a concentration of 2 × 104 cells/well and, when reached cellular confluence, were trypsinized and counted with Trypan blue (Invitrogen, Carlsbad, CA). Population doublings (PD) were determined according to the formula PD = log2 (Nf) – log2 (Ni), in which Nf is the number of cells per well at the time of passage and Ni is the number of cells seeded at the previous passage. The sum of PDs was then plotted against time of culture. Cells were followed for approximately 3 months.
Cell senescence was determined using the Senescence Cells Histochemical Staining Kit (Sigma-Aldrich, MO, USA), following the manufacturer’s recommendations.
MSCs and MSCs-TERT Immunophenotyping
Immunophenotypic characterization to evaluate the MSCs markers profile was performed by flow cytometry using the following antibodies: CD90-PERCP, CD29-PE (Molecular Probe, Waltham, MA), CD45-PE and CD11b-FITC (Invitrogen, Carlsbad, CA) (dilution 1:200). To characterize the purinergic system enzymes on the cell surface, the following antibodies were used: Rabbit or Guinea Pig anti-rat NTPDase1 (rN1-6LI5), NTPDase2 (rN2-6 L), NTPDase3 (rN3-1LI5), CD73 (rNu-9LI5) and NPP1 (mNPP1-2cL5) (dilution 1:100) (http://ectonucleotidases-ab.com).
For staining, 1 × 106 cells (MSCs or MSCs-TERT) were dissociated with trypsin, washed once with PBS, centrifuged, incubated for 60 min at 4 °C with the antibodies and washed twice with PBS. For primary antibodies, after washing with PBS, cells were incubated with anti-rabbit or Guinea Pig secondary antibody Alexa Fluor 488 (Invitrogen, Carlsbad, CA) for 30 min, with a minimum of two washes after each incubation.
In all analysis, at least 10,000 events were acquired on a FACS Calibur flow cytometer (Becton-Dickinson, Mountain View, CA). The following excitation (Ex) / emission (Em) wavelengths were used: PERCP (Ex: 482 nm / Em: 695 nm), PE (Ex: 496 nm / Em: 578 nm), FITC (Ex: 494 nm / Em: 520 nm) and Alexa fluor 488 (Ex: 495 nm / Em: 519 nm).
For cellular analysis, unlabeled cells or labeled only with the secondary antibody were used as a negative control and acquired in order to set the forward and side scatter parameters to center the cell population on the scatter plot, excluding dead cells and doublets. Fluorescence intensity was adjusted to set negative control cells within 100–101 on the log scale axis and cells were then acquired with an event count set. Cells were considered as positive when fluorescence intensity was above the maximum fluorescence of the negative control, and reported as percentage of positive cells, or used geometric mean fluorescence intensity (MFI). Data analysis and representative histograms were created using FlowJo V10 software.
The cell size of MSCs and MSCs-TERT was evaluated by measurement of forward scatter (FSC) from flow cytometry data. In order to normalize the expression levels of purinergic enzymes considering cell size, the ratio between fluorescence intensity and forward scatter (FSC) values was calculated for individual cells.
MSCs and MSCs-TERT Differentiation
After cell transduction, both MSCs and MSCs-TERT were induced to differentiate into adipogenic and osteogenic lineages, as previously described [6]. Briefly, cells were plated at a density of approximately 3 × 104 cells in 24-well plates and cultured with osteogenic or adipogenic medium. For the adipogenic differentiation, medium consisted of high-glucose DMEM (DMEM-HG) + 10% FBS, 10 μM dexamethasone, 200 mM indomethacin and 10 μg/mL insulin and the cells were cultured for 21 days. Next, samples were fixed in 3.7% (w/v) formaldehyde and photographed to identify lipid vacuoles. For the osteogenic differentiation, medium consisted of minimal alpha essential medium (αMEM), 10% FBS, 0.1 μM dexamethasone, 200 μM ascorbic acid, 10 mM β-glycerophosphate and the cells were cultured for 30 days. After, cells were fixed in 3.7% (w/v) formaldehyde, washed once with PBS, and incubated with alizarin red (40 mM, pH 4.2) for 45 min in the dark to stain calcium deposits.
All experiments were analyzed by phase microscopy using an inverted microscope Olympus IX51 equipped with a digital camera Olympus DP71®.
Immunocytochemistry
For the CD73 staining, cells were seeded on coverslips, cultured for 48 h and fixed with 4% paraformaldehyde solution in PBS for 30 min at 4 °C. Next, cells were stained with Rabbit anti-rat CD73 (rNu-9LI5) (1:200) (http://ectonucleotidases-ab.com) for 60 min, followed by the anti-rabbit secondary antibody Alexa Fluor 488 for 30 min, with a minimum of two washes after each incubation. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probe, Waltham, MA) for 20 min at 4 °C in a dark room. Images were obtained with inverted microscope Olympus IX51 equipped with a digital camera Olympus DP71®.
Ecto-Nucleotidases Assay
To determine the activity of ecto-nucleotidases in MSCs and MSCs-TERT, cells were cultured in 24-well microplates until confluence and washed three times with incubation medium (IM) containing (final concentration) 120 mM NaCl, 5 mM KCl, 10 mM glucose, 20 mM Hepes and 2 mM CaCl2 (for ATP and ADP) or 2 mM MgCl2 (for AMP) (Sigma-Aldrich, MO, USA) (pH 7.4). The reaction was started by the addition of 200 μL of the IM containing ATP, ADP or AMP (1 mM) (Sigma-Aldrich, MO, USA) for 20 min at 37 °C [40]. To stop the reaction, an aliquot of the cell incubation medium (150 μL) was transferred to a tube containing trichloroacetic acid (TCA) to a final concentration of 5% (w/v). The production of inorganic phosphate (Pi) was measured using the Malachite green method [41]. To determine the spontaneous hydrolysis of nucleotides during the incubation, the medium was incubated with its respective nucleotide without cells. Cells in the 24 well-microplates were dried and solubilized with 100 μL of NaOH 1 N and the protein was measured by the Coomassie blue method [42], using bovine serum albumin as standard. ATP, ADP and AMP hydrolysis are expressed as nmol Pi/min/mg protein.
The profile of extracellular nucleotide metabolism was also analyzed by HPLC. Cells were cultivated as described above and incubated with ATP or AMP in a final concentration of 100 μM at 37 °C. Aliquots (150 μL) of the samples were collected at different times of incubation (0, 10, 30, 60, 120 and 180 min) and the reaction was stopped on ice. All samples were centrifuged at 16,000 g in a refrigerated centrifuge at 4 °C for 15 min and frozen at −80 °C. To investigate the uptake of adenosine by cells, MSCs and MSCs-TERT were previously treated with 10 μM dipyridamole, a pharmacological inhibitor of adenosine transport, 30 min before AMP exposure and the assay was performed as described above.
