Abstract
Mesenchymal stem cells (MSCs) represent a population of cells which have the ability to regulate reactivity of T and B lymphocytes by multiple mechanisms. The immunoregulatory activities of MSCs are strictly influenced by the cytokine environment. Here we show that two functionally distinct cytokines, interleukin-4 (IL-4) and interferon-γ (IFN-γ), significantly potentiate the ability of MSCs to inhibit IL-10 production by activated regulatory B cells (Bregs). However, MSCs in the presence of IL-4 or IFN-γ inhibit the IL-10 production by different mechanisms. Preincubation of MSCs with IFN-γ led to the suppression, but pretreatment with IL-4 of neither MSCs nor B cells resulted in the suppression of IL-10 production. The search for candidate regulatory molecules expressed in cytokine-treated MSCs revealed different patterns of the gene expression. Pretreatment of MSCs with IFN-γ, but not with IL-4, induced expression of indoleamine-2,3-dioxygenase, cyclooxygenase-2 and programmed cell death-ligand 1. To identify the molecule(s) responsible for the suppression of IL-10 production, we used specific inhibitors of the putative regulatory molecules. We found that indomethacine, an inhibitor of cyclooxygenase-2 (Cox-2) activity, completely abrogated the inhibition of IL-10 production in cultures containing MSCs and IFN-γ, but had no effect on the suppression in cell cultures containing MSCs and IL-4. The results show that MSCs can inhibit the response of B cells to one stimulus by different mechanisms in dependence on the cytokine environment and thus support the idea of the complexity of immunoregulatory action of MSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The final manifestation of immune response is a result of a complex interaction and cooperation of phenotypically and functionally different cell populations and cytokines. One of cell populations which modulate reactivity of cells of natural and adaptive immunity, are mesenchymal stem cells (MSCs). These cells can be found in nearly all tissues and organs and are characterized by the ability to differentiate into various cell types. In addition to their differentiation potential, MSCs are a potent source of a number of various cytokines and other growth factors and regulate immune response in both positive and negative manner. It has been shown that MSCs inhibit proliferation of T and B lymphocytes [1, 2] regulate production of cytokines [3, 4], modulate functions of antigen-presenting cells [5, 6] and suppress development of cytotoxic T and NK cells in vitro [7, 8]. MSCs also inhibit autoimmune, transplantation and inflammatory reactions in vivo [9–11]. For their immunomodulatory activity MSCs utilize multiple mechanisms which include secretion of regulatory cytokines and other factors, the expression of inhibitory membrane molecules, negative effects on antigen-presenting cells and activation of regulatory T cells [12] Although MSCs themselves are a potent source of cytokines and other immunoregulatory molecules, their activity is regulated by other cytokines. It has been shown that cytokine production and phenotypic profiles of MSCs change in dependence on cytokine environment [13, 14]. The complexity of immunoregulatory action of MSCs is supported by the observation that MSCs inhibited lymphocyte proliferation induced by mitogens and alloantigens by different mechanisms [15].
Although there are extensive published data on the effects of MSCs on T cells or antigen-presenting cells, considerably less knowledge is available about the effects of MSCs on B lymphocytes. In these studies, the suppression of B cell differentiation and inhibition of antibody production was demonstrated [16, 17]. Within the population of B cells, a subpopulation called regulatory B cells (Bregs) which is characterized by the ability to suppress immune reactions in antibody-independent manner, has been identified [18, 19]. These cells produce high concentrations of IL-10 which is considered as the main effector molecule of Breg-mediated immunosuppression [19, 20]. Bregs play an important role in autoimmune, transplantation, antitumour and other immune reactions. It has been shown that production of IL-10 by Bregs is regulated in a positive and negative manner by cytokines [21, 22]. Furthermore, we have shown that production of IL-10 by activated B cells is strongly inhibited by MSCs in the presence of IFN-γ [23]. Activation of cyclooxygenase-2 pathway and the synthesis of prostaglandin E2 (PGE2) has been identified as the mechanism of the suppression of IL-10 production by MSCs in the presence of IFN-γ [23]. We recently observed a similar inhibitory effect of MSCs on IL-10 production by B cells in the presence of IL-4. However, IFN-γ induces in MSCs the expression of a different spectrum of immunoregulatory molecules than IL-4 and the mechanisms of MSC-mediated suppression of IL-10 production are different in the presence of IL-4 or IFN-γ. It suggests that MSCs can inhibit not only the response to different antigens by distinct mechanisms, as it had been published [15], but also the response to the one stimulus by different mechanisms in dependence on the cytokine environment. These findings support the idea of the complexity of the immunoregulatory actions of MSCs.