Aliquots of 20 μL were applied to a reverse phase HPLC system using a C18 Shimadzu column (Shimadzu, Japan) with absorbance measured at 250 nm. The mobile phase was 60 mM KH2PO4, 5 mM tetrabutylammonium chloride, pH 5.0, in 30% methanol (Sigma-Aldrich, MO, USA) as described [40]. Retention times were assessed using standard samples of nucleotides and nucleoside, and concentrations are expressed as μmol of nucleotide.
Adenosine Deaminase (ADA) Activity
ADA activity in MSCs and MSCs-TERT was determined spectrophotometrically using the colorimetric method described by Giusti&Galanti [43], which is based on the indirect measurement of the formation of ammonia, using adenosine as a substrate. Briefly, cells were cultured in 24-well microplates until confluence, washed with PBS and incubated with sodium phosphate buffer (pH 7,4) for 10 min. Next, 2.5 mM adenosine was added and incubated for 60 min at 37 °C. To stop the reaction, 500 μL of “Solution A” (50.4 mg/mL phenol +0.4 mg/mL Sodium nitroprusside) and 500 μL of “Solution B” (0.125% NaClO +0.6 M NaOH) were added, respectively. The samples were incubated for 15 min at 37 °C and read in a spectrophotometer (635 nm). Ammonia was used as standard and the results are expressed as nmol NH3/min/mg protein.
T Cell Suppression Assay
To evaluate the capacity of MSCs and MSCs-TERT to modulate lymphocyte proliferation, 1 × 105 cells were seeded into 24-well plates overnight. Next, Jurkat cells were incubated with 5 μM of carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) for 15 min and washed twice with PBS. 1 × 105 Jurkat cells were co-cultured with MSCs or MSCs-TERT (ratio of 1:1) for a period of 5 days in RPMI-1640 complete medium supplemented with 2 μg/mL phytohemagglutinin-M (PHA-M) (Invitrogen, Carlsbad, CA). Fluorescent Jurkat cells were analyzed in FACS Calibur flow cytometer (Becton-Dickinson, Mountain View, CA).
Statistical Analysis
Student’s T-test was performed to compare two groups while one-way ANOVA with post-hoc Tukey was used to compare three or more groups. Results were considered significant when p < 0.05 (*), p < 0.001 (**) and p < 0.0001 (***).
Results
Establishment and Characterization of MSCs Immortalized with the TERT Gene
MSCs cultures were obtained from rat adipose tissue and characterized before the cellular transduction to confirm their MSCs profile, as already published [44]. To extend the life span of these cells, the TERT gene was transduced into MSCs by retroviruses and its expression was confirmed by RT-qPCR analysis, whereas non-immortalized MSCs were negative to the TERT gene (Fig. 1a). After immortalization, both cells (MSCs and MSCs-TERT) were expanded for about 3 months and a significant difference was detected in proliferation potential, in which non-immortalized MSCs exhibited slower growth when compared to MSCs-TERT (Fig. 1b). At the end of the assay, cellular senescence features, such as irregular morphology, larger cell size and granular cytoplasm, could be observed in MSCs, but not in MSCs-TERT, which was confirmed by the presence of higher activity of the acidic β-galactosidase (Fig. 1c).
In addition, MSCs-TERT maintained their in vitro characteristics of MSCs, such as differentiation potential into osteogenic and adipogenic lineages (Fig. 2A), expression of MSCs markers (CD90 and CD29) and absence of hematopoietic surface markers (CD11b and CD45) (Fig. 2B).
Differentiation potential and MSCs markers expression after cell immortalization. A MSCs and MSCs-TERT were induced to differentiate to osteogenic or adipogenic lines. Osteogenic induction was characterized by alizarin red staining (c-g) and adipogenic induction by the presence of lipid vacuoles (d-h), as observed by representative photomicrographs (×40 magnification). Control cells did not show any differentiation (a, b, e and f). B The representative histograms of flow cytometry showing the immunophenotypic profile of MSCs: White histograms represent isotype controls
Immortalized MSCs Display Changes in Expression of the CD39/NTPDase1 and CD73/NT5E Enzymes
The purinergic signaling plays important roles in key mechanisms of MSCs, such as potentiating the chemotactic response to the chemokine CXCL12 and increasing their migration [34, 45, 46], immunosuppression [47, 48] and stem cell maintenance and differentiation [49, 50]. Thus, we evaluated the expression of the purinergic enzymes after cell immortalization. As shown in Fig. 3a, both cells exhibited gene expression of Entpd1, Entpd5, Entpd6, Nt5e, Npp1 and Npp3. Interestingly, MSCs-TERT showed significant upregulation of Entpd1 and downregulation of Nt5e, genes that encode two of the major enzymes involved in ATP degradation and adenosine (ADO) formation, CD39/NTPDase1 and CD73/NT5E, respectively. Entpd2, Entpd3, Entpd8 and Npp2 genes were not detectable by RT-qPCR.
Expression of genes involved in the metabolism of extracellular nucleotides and nucleosides. a Quantitative RT-qPCR showed significative alterations in gene expression of two of the main enzymes of the purinergic system, Entpd1 and Nt5e (n = 6), while Entpd 2, 3, 8 and Npp2 genes were not detected (ND) (n = 3). mRNA levels were normalized by Actb gene expression and results are expressed as mean ± SEM. b Representative histograms of flow cytometry demonstrating absence of NTPDase2 and NTPDase3 enzymes and presence of CD73/NT5E marker in both cells, while CD39/NTPDase1 immunoreactivity was increased in MSCs-TERT. c Analysis of flow cytometry data confirmed a significant increase in the percentage of total cells expressing CD39/NTPDase1 marker, but no difference in the medium fluorescence intensity (MFI) between them (n = 4). d For CD73 marker, both cell populations exhibited high percentage of positive cells. However, MFI analysis reveals a significant decrease in fluorescence intensity of CD73 marker in MSCs-TERT (n = 4). Results are expressed as mean ± SD and T-test was used to determine the statistical difference with the paired samples
In order to verify the expression of these proteins on the cell surface we performed flow cytometry analysis. The representative histograms revealed low expression of the NTPDase2 and NTPDase3 enzymes in both cells (Fig. 3b). In addition, a significant increase in the number of CD39/NTPDase1 positive cells was observed in the MSCs-TERT (19.2% ± 7.6%) when compared to MSCs (7.8% ± 4.4%), although there was no statistical difference in medium fluorescence intensity (MFI) (MSCs = 31.6 ± 7.8 and MSCs-TERT = 37.6 ± 6.3) (Fig. 3c). When CD73 protein expression was evaluated, we did not observe any difference in the percentage of positive cells CD73 (MSCs = 98.3% ± 0.9% and MSCs-TERT = 95.9% ± 1.1%). However, a significant decrease in MFI was detected (MSCs = 353.6 ± 100.5 and MSCs-TERT = 124.4 ± 54.0) (Fig. 3d).
In addition, after cell immortalization, we observed a reduction in the cell size of MSCs-TERT in comparison to non-immortalized MSCs (Fig. 4A). To rule out the possibility that this cell size difference would be affecting the quantification of CD73, we normalized the expression of this protein by the cell size of each cell and confirmed that MSCs-TERT still had a significant reduction in the MFI of the CD73 marker (Fig. 4B). Immunocytochemistry analysis confirmed this decrease in CD73 labeling in MSCs-TERT cells (Fig. 4C).
CD73 immunocytochemistry in MSCs and MSCs-TERT. A The comparison of the cell size between MSCs and MSCs-TERT was evaluated by measurement of forward-scatter (FSC) in flow cytometry. A significant decrease in cell size was observed in MSCs-TERT, as observed by the representative histogram (n = 4). B Paired analysis of cell size and CD73 MFI was evaluated by the ratio between fluorescence intensity and forward scatter (FSC) values calculated for each cell in both MSCs and MSCs-TERT, confirming that immortalized MSCs have a CD73 lower expression. Four independent experiments were carried and T test was used, *p < 0.05. C Representative photomicrographs of CD73 immunocytochemistry show a lower labeling intensity of this enzyme in MSCs-TERT (×40 magnification)
To ensure that these observed results did not come from a random insertion event, we performed a second transfection with the TERT gene in another primary rat MSCs. The TERT gene insertion was confirmed with RT-qPCR (Supplementary Fig. 1a) and similarly to previous results, we observed a reduction in the MFI of the CD73 marker in TERT-transfected MSCs (Supplementary Fig. 1B).
Immortalized MSCs Quickly Degrade Extracellular ATP and Promote Adenosine Uptake
Corroborating the data of gene and protein expression, we observed a significant increase in the degradation of ATP and ADP, in agreement with the increased expression of CD39/NTPDase1, and decrease in AMP hydrolysis, controlled by CD73/NT5E, in MSCs-TERT, as shown in the specific enzymatic activity assay (Fig. 5a). This reduction in CD73/NT5E enzymatic activity was also observed in a second TERT-transfected MSCs experiment (Supplementary Fig. 1C).
Nucleotides metabolism by the MSCs and MSCs-TERT. a Specific enzymatic activities from MSCs and MSCs-TERT measured by release of inorganic phosphate after incubation with ATP, ADP or AMP (Malachite Green). Data are expressed as nmol Pi/min/mg of protein, using mean ± SD of six independent experiments. b-c Kinetics of the metabolism of both cells after adding 100 μM ATP (b) or 100 μM AMP (c) was evaluated during the time of 0, 10, 30, 60, 120 and 180 min. The presence of nucleotides and nucleosides was determined by HPLC. HPLC data are represented as μMol of nucleotide and expressed as mean ± SD of three independent experiments carried out in duplicate
To further investigate the pattern of nucleotides hydrolysis in these cells, we also evaluated their metabolism through HPLC assay. Figure 5b shows the degradation profile when exogenous ATP was added, demonstrating that non-immortalized MSCs slowly hydrolyzed ATP, producing a small accumulation of ADP over 180 min. On the other hand, MSCs-TERT hydrolyzed ATP almost completely in 30 min, displaying accumulation of inosine and hypoxanthine in 180 min. Although the hydrolysis pattern of ATP was consistent with our previous findings, we did not observe accumulation of ADO by MSCs and AMP by MSCs-TERT and therefore we investigated the AMP metabolism by both cells. After addition of exogenous AMP, MSCs rapidly degraded the nucleotide accumulating ADO up to approximately 120 min, subsequently producing inosine and hypoxanthine. Surprisingly, we observed a complete degradation of AMP by the MSCs-TERT within 30 min and there was no accumulation of ADO in the supernatant of these cells (Fig. 5c).
To investigate the fate of this nucleoside in MSCs-TERT, we incubated both cells with dipyridamole, which is a pharmacological inhibitor of nucleoside transporters in the cell membrane. As observed in the Fig. 6a, AMP hydrolysis profile by MSCs was similar in cells previously incubated with dipyridamole and those without treatment. On the other hand, the treatment with dipyridamole in the MSCs-TERT, produced an increase in extracellular ADO, due to the blockade of ADO uptake generated from AMP hydrolysis (Fig. 6a). In addition, we evaluated the gene expression of the adenosine deaminase enzyme (Ada) and its ligand CD26 (Dpp4), which are responsible for the hydrolysis of ADO into inosine. As observed in Fig. 6b, MSCs-TERT exhibited a significant decrease in gene expression of this enzyme. Similarly, a decrease in enzymatic activity of ADA was detected in MSCs-TERT (Fig. 6c).
Adenosine uptake and ADA activity in MSCs and MSCs-TERT. a Kinetics of the adenosine metabolism by both cells with or without pre-incubation with dipyridamole, an inhibitor of nucleoside transporters, demonstrating the adenosine uptake by MSCs-TERT. Data are represented as μMol of nucleotide/nucleoside and expressed as mean ± SD of three independent experiments carried out in duplicate. b RT-qPCR showed a significative decrease in gene expression of Ada (Adenosine deaminase) and Dpp4 (CD26) in MSCs-TERT (n = 3). mRNA levels were normalized by Actb gene expression. c Specific enzymatic activity of adenosine deaminase in MSCs and MSCs-TERT (n = 3). Results are expressed as mean ± SD and T-test was used to determine the statistical difference
Immortalized MSCs Have Impaired Immunosuppressive Capacity
MSCs can suppress T cell proliferation via adenosinergic signaling [47]. Since there is no accumulation of ADO in the supernatant of the MSCs-TERT culture, we investigated whether this nucleoside metabolism profile could alter their in vitro immunosuppressive role. The co-culture assay with lymphocytes demonstrated that MSCs-TERT exhibited a lower capacity of inhibiting lymphocyte proliferation when compared to non-immortalized MSCs (Fig. 7a-b). Curiously, gene expression data revealed that adenosinergic receptors (AdoraA1, A2a, A2b and A3), were also downregulated in the MSCs-TERT (Fig. 7c).