Materials and Methods
Mice
Mice of both sexes of the inbred strain BALB/c were used in the experiments at the age of 7–9 weeks. The animals were purchased from the Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Prague. The use of the animals was approved by the Local Ethical Committee of the Institute of Experimental Medicine.
MSC and B-Cell Enrichment Procedure
MSCs were prepared from bone marrow isolated from the femurs and tibias of female BALB/c mice. The bone marrow was flushed out and a single-cell suspension was seeded at a concentration of 4 x 106 cells/ml in Dulbecco’s modified Eagle’s medium (DMEM, Sigma, St. Louis, MO, USA) containing 10 % fetal calf serum (FCS, Gibco BRL, Grand Island, NY, USA), antibiotics (100 U/ml ml penicillin, 100 μg/ml ml streptomycin) and 10 mM Hepes buffer (hereafter referred to as complete DMEM) in 75-cm2 tissue culture flasks (TPP, Trasadingen, Switzerland). Nonadherent cells were washed out after 48 hrs of cultivation, and the remaining adherent cells were cultured for an additional 3 weeks (2–3 passages) at 37 °C in an atmosphere of 5 % CO2. Plastic adherent cells were harvested by a short trypsinization and subsequent gentle scraping. The resulting cell suspension was incubated for 15 min with CD11b MicroBeads and CD45 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. The cell suspension was then immunodepleted of CD11b+ and CD45+ contaminating cells using a magnetic activated cell sorter (AutoMACS, Miltenyi Biotec). The remaining CD11b− and CD45− cells were characterized in terms of their purity and differentiation potential.
For preparation of B cells, a single cell suspension of spleen cells was prepared in RPMI 1640 medium (Sigma Corp., St. Louis, CA, USA) containing 10 % FCS (Sigma), antibiotics (penicillin, streptomycin), 10 mM Hepes buffer and 5 x 10−5 M 2-mercaptoethanol. The B cells were isolated by a positive selection using the CD19 MicroBeads isolation kit (Miltenyi Biotec). The flow cytometry analysis showed that this procedure yielded a cell population containing more than 95 % CD19+ cells and less than 2 % cells were CD3+.
Characterization of Isolated B-Cell and MSC Populations
The purity and phenotype of the enriched B cell population were characterized by a flow cytometry using the following monoclonal antibodies (mAb): fluorescein isothiocyanate (FITC)-labelled anti-CD19 (clone 6D5), Alexa Fluor 647-labelled anti-CD5 (clone 53–73), Alexa Fluor 647-labelled anti-CD22 (clone OX-97), phycoerythrin (PE)-labelled anti-CD1d (clone 1B1), allophycocyanine (APC)-labelled anti-CD11b (clone M1/70), and APC-labelled anti-CD3 (clone 17A2). All antibodies were purchased from BioLegend (San Diego, CA, USA).