Immunosuppression potential by MSCs and MSCs-TERT in co-culture with Jurkat lymphocytes. a Representative photomicrographs of the co-culture between MSCs or MSCs-TERT with Jurkat cells (×40 magnification). b Jurkat lymphocytes were pre-incubated with CFSE marker and co-cultured with MSCs or MSCs-TERT (ratio 1:1). Representative histograms show that two lymphocyte populations can be seen when co-cultured with MSCs-TERT, demonstrating the increase in lymphocyte proliferation and consequent decrease in CFSE dye. CFSE medium fluorescence intensity (MFI) confirms this significant difference between MSCs and MSCs-TERT regarding the potential to inhibit lymphocyte proliferation. Lymphocytes without or with Phytohemagglutinin (PHA) were used as control. Data are expressed as mean ± SD of three independent experiments carried out in quadruplicate. One-way ANOVA was used to determine the statistical difference. ****p < 0.0001. c Gene expression of adenosinergic receptors in both cells was analyzed by RT-qPCR. Data are expressed as mean ± SEM (n = 3) and T-test was used to determine the statistical difference
Discussion
The ability to release anti-inflammatory factors and modulate immune responses is a fundamental feature of MSCs. It is necessary for several of its potential therapeutic applications, including hematological diseases, organ transplantation, inflammatory diseases, musculoskeletal regeneration, autoimmune diseases, among others [51,52,53]. However, their limited proliferation rate and the possibility to reach cellular senescence after long periods of culture, limit the use of MSCs in clinical applications [15].
In this study, we investigated the purinergic signaling in MSCs after immortalization with TERT, which has been an alternative to overcome the replicative constraints from primary MSCs and prolong their lifespan. We found that the immortalization process with the TERT gene may lead to in vitro alterations in the purinergic metabolism in MSCs, especially in the adenosinergic pathway. The results show an upregulation of gene and protein expression of the CD39/NTPDase1 and a reduction of CD73/NT5E, which impacted their enzymatic activities when compared to non-immortalized cells. More importantly, immortalized MSCs had a reduction in gene expression and enzymatic activity of the ADA enzyme, but an increase in adenosine uptake, decreasing the nucleoside concentration in the extracellular space and consequently reducing its immunosuppressive function.
Immortalized MSCs have been used to replace primary MSCs in different diseases models, such as bone repair [24, 54, 55], ischemia [56,57,58], treatment and monitoring of diabetes [59, 60], muscle [61] and neural [62] regeneration, as well as, in strategies that could use these cells as a therapeutic vehicle with controlled cell death through inducible caspase 9 (iCasp9) [63]. In this context, the changes in the purinergic pathway observed here could result in different therapeutic properties of the immortalized MSCs when compared to non-immortalized MSCs.
Purinergic signaling plays an important role in MSC-based therapies, as suggested by the large number of studies that link MSCs release of ATP and other nucleotides to their functions and effects on the microenvironment [33]. Ferrari and collaborators [34] demonstrated that ATP enhances the secretion of IL-2 and the proinflammatory Th1 cytokines in bone marrow-MSCs, while downregulates IL-10 secretion, partially reverting the well-known inhibitory activity of MSCs on T cell proliferation. However, the main role of ATP in these cells is to stimulate their mobilization, increasing the proliferation and migration rates and effectiveness of differentiation capacity [32, 33]. On the other hand, MSCs can also mediate kidney allograft tolerance through secretion of the T cell suppressor IDO [64], whose expression can be triggered by ATP [65]. Similarly, this nucleotide can control the secretion of nitric oxide (NO) and prostaglandin E2 (PGE2) [66, 67], which, via MSCs, suppressed graft-versus-host disease (GVHD) [68] and ameliorated myocardial fibrosis in diabetic cardiomyopathy [69].
However, it is mainly through the CD73/adenosine pathway, mediating the crosstalk between MSCs and immune cells, that researchers have demonstrated the importance of the purinergic pathway on the immunosuppressive effects from MSCs [31, 33]. Through this pathway, MSCs can inhibit T cell proliferation and activation [47, 48, 70] and modulate Treg lymphocytes [71], NK cells [72], Th17 cells [73] and monocytes [74]. Amarnath and collaborators [75] showed that MSCs were effective in reversing the lethal mouse GVHD model through immunomodulatory effects mediated by adenosine. Also, in a model of myocardial ischemia/reperfusion injury, adenosine secreted by implanted MSCs strongly reduced the inflammatory response and facilitated cardiac recovery [76].
We observed that TERT-immortalized MSCs, which exhibited a reduction in the extracellular adenosine concentration, had less potential to inhibit the in vitro T cell proliferation. A similar result was described with peritoneal macrophages (pMϕ) co-cultured with MSCs obtained from CD73 knockout mice, in which a decrease of their anti-inflammatory potential was observed, in comparison to those co-cultured with normal MSCs [77]. Rodriguez and collaborators [78] also showed that human MSCs can lose in vitro and in vivo immunosuppressive and anti-inflammatory function after spontaneous or induced oncogenic transformation. Although in their analysis these results were not associated with the CD73/adenosine pathway, our data suggest that the immortalization / transformation process could alter this signaling pathway, which appears to be responsible for the immunosuppressive properties of MSCs. Indeed, many of the upregulated pro-inflammatory cytokines, chemokines and receptors in transformed MSCs [78] are influenced by adenosine and its receptors [79, 80]. In addition, even though it is one of the classical markers of MSCs [81], we and others have already shown that there is no uniform pattern of expression and activity of CD73/NT5E in cultivated MSCs, which can vary depending on tissue source and species [77, 82,83,84], and this can result in different immunosuppressive and reparative properties [77, 85, 86].
Still on the adenosinergic pathway, another interesting point observed in our work is that even with a downregulated expression and activity of CD73/NT5E, TERT-immortalized MSCs still slowly hydrolyze the AMP nucleotide, which could be the result of the action of alkaline phosphatases, for example. However, the generated nucleoside is quickly taken up by the cell. In this context, although the role of intracellular adenosine is not yet fully understood, it is possible that this mechanism is being used for replenishing the intracellular ATP pool through complex phosphotransferase reactions [87], considering the energy expenditure by the rapidly dividing cells. Rodríguez-Serrano and collaborators [88] showed that exogenous nucleosides appeared to be a major factor for optimizing the cell population doubling time of MSCs, stimulating protein synthesis activity and concentrations of RNA and DNA. Indeed, a study of proteomic analysis in TERT-immortalized MSCs demonstrated that the TERT gene affects many aspects of cellular functions, such as energy generation, protein folding and binding, transcription, translation, antioxidant functions, intermediary metabolism and Ca2+ binding [29].
On the other hand, researchers recently described a new family of multifunctional purine enzymes (FAMIN) with activities analogous to ADA, purine nucleoside phosphorylase (PNP) and methylthioadenosine phosphorylase (MTAP), which are conserved in prokaryotes and eukaryotes [89]. In this way, we cannot rule out the possibility that other enzymes present in MSCs may cleave adenosine, which would also lead to the reduction of this nucleoside in the extracellular space. Further analysis will be necessary to clarify the role of intracellular adenosine and other possible purine metabolism enzymes. However, it is increasingly accepted that this nucleoside mediates crucial biological effects beyond their extracellular role, as recently reviewed [87].
Parallel to changes in the adenosine pathway, we also identified alterations on ATP metabolism, which can influence other properties of MSCs-TERT in addition to changes in their immunosuppressive capacity. The expression and activity of CD39/NTPDase1 enzyme were upregulated in MSCs-TERT when compared to non-immortalized MSCs. Similarly, we also observed a slight increase in NPP1 expression, which could contribute to the reduction of extracellular ATP levels. In vivo, this modulation could impact, for example, the homing and engrafting capacity of MSCs-TERT to injured tissues [34, 90, 91], considering that this nucleotide modulates the migration potential of MSCs [45, 46].
Although these results only reflect in vitro alterations in enzymes expression and nucleotides / nucleoside concentration from immortalized MSCs, these changes may have important implications for the use of these cells, both in the field of regenerative medicine and in tissue engineering. The observed disturbances in the purinergic pathway and expected alterations in the immunomodulatory potential of MSCs-TERT may become a setback to implement cell-based therapies with these immortalized cells, and the role of this signaling in the in vivo microenvironment complexity has to be taken into account.
In conclusion, our results indicate that although TERT-immortalized MSCs are able to maintain some of the major MSC hallmarks and circumvent replicative limitations, their purinergic metabolism profile is altered. In this context, as the adenosinergic pathway has been emerging as a key mechanism by which MSCs exert hemostatic and immunomodulatory functions, our data may have strong implications for the risk and benefit assessment regarding the use of immortalized MSCs.
References
Galipeau, J., & Sensébé, L. (2018). Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell, 22, 824–833.
da Silva, M. L., Fontes, A. M., Covas, D. T., & Caplan, A. I. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & Growth Factor Reviews, 20, 419–427.
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews. Immunology, 8, 726–736.
Salem, H. K., & Thiemermann, C. (2010). Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells, 28, 585–596.
Iser, I. C., Ceschini, S. M., Onzi, G. R., Bertoni, A. P. S., Lenz, G., & Wink, M. R. (2016). Conditioned medium from adipose-derived stem cells (ADSCs) promotes epithelial-to-Mesenchymal-like transition (EMT-like) in Glioma cells in vitro. Molecular Neurobiology, 53, 7184–7199.
Beckenkamp, L. R., Souza, L. E. B., Melo, F. U. F., Thomé, C. H., Magalhães, D. A. R., Palma, P. V. B., & Covas, D. T. (2018). Comparative characterization of CD271 + and CD271 − subpopulations of CD34 + human adipose-derived stromal cells. Journal of Cellular Biochemistry, 119, 3873–3884.
Onzi, G. R., Ledur, P. F., Hainzenreder, L. D., Bertoni, A. P. S., Silva, A. O., Lenz, G., & Wink, M. R. (2016). Analysis of the safety of mesenchymal stromal cells secretome for glioblastoma treatment. Cytotherapy, 18, 828–837.
Sous Naasani, L. I., Rodrigues, C., Azevedo, J. G., Damo Souza, A. F., Buchner, S., & Wink, M. R. (2018). Comparison of human denuded amniotic membrane and porcine small intestine submucosa as scaffolds for Limbal Mesenchymal stem cells. Stem Cell Rev Reports, 14, 744–754.
Rodrigues, C., Naasani, L. I. S., Zanatelli, C., Paim, T. C., Azevedo, J. G., de Lima, J. C., da Cruz Fernandes, M., Buchner, S., & Wink, M. R. (2019). Bioglass 45S5: Structural characterization of short range order and analysis of biocompatibility with adipose-derived mesenchymal stromal cells in vitro and in vivo. Materials Science and Engineering: C, 103, 109781.
Sous Naasani, L. I., Damo Souza, A. F., Rodrigues, C., Vedovatto, S., Azevedo, J. G., Santin Bertoni, A. P., da Cruz Fernandes, M., Buchner, S., & Wink, M. R. (2019). Decellularized human amniotic membrane associated with adipose derived mesenchymal stromal cells as a bioscaffold: Physical, histological and molecular analysis. Biochemical Engineering Journal, 152, 107366.
Glaser, T., Cappellari, A. R., Pillat, M. M., Iser, I. C., Wink, M. R., Battastini, A. M. O., & Ulrich, H. (2012). Perspectives of purinergic signaling in stem cell differentiation and tissue regeneration. Purinergic Signal, 8, 523–537.
Naji, A., Eitoku, M., Favier, B., Deschaseaux, F., Rouas-Freiss, N., & Suganuma, N. (2019). Biological functions of mesenchymal stem cells and clinical implications. Cellular and Molecular Life Sciences, 76, 3323–3348.
Andrzejewska, A., Lukomska, B., & Janowski, M. (2019). Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells, 37, 855–864.
Oedayrajsingh-Varma, M. J., van Ham, S. M., Knippenberg, M., Helder, M. N., Klein-Nulend, J., Schouten, T. E., Ritt, M. J. P. F., & van Milligen, F. J. (2006). Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy, 8, 166–177.
Turinetto, V., Vitale, E., & Giachino, C. (2016). Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. International Journal of Molecular Sciences, 17(7), 1164.
Wagner, W., Horn, P., Castoldi, M., Diehlmann, A., Bork, S., Saffrich, R., Benes, V., Blake, J., Pfister, S., Eckstein, V., & Ho, A. D. (2008). Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS One, 3(5), e2213.
Baxter, M. A. (2004). Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells, 22, 675–682.
Lee, W. Y. W., Zhang, T., Lau, C. P. Y., Wang, C. C., Chan, K. M., & Li, G. (2013). Immortalized human fetal bone marrow-derived mesenchymal stromalcell expressing suicide gene for anti-tumor therapy in vitro andin vivo. Cytotherapy, 15, 1484–1497.
Nishioka, K., Fujimori, Y., Hashimoto-Tamaoki, T., Kai, S., Qiu, H., Kobayashi, N., Tanaka, N., Westerman, K. A., Leboulch, P., & Hara, H. (2003). Immortalization of bone marrow-derived human mesenchymal stem cells by removable simian virus 40T antigen gene: Analysis of the ability to support expansion of cord blood hematopoietic progenitor cells. International Journal of Oncology, 23, 925–932.
Hung, S. C., Yang, D. M., Chang, C. F., Lin, R. J., Wang, J. S., Low-Tone Ho, L., & Yang, W. K. (2004). Immortalization without neoplastic transformation of human mesenchymal stem cells by transduction with HPV16 E6/E7 genes. International Journal of Cancer, 110, 313–319.
Balducci, L., Blasi, A., Saldarelli, M., Soleti, A., Pessina, A., Bonomi, A., Coccè, V., Dossena, M., Tosetti, V., Ceserani, V., Navone, S., Falchetti, M., Parati, E., & Alessandri, G. (2014). Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Research & Therapy, 5(3), 63.
Piper, S. L., Wang, M., Yamamoto, A., Malek, F., Luu, A., Kuo, A. C., & Kim, H. T. (2012). Inducible immortality in hTERT-human mesenchymal stem cells. Journal of Orthopaedic Research, 30, 1879–1885.