To characterize the phenotype of MSCs, the cells were washed in PBS containing 0.5 % bovine serum albumin and then incubated for 30 min on ice with the following anti-mouse mAb: APC-labelled anti-CD44 (clone IM7, BD PharMingen, San Jose, CA, USA), PE-labelled anti-CD105 (clone MJ7/18, eBioscience, San Diego, CA, USA), PE-labelled anti-CD73 (clone TY/11.8), eBioscience), APC-labelled anti-CD11b (clone M1/70, BioLegend) or FITC-labelled anti-CD45 (clone 30-F11, BioLegend). Dead cells were stained using Hoechst 33258 fluorescent dye (Invitrogen, Carlsbad, CA, USA) added to the samples 10 min before flow cytometry analysis. Data were collected using an LSRII cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA). The morphological characteristics and differentiation potential of purified MSCs we have described in details elsewhere [23, 24].
Production and Detection of IL-10
B cells at a concentration 0.9 x 106 cells/ml were incubated in 48-well tissue culture plates (Corning Inc., Corning, NY, USA) in a final volume of 0.6 ml of complete RPMI 1640 medium unstimulated or stimulated with LPS (5 μg/ml, Difco Laboratories, Detroit, MI, USA). After a 72-hr incubation, the supernatants were harvested and tested for the presence of IL-10 by ELISA. To test effects of MSCs and IFN-γ on IL-10 production by B cells, MSCs at a ratio 1 : 30 or 10 ng/ml of recombinant mouse IL-4 or IFN-γ (PeproTech, Rocky Hill, NJ, USA) was added to the cultures of LPS-activated B cells and the production of IL-10 was determined.
The production of IL-10 was determined by ELISA using capture and detection mAb anti-IL-10 purchased from R & D Systems (Minneapolis, MN, USA) and following the instructions of manufacturer. The reaction was quantified by spectrophotometry using a Sunrise Remote ELISA Reader (Gröding, Austria).
Effects of Separation of B Cells and MSCs in a Transwell System on IL-10 Production
To test the effect of separation of MSCs from B cells on the suppression of IL-10 production, MSCs were separated from B cells by cell culture inserts (Nunc, Roskilde, Denmark) and cultured for 72 hrs in the presence of 5 μg/ml LPS and cytokines. The production of IL-10 in cultures of LPS-stimulated B cells alone, B cells mixed with MSCs or B cells separated from MSCs by semipermeabile membrane was determined.
In the other set of experiments, the supernatants were prepared by a 48-h incubation of untreated MSCs, or MSCs stimulated with 10 ng/ml of IL-4 or IFN-γ, LPS (5 μg/ml) or LPS plus IL-4 or IFN-γ. The supernatants were added to the cultures of B cells stimulated with LPS to make s final concentration 50 % of cell culture volume, and the production of IL-10 cells was determained after a 72-hr incubation.
Pretreatment of MSCs and B Cells with IL-4 or IFN-γ
MSCs (105 cells/well) were cultured in a volume 1 ml in 24-well tissue culture plates (Techno Plastic Products, Trasadinger, Switzerland) untreated or in the presence of 10 ng/ml of IL-4 or IFN-γ. After a 24-hr incubation, the adherent MSCs were washed with an excess of culture medium. B cells which were preincubated for 24 h alone or with 10 ng/ml of IL-4 or IFN-γ and then thoroughly washed, were added (0.9 x 106 cells/ml) to cultures of MSCs and were stimulated with LPS (5 μg/ml). The supernatants were harvested after a 72-h incubation and the concentrations of IL-10 were determined by ELISA.
Inhibition of MSC-Mediated Suppression of IL-10 Production
Purified B cells were stimulated with LPS in the presence of MSCs and IL-4 or IFN-γ and 1-methyl-D-tryptophane (1-MT, Sigma), i.e. selective inhibitor of indoleamine-2,3-dioxygenase (IDO), or inhibitor of Cox-2 synthesis indomethacine (Sigma), was added to the cultures to make a final concentration 10−3 M or 10−6 M, respectively. To block the progammed cell death-ligand 1 (PD-L1, CD274) – PD-1 (CD279) pathway, an inhibitory mAb anti-PD-L1 (clone MH5, eBioscience, San Jose, CA, USA) was added into the cultures at a concentration 5 μg/ml. To inhibit possible involvement of the Fas - FasL pathway in the immunosuppression, a mAb anti-Fas (anti-CD178, clone MFL3, eBioscience) was included into culture containing B cells, MSCs and cytokines at a final concentration 5 μg/ml.
Detection of Gene Expression
The expression of genes for IDO, Cox-2, trasnsforming growth factor-β (TGF-β), IL-6, hepatocyte growth factor (HGF), PD-L1, Fas and Fas-L was detected using real-time PCR, as we have described [23]. In brief, MSCs were cultured for 48 hrs unstimulated or stimulated with 10 ng/ml of IL-4 or IFN-γ, LPS (5 μg/ml) or LPS and IL-4 or IFN-γ, and total RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions. One μg of total RNA was treated with deoxyribonuclease I (Promega, Madison, WI, USA) and used for subsequent reverse transcription. The first-strand cDNA was synthesized using random hexamers (Promega) in a total reaction volume of 25 μl using M-MLV Reverse Transcriptase (Promega). Quantitative real-time PCR was performed in a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA) as we have previously described [23]. The sequences of primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), IDO, Cox-2, IL-6, TGF-β, HGF, PD-L1, Fas and FasL used for amplification are presented in Table 1. The PCR parameters included denaturation at 95 °C for 3 min, 40 cycles at 95 °C for 20 s, annealing at 60 °C for 30 s and elongation at 72 °C for 30 s. Fluorescence data were collected at each cycle after an elongation step at 80 °C for 5 s and were analyzed using StepOne Software version 2.2.2 (Applied Biosystems).
Statistical Analysis
The results are expressed as the mean ± SD. Comparisons between two groups were analyzed by Student’s t-test, and multiple comparisons were calculated by ANOVA. A value of p < 0.05 was considered statistically significant.
Results
Characterization of Purified B-Cell and MSC Populations
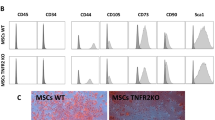
The MACS-purified B-cell and MSC populations were phenotypically characterized by flow cytometry. The purified B cell population contained more than 95 % CD19+ cells and less than 2 % CD3+ cells or CD11b+ cells. Within CD19+ cell populations, 93.8 ± 2.2 % cells were CD19+CD22+, 86.9 ± 1.8 % cells were CD19+CD1d+ and 7.6 ± 0.8 % cells were CD19+CD5+ (Fig. 1a). The growing MACS-purified MSCs had a typical fibroblast-like morphology, adhered to plastic and were positive with corresponding intensity for CD44, CD73 and CD105, which are markers attributed to murine MSCs (Fig. 1b). The MSC populations contained less than 1.0 % CD11b+ cells and less than 3 % CD45+ cells. In addition, MSCs were able to undergo adipogenic and osteogenic differentiation, when cultured in specific differentiation media, as we have described [24].
The flow cytometry characterization of MACS-separated B-cell and MSC populations. Representative dot blots indicate the percentage of CD19+CD22+, CD19+CDld+, CD19+CD5+, CD3+ and CD11b+ cells within the B-cell population (a). Histograms show the expression of CD44, CD73 , CD105, CD45 and CD11b markers within the MSCs (b). One of four similar experiments is shown
MSCs in the Presence of IL-4 or IFN-γ Strongly Inhibit IL-10 Production by B Cells
Purified B cells stimulated with LPS produced significant levels of IL-10 (Fig. 2). This production was even increased in the presence of IFN-γ and remained preserved or only slightly decreased in the presence of IL-4. Similarly, only a weak inhibitory effect on IL-10 production was detected, if B cells were stimulated with LPS in the presence of MSCs. However, a strong suppression of IL-10 production was observed when B cells were stimulated with LPS in the presence of MSCs and IL-4 (Fig. 2a) or MSCs and IFN-γ (Fig. 2b). The suppression was dependent on a concentration of MSCs (the suppression disappeared at ratios of MSCs cells to B cells below 1 : 30) and depended also on cytokine concentrations (data not shown).