Bodnar AG, Ouellette M, Frolkis M, et al (1998) Extension of life-span by introduction of telomerase into normal human cells. Science (80- ) 279:349–352.
Lu, S., Wang, J., Ye, J., Zou, Y., Zhu, Y., Wei, Q., Wang, X., Tang, S., Liu, H., Fan, J., Zhang, F., Farina, E. M., Mohammed, M. M., Song, D., Liao, J., Huang, J., Guo, D., Lu, M., Liu, F., Liu, J., Li, L., Ma, C., Hu, X., Lee, M. J., Reid, R. R., Ameer, G. A., Zhou, D., & He, T. (2016). Bone morphogenetic protein 9 (BMP9) induces effective bone formation from reversibly immortalized multipotent adipose-derived (iMAD) mesenchymal stem cells. American Journal of Translational Research, 8, 3710–3730.
Hu, X., Li, L., Yu, X., Zhang, R., Yan, S., Zeng, Z., Shu, Y., Zhao, C., Wu, X., Lei, J., Li, Y., Zhang, W., Yang, C., Wu, K., Wu, Y., An, L., Huang, S., Ji, X., Gong, C., Yuan, C., Zhang, L., Liu, W., Huang, B., Feng, Y., Zhang, B., Haydon, R. C., Luu, H. H., Reid, R. R., Lee, M. J., Wolf, J. M., Yu, Z., & He, T. C. (2017). CRISPR/Cas9-mediated reversibly immortalized mouse bone marrow stromal stem cells (BMSCs) retain multipotent features of mesenchymal stem cells (MSCs). Oncotarget, 8, 111847–111865.
Harley, C. B. (2002). Telomerase is not an oncogene. Oncogene, 21, 494–502.
Jiang, X. R., Jimenez, G., Chang, E., Frolkis, M., Kusler, B., Sage, M., Beeche, M., Bodnar, A. G., Wahl, G. M., Tlsty, T. D., & Chiu, C. P. (1999). Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nature Genetics, 21, 111–1114.
Kassem, M., Abdallah, B. M., Yu, Z., Ditzel, N., & Burns, J. S. (2004). The use of hTERT-immortalized cells in tissue engineering. Cytotechnology, 45, 39–46.
Huang, G. P., Pan, Z. J., Huang, J. P., Yang, J. F., Guo, C. J., Wang, Y. G., Zheng, Q., Chen, R., Xu, Y. L., Wang, G. Z., Xi, Y. M., Shen, D., Jin, J., & Wang, J. F. (2008). Proteomic analysis of human bone marrow mesenchymal stem cells transduced with human telomerase reverse transcriptase gene during proliferation. Cell Proliferation, 41, 625–644.
Burnstock, G. (2018). The therapeutic potential of purinergic signalling. Biochemical Pharmacology, 151, 157–165.
de Oliveira, B. M., Carvalho, J. L., & Saldanha-Araujo, F. (2016). Adenosine production: A common path for mesenchymal stem-cell and regulatory T-cell-mediated immunosuppression. Purinergic Signal, 12, 595–609.
Kaebisch, C., Schipper, D., Babczyk, P., & Tobiasch, E. (2015). The role of purinergic receptors in stem cell differentiation. Computational and Structural Biotechnology Journal, 13, 75–84.
Roszek, K., & Wujak, M. (2018). How to influence the mesenchymal stem cells fate? Emerging role of ectoenzymes metabolizing nucleotides. Journal of Cellular Physiology, 234, 320–334.
Ferrari Davide D, Gulinelli S, Salvestrini V, et al (2011) Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12 and increases the homing capacity and production of proinflammatory cytokines. Exp Hematol 39:360-374.e5.
Coppi E, Pugliese AM, Urbani S, et al (2007) ATP modulates cell proliferation and elicits two different electrophysiological responses in human Mesenchymal stem cells. Stem Cells 25:1840–1849.
Ciciarello, M., Zini, R., Rossi, L., et al. (2012). Extracellular purines promote the differentiation of human bone marrow-derived Mesenchymal stem cells to the Osteogenic and Adipogenic lineages. Stem Cells and Development, 22, 1097–1111.
Counter, C. M., Hahn, W. C., Wei, W., Caddle, S. D., Beijersbergen, R. L., Lansdorp, P. M., Sedivy, J. M., & Weinberg, R. A. (1998). Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proceedings of the National Academy of Sciences of the United States of America, 95, 14723–14728.
Tamajusuku, A. S. K., Villodre, E. S., Paulus, R., Coutinho-Silva, R., Battasstini, A. M. O., Wink, M. R., & Lenz, G. (2010). Characterization of ATP-induced cell death in the GL261 mouse glioma. Journal of Cellular Biochemistry, 109, 983–991.
Albesiano, E., Messmer, B. T., Damle, R. N., et al. (2003). Activation induced cytidine deaminase in chronic lymphocytic leukemia B cells: Expression as multiple forms in a dynamic, variably sized fraction of the clone. Neoplasia, 102, 3333–3340.
Wink, M. R., Braganhol, E., Tamajusuku, A. S. K. K., et al. (2003). Extracellular adenine nucleotides metabolism in astrocyte cultures from different brain regions. Neurochemistry International, 43, 621–628.
Chan, K. M., Delfert, D., & Junger, K. D. (1986). A direct colorimetric assay for Ca2+ −stimulated ATPase activity. Analytical Biochemistry, 157, 375–380.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Galanti, B., & Giusti, G. (1966). Direct colorimetric method for the determination of adenosine deaminase and 5-AMP deaminase in the blood. Bollettino della Società Italiana di Biologia Sperimentale, 42, 1316–1320.
Iser IC, Ceschini SM, Onzi GR, et al (2015) Conditioned medium from adipose-derived stem cells ( ADSCs ) promotes epithelial-to-mesenchymal-like transition ( EMT-Like ) in glioma cells in vitro. 53 (10):7184–7199.
Peng, H., Hao, Y., Mousawi, F., Roger, S., Li, J., Sim, J. A., Ponnambalam, S., Yang, X., & Jiang, L. H. (2016). Purinergic and store-operated Ca 2+ signaling mechanisms in Mesenchymal stem cells and their roles in ATP-induced stimulation of cell migration. Stem Cells, 34, 2102–2114.
Jiang, L. H., Mousawi, F., Yang, X., & Roger, S. (2017). ATP-induced Ca2+−signalling mechanisms in the regulation of mesenchymal stem cell migration. Cellular and Molecular Life Sciences, 74, 3697–3710.
Saldanha-Araujo, F., Ferreira, F. I. S., Palma, P. V., Araujo, A. G., Queiroz, R. H. C., Covas, D. T., Zago, M. A., & Panepucci, R. A. (2011). Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Research, 7, 66–74.
Sattler, C., Steinsdoerfer, M., Offers, M., Fischer, E., Schierl, R., Heseler, K., Däubener, W., & Seissler, J. (2011). Inhibition of T-cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39 expression and adenosine generation. Cell Transplantation, 20, 1221–1230.
Cavaliere, F., Donno, C., & Ambrosi, N. (2015). Purinergic signaling: A common pathway for neural and mesenchymal stem cell maintenance and differentiation. Frontiers in Cellular Neuroscience, 9, 1–8.
Zippel, N., Limbach, C. A., Ratajski, N., et al. (2011). Purinergic receptors influence the differentiation of human Mesenchymal stem cells. Stem Cells and Development, 21, 884–900.
Bernardo, M. E., Pagliara, D., & Locatelli, F. (2012). Mesenchymal stromal cell therapy: A revolution in regenerative medicine? Bone Marrow Transplantation, 47, 164–171.
Jiang, W., & Xu, J. (2019). Immune modulation by mesenchymal stem cells. Cell Proliferation, 53(1), e12712.
Gao, F., Chiu, S. M., Motan, D. A. L., Zhang, Z., Chen, L., Ji, H. L., Tse, H. F., Fu, Q. L., & Lian, Q. (2016). Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death & Disease, 7(1), e2062.
Nakahara, H., Misawa, H., Hayashi, T., Kondo, E., Yuasa, T., Kubota, Y., Seita, M., Kawamoto, H., Hassan, W. A. R. A., Hassan, R. A. R. A., Javed, S. M., Tanaka, M., Endo, H., Noguchi, H., Matsumoto, S., Takata, K., Tashiro, Y., Nakaji, S., Ozaki, T., & Kobayashi, N. (2009). Bone repair by transplantation of hTERT-immortalized human mesenchymal stem cells in mice. Transplantation, 88, 346–353.
Burns, J. S., Rasmussen, P. L., Larsen, K. H., Schrøder, H. D., & Kassem, M. (2010). Parameters in three-dimensional Osteospheroids of Telomerized human Mesenchymal (stromal) stem cells grown on Osteoconductive scaffolds that predict In Vivo bone-forming potential. Tissue Engineering. Part A, 16, 2331–2342.
Honma, T., Honmou, O., Iihoshi, S., Harada, K., Houkin, K., Hamada, H., & Kocsis, J. D. (2006). Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Experimental Neurology, 199, 56–66.
Zhao, F., Qu, Y., Liu, H., du, B., & Mu, D. (2014). Umbilical cord blood mesenchymal stem cells co-modified by TERT and BDNF: A novel neuroprotective therapy for neonatal hypoxic-ischemic brain damage. International Journal of Developmental Neuroscience, 38, 147–154.
Li, J., Liu, W., & Yao, W. (2019). Immortalized human bone marrow derived stromal cells in treatment of transient cerebral ischemia in rats. J Alzheimer’s Dis, 69, 871–880.
Weber, C., Pohl, S., Poertner, R., et al. (2008). Development of a production process for stem cell based cell therapeutic implants using disposable bioreactor systems. In IFMBE proceedings (pp. 2277–2280). Berlin, Heidelberg: Springer.
Siska, E. K., Weisman, I., Romano, J., Ivics, Z., Izsvák, Z., Barkai, U., Petrakis, S., & Koliakos, G. (2017). Generation of an immortalized mesenchymal stem cell line producing a secreted biosensor protein for glucose monitoring. PLoS One, 12(9), e0185498.
Chiu, C.-H., Chang, T.-H., Chang, S.-S., Chang, G. J., Chen, A. C. Y., Cheng, C. Y., Chen, S. C., Fu, J. F., Wen, C. J., & Chan, Y. S. (2020). Application of bone marrow–derived Mesenchymal stem cells for muscle healing after contusion injury in mice. The American Journal of Sports Medicine, 48(5), 1226–1235.
Zhu, G. Q., Jeon, S. H., Lee, K. W., et al. (2020). Engineered stem cells improve neurogenic bladder by overexpressing SDF-1 in a pelvic nerve injury rat model. Cell Transplantation, 29, 963689720902466.
Bourgine, P., Le Magnen, C., Pigeot, S., et al. (2014). Combination of immortalization and inducible death strategies to generate a human mesenchymal stromal cell line with controlled survival. Stem Cell Research, 12, 584–598.
Ge, W., Jiang, J., Arp, J., Liu, W., Garcia, B., & Wang, H. (2010). Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation, 90, 1312–1320.
Lotfi, R., Steppe, L., Hang, R., Rojewski, M., Massold, M., Jahrsdörfer, B., & Schrezenmeier, H. (2018). ATP promotes immunosuppressive capacities of mesenchymal stromal cells by enhancing the expression of indoleamine dioxygenase. Immunity, Inflammation and Disease, 6, 448–455.
Toki, Y., Takenouchi, T., Harada, H., Tanuma, S. I., Kitani, H., Kojima, S., & Tsukimoto, M. (2015). Extracellular ATP induces P2X7 receptor activation in mouse Kupffer cells, leading to release of IL-1β, HMGB1, and PGE2, decreased MHC class I expression and necrotic cell death. Biochemical and Biophysical Research Communications, 458, 771–776.
Ulker, P., Özen, N., Abdullayeva, G., Köksoy, S., Yaraş, N., & Basrali, F. (2018). Extracellular ATP activates eNOS and increases intracellular NO generation in red blood cells. Clinical Hemorheology and Microcirculation, 68, 89–101.
Ren, G., Zhang, L., Zhao, X., Xu, G., Zhang, Y., Roberts, A. I., Zhao, R. C., & Shi, Y. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell, 2, 141–150.
Jin, L., Zhang, J., Deng, Z., Liu, J., Han, W., Chen, G., Si, Y., & Ye, P. (2020). Mesenchymal stem cells ameliorate myocardial fibrosis in diabetic cardiomyopathy via the secretion of prostaglandin E2. Stem Cell Research & Therapy, 11, 122.
Kerkela; E, Laitinen A, Rabina J, et al (2016) Adenosinergic immunosuppression by human Mesenchymal stromal cells requires co-operation with T cells. Stem Cell 34:781–790.
Sivanathan, K. N., Rojas-Canales, D. M., Hope, C. M., Krishnan, R., Carroll, R. P., Gronthos, S., Grey, S. T., & Coates, P. T. (2015). Interleukin-17A-induced human Mesenchymal stem cells are superior modulators of immunological function. Stem Cells, 33, 2850–2863.
Chatterjee, D., Tufa, D. M., Baehre, H., et al. (2014). Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells we. Blood, 123, 595–597.
Lee, J. J., Jeong, H. J., Kim, M. K., Wee, W. R., Lee, W. W., Kim, S. U., Sung, C., & Yang, Y. H. (2014). CD39-mediated effect of human bone marrow-derived mesenchymal stem cells on the human Th17 cell function. Purinergic Signal, 10, 357–365.