The suppression of IL-10 production by LPS-stimulated B cells in the presence of MSCs and IL-4 (a) or MSCs and IFN-γ (b). Purified B cells were cultured unstimulated (−) or stimulated with LPS in the presence or absence of MSCs and/or IL-4 or IFN-γ. The production of IL-10 was determined in the supernatants after a 72-hr incubation period by ELISA. Each bar represents the mean ± SD from 4 independent experiments. Values with asterisks are significantly different (***p < 0.001) from the control (B cells stimulated with LPS only)
The levels of IL-10 found in the supernatants after co-culture of B cells and MSCs originate exclusively from stimulated B cells, since highly purified mouse MSCs (devoid of cells with leukocyte or myeloid cell markers) did not produce detectable IL-10 (data not shown).
The Suppression of IL-10 Production Requires Contact Between B Cells and MSCs
To test whether the MSC plus cytokine-mediated suppression of IL-10 production requires cell-cell contact or is caused by a cell-free molecule produced by MSCs, B cells were separated from MSCs by semipermeabile membrane and the production of IL-10 was determined. As demonstrated in Fig. 3a, separation of B cells and MSCs completely abrogated the suppression of IL-10 production occurring in the cultures containing MSCs and IL-4 or IFN-γ. In addition, the supernatants obtained after incubation of MSCs with IL-4, IFN-γ, LPS, LPS plus IL-4 or LPS plus IFN-γ did not suppress IL-10 production by LPS-stimulated B cells (Fig. 3b).
The suppression of IL-10 production by B cells in cultures containing MSCs and IL-4 or IFN-γ requires contact between B cells and MSCs. (a) B cells were stimulated with LPS in the presence of MSCs and IL-4 or IFN-γ in standard culture conditions or in cultures where B cells were separated from MSCs (trMSCs) by a semipermeabile trasnswell system. (b) Production of IL-10 in cultures of B cells stimulated with LPS in the presence of supernatants obtained after cultivation of MSCs that were either unstimulated or stimulated for 48 h with IL-4, IFN-γ, LPS or LPS plus IL-4 or LPS plus IFN-γ. Each bar represents the mean ± SD from 3 independent experiments. Values with asterisks are significantly different (***p < 0.001) from the control (B cells stimulated with LPS only)
Expression of Genes for Immunoregulatory Molecules in MSCs Treated with IL-4 or IFN-γ
To search for the putative molecule(s) produced by MSCs and responsible for the suppression of IL-10 production by B cells, MSCs were cultured for 48 h alone or in the presence of IL-4, IFN-γ, LPS, LPS and IL-4 or LPS and IFN-γ, and the expression of genes for the putative immunomodulatory molecules was determined by RT-PCR. We identified three genes (IDO, Cox-2 and PD-L1) which were upregulated in the presence of IFN-γ and one molecule (Fas) slightly upregulated in the presence of IL-4 (Fig. 4). However, there were apparently distinct patterns of gene expression between IL-4- and IFN-γ − treated MSCs.
The expression of genes for immunomodulatory molecules in purified MSCs which were cultured for 48 hrs unstimulated (1) or stimulated with IL-4 (2), IFN-γ (3), LPS (4) or with LPS and IL-4 (5) or LPS and IFN-γ (6). The expression of genes for IDO (a), Cox-2 (b), IL-6 (c), TGF-β (d), HGF (e), PDL-1 (f), Fas (g) or Fas-L (h) was determined by real-time PCR. Each bar represents the mean ± SD from 3 independent experiments. Values with asterisks are significantly different (*p < 0.05, **p < 0.01, ***p < 0.001) from those for unstimulated MSCs
Distinct Effects of Preincubation with IL-4 or IFN-γ on the Immunosuppressive Properties of MSCs
Purified MSCs were preincubated for 24 h alone or in the presence of IL-4 or IFN-γ and then cultured with LPS-stimulated B cells. The suppression of IL-10 production occurred only in the cultures containing MSCs pretreated with IFN-γ (Fig. 5a). If B cells were preincubated alone or with IL-4 or IFN-γ and stimulated with LPS in the presence of MSCs, no significant suppression of IL-10 production was detected, irrespective of whether B cells were pretreated with IL-4 (Fig. 5b) or IFN-γ (Fig. 5c). Rather, B cells preincubated with IL-4 produced in the presence of MSCs even more IL-10 than in the absence of MSCs.
The effects of MSCs which were preincubated with IL-4 or IFN-γ, on production of IL-10 by B cells. Purified MSCs were preincubated for 24 hrs untreated or in the presence of IL-4 or IFN-γ and were added to cultures of LPS-stimulated B cells that were either untreated (a) or preincubated with IL-4 (b) or IFN-γ (c). The production of IL-10 was determined after a 72-hr incubation by ELISA. Each bar represents the mean ± SD from 3 independent experiments. Values with asterisks are significantly different (***p < 0.001) from the control (B cells stimulated with LPS in the absence of MSCs)
The Effects of Inhibitors on MSC-Mediated Suppression of IL-10 Production by B Cells
Since we identified 3 molecules (IDO, Cox-2 and PD-L1) which were upregulated in IFN-γ − treated MSCs and one molecule (Fas) with slightly enhanced expression in IL-4-treated MSCs, we made attempts to block these molecules and thus inhibit their possible role in the suppression. We used indomethacine (an inhibitor of Cox-2 and PGE2 synthesis), 1-MT (a selective inhibitor of IDO) or inhibitory mAb anti-PD-L1 and anti-Fas). These inhibitors were added to the cultures of B cells stimulated with LPS in the presence of MSCs and IL-4 or IFN-γ, and the production of IL-10 was determined by ELISA after a 72-h incubation period. The production of IL-10 was strongly inhibited in cultures containing MSCs and IL-4 or IFN-γ. As demonstrated in Fig. 6, indomethacine completely abrogated the suppression in cultures containing MSCs and IFN-γ, but had no detectable effect on the suppressioon in cultures containing MSCs and IL-4. The suppression of IL-10 production in the cultures containing MSCs and IL-4 was not abrogated by any inhibitors or mAb.
The effects of IDO or Cox-2 inhibitors and neutralization mAb anti-PD-L1 or anti-Fas on the suppression of IL-10 production by B cells stimulated with LPS in the presence of MSCs and IL-4 or IFN-γ. Indomethacine (a selective inhibitor of Cox-2), 1-MT (an inhibitor of IDO), mAb anti-PD-L1 or mAb anti-Fas were added into the cultures of B cells stimulated with LPS in the presence of MSCs and IL-4 (a) or IFN-γ (b). The production of IL-10 was determined after a 72-hr incubation by ELISA. Each bar represents the mean ± SD from 3 independent experiments. Values with asterisks are significantly different (***p < 0.001) from the control (B cells stimulated with LPS in the absence of MSCs and IL-4 or IFN-γ)
Discussion
The immunomodulatory properties of MSCs have been well documented in various models and it has been shown that MSCs regulate the reactivity of T cells, macrophages, dendritic cells, NK cells and B cells by multiple different mechanisms. Although extensive data are available on the effects of MSCs on T cells, T cell subpopulations or antigen-presenting cells, less knowledge exists about the effects of MSC on B cells or even their subpopulations.
It has been shown that MSCs inhibit proliferation of B cells and decrease antibody production [25]. Within B-cell population, a subpopulation of cells producing IL-10 has been identified and called Bregs or B10 cells for their potential to inhibit immune responses by a production of IL-10 as a regulatory cytokine [20]. We recently described that the production of IL-10 by activated B cells is strongly suppressed by MSCs in the presence of IFN-γ [23]. This observation supported the findings that the immunomodulatory properties of MSCs depend on cytokine environment. It has been shown that MSCs sense their environment and can be polarized towards either a pro-inflammatory or anti-inflammatory phenotype depending on the TLR signals received [26, 27]. Other studies also confirmed the sensitivity of the immunoregulatory properties of MSCs to the cytokine environment [28–30]. Since the immunoregulatory activities of MSCs depend on their priming with cytokines, we tested the effects of other cytokines on MSC-mediated regulation of IL-10 production. The results revealed that IL-4 is another cytokine synergizing with MSCs in the suppression of IL-10 production by B cells, but that MSCs inhibit IL-10 production in the presence of IL-4 by a different mechanism than in the presence of IFN-γ.
The observation that MSCs, which are generally considered as immunosuppressive cells, inhibit production of immunosuppressive molecule IL-10, deserves more attention. Our data suggest that MSCs protect themselves (expression of inhibitory molecule PD-L1, production of suppressive molecules Cox-2 and IDO and in dependecnce on the cytokine environment modulate immune response to keep immunological homeostasis. MSCs suppress harmful immune responses, but simultaneously they can attenuate a strong immunosuppression by inhibition of IL-10 production. Multiple immunoregulatory mechanisms expressed in MSCs could ensure such complexity of immunomodulatory action of MSCs.
To search for a putative inhibitory molecule produced by IL-4- or IFN-γ − treated MSCs, different patterns of the expression of genes for immunoregulatory molecules were found. While IFN-γ stimulated the expression of IDO, Cox-2 and PD-L1 genes, IL-4 had no effect on the expression of these genes, but rather enhanced the expression of Fas molecule. While the involvement of IDO or Cox-2 in MSC-mediated immunosuppression has been well documented [8, 12], the high expression of PD-L1 molecules on IFN-γ-treated MSC might suggest the role of PD-L1 in the protection of MSCs and in their lower immunogenicity. Similarly, the expression of PD-L1 was found in some types of cancers, where this molecule contributes to their low immunogenicity and has become a target for cancer immune therapy [31, 32]. The use of selective inhibitors or mAb against PD-L1 or Fas molecules showed that only indomethacine, a selective inhibitor of Cox-2 and PGE2 production, completely abrogated IFN-γ-induced suppression. However, indomethacine had no effect on the IL-4-induced suppression of IL-10 production. The results also showed that MSC-mediated suppression of IL-10 production in the presence of IFN-γ or IL-4 was completely abrogated, if B cells and MSCs were separated by semipermeabile membrane. Furthermore, MSCs preincubated with IFN-γ and then carefully washed strongly inhibited IL-10 production by B cells. On the other hand, MSCs preincubated with IL-4 rather enhance IL-10 secretion, but the incubation of B cells with MSCs and IL-4 simultaneously strongly inhibited IL-10 production. These observations suggest that IL-4 and IFN-γ activate different regulatory mechanisms in MSCs, one of which involves the Cox-2 - PGE2 pathway and other remains to be resolved. Even Akiyama et al. [33] described the involvement of Fas/FasL-induced apoptosis in MSC-mediated immunosuppression and we observed the enhanced Fas expression in IL-4-treated MSCs, we were not able to abrogate the suppression of IL-10 production by inclusion of anti-Fas antibodies into cultures containing B cells, MSCs and IL-4.
It has been well documented that MSCs modulate immune response by multiple mechanisms. Some of these mechanisms (TGF-β, HGF or IL-6 production, Fas and FasL expression) are constitutively expressed by MSCs, while others (such IDO, PD-L1, Cox-2) are activated only after stimulation with cytokines or through Toll-like receptors [12]. We have shown that two of these mechanisms are involved in the MSC-mediated suppression of IL-10 production by activated B cells. As it is summarized in Fig. 7, one inhibitory mechanism is induced by the proinflammatory cytokine IFN-γ and involves the Cox-2 pathway. The other mechanism is activated by the Th2 cytokine IL-4 and is independent of Cox-2. These observations show that MSCs can inhibit response to the same stimulus by different mechanisms in dependence on cytokine environment. The