Monguió-Tortajada, M., Roura, S., Gálvez-Montón, C., et al. (2017). Mesenchymal stem cells induce expression of CD73 in human monocytes in vitro and in a swine model of myocardial infarction in vivo. Frontiers in Immunology, 8, 1–13.
Amarnath S, Foley JE, Farthing DE, et al (2015) Bone marrow derived Mesenchymal stromal cells harness Purinergenic signaling to Tolerize human Th1 cells in vivo. Stem cell 1200–1212.
Shin, E. Y., Wang, L., Zemskova, M., et al. (2018). Adenosine production by biomaterial-supported mesenchymal stromal cells reduces the innate inflammatory response in myocardial ischemia/reperfusion injury. Journal of the American Heart Association, 7(2), e006949.
Tan, K., Zhu, H., Zhang, J., et al. (2019). CD73 expression on mesenchymal stem cells dictates the reparative properties via its anti-inflammatory activity. Stem Cells International, 2019, 8717694.
Rodriguez, R., Rosu-Myles, M., Aráuzo-Bravo, M., Horrillo, A., Pan, Q., Gonzalez-Rey, E., Delgado, M., & Menendez, P. (2014). Human bone marrow stromal cells lose immunosuppressive and anti-inflammatory properties upon oncogenic transformation. Stem Cell Reports, 3, 606–619.
Antonioli L, Blandizzi C, Pacher P, Haskó G (2013) Immunity , inflammation and cancer : a leading role for adenosine. Nat Publ Gr 13:842–857.
Borea, P. A., Gessi, S., Merighi, S., & Varani, K. (2016). Adenosine as a multi-Signalling Guardian angel in human diseases: When, where and how does it exert its protective effects? Trends in Pharmacological Sciences, 37, 419–434.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317.
Iser IC, Bracco P a., Gonçalves CEI, et al (2014) Mesenchymal stem cells from different murine tissues have differential capacity to metabolize extracellular nucleotides. Journal of Cellular Biochemistry 115:1673–1682.
Naasani, L. I. S., Rodrigues, C., de Campos, R. P., Beckenkamp, L. R., Iser, I. C., Bertoni, A. P. S., & Wink, M. R. (2017). Extracellular nucleotide hydrolysis in dermal and Limbal Mesenchymal stem cells: A source of adenosine production. Journal of Cellular Biochemistry, 118, 2430–2442.
Roszek, K., Bomastek, K., Drożdżal, M., & Komoszyński, M. (2013). Dramatic differences in activity of purines metabolizing ecto-enzymes between mesenchymal stem cells isolated from human umbilical cord blood and umbilical cord tissue. Biochemistry and Cell Biology, 91, 519–525.
Chen, X., Shao, H., Zhi, Y., Xiao, Q., Su, C., Dong, L., Liu, X., Li, X., & Zhang, X. (2016). CD73 pathway contributes to the immunosuppressive ability of Mesenchymal stem cells in intraocular autoimmune responses. Stem Cells and Development, 25, 337–346.
Netsch, P., Elvers-Hornung, S., Uhlig, S., Klüter, H., Huck, V., Kirschhöfer, F., Brenner-Weiß, G., Janetzko, K., Solz, H., Wuchter, P., Bugert, P., & Bieback, K. (2018). Human mesenchymal stromal cells inhibit platelet activation and aggregation involving CD73-converted adenosine. Stem Cell Research & Therapy, 9(1), 184.
Boison, D., & Yegutkin, G. G. (2019). Adenosine metabolism: Emerging concepts for Cancer therapy. Cancer Cell, 36, 582–596.
Rodríguez-Serrano, F., Álvarez, P., Caba, O., et al. (2010). Promotion of human adipose-derived stem cell proliferation mediated by exogenous nucleosides. Cell Biology International, 34, 917–924.
Cader MZ, de Almeida Rodrigues RP, West JA, et al (2020) FAMIN is a multifunctional purine enzyme enabling the purine nucleotide cycle. Cell 180:278-295.e23.
Mousawi, F., Peng, H., Li, J., Ponnambalam, S., Roger, S., Zhao, H., Yang, X., & Jiang, L. H. (2020). Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells, 38, 410–421.
Zhou, Q., Yang, C., & Yang, P. (2017). The promotional effect of Mesenchymal stem cell homing on bone tissue regeneration. Current Stem Cell Research & Therapy, 12, 365–376.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001; All students are recipients of fellowships from CAPES. MRW, GL and RPC are recipients of research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brasil (CNPq). This study was supported by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul - Brasil (FAPERGS/CAPES 06/2018 - Programa de Internacionalização da pós-graduação no RS (19/2551-0000679-9) and FAPERGS/MS/CNPq/SESRS n.03/2017 – PPSUS (17/2551-0001)); and CNPqMS-SCTIE-Decit/CNPqn°12/2018(441575/2018-8). JS received support from the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2016-05867).
Author information
Authors and Affiliations
Contributions
LRB performed cell culture experiments, HPLC assays, flow cytometry, immunosuppression assay and wrote the manuscript. GRO assisted the stable transfection cell assays and wrote the manuscript. DMF and VGK performed cell culture experiments, enzymatic activity and cell differentiation assay, flow cytometry and doubling population experiments. RPC performed the stable transfection of cells. ICI performed the ADA enzymatic assay. APSB performed RT-qPCR and HPLC assays. JS contributed to the interpretation of the results and provided the NTPDases antibodies. MRW and GL supervised the experiments, assisted in drafting and critical reading. All the authors discussed the results and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Disclosure Statement
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Supplementary Figure 1
– CD73 activity and expression in a new cell transduction with TERT. To confirm that cell immortalization process alters the activity and expression of CD73 enzyme, a new stable transfection with the TERT gene was performed. (A) The TERT gene insertion was confirmed by RT-qPCR, as demonstrated by melt curve peak chart collected using the StepOnePlusTM (Applied Biosystems). (B) Flow cytometry data analysis also confirmed a decrease of CD73 MFI in MSCs-TERT (n = 3). (C) Specific enzymatic activity from MSCs and MSCs-TERT measured by release of inorganic phosphate after incubation with AMP (n = 3). Data are expressed as nmol Pi/min/mL, using mean ± SD. T-test was used to determine the statistical difference. (PNG 285 kb)
Rights and permissions
About this article
Cite this article
Beckenkamp, L.R., da Fontoura, D.M.S., Korb, V.G. et al. Immortalization of Mesenchymal Stromal Cells by TERT Affects Adenosine Metabolism and Impairs their Immunosuppressive Capacity. Stem Cell Rev and Rep 16, 776–791 (2020). https://doi.org/10.1007/s12015-020-09986-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-09986-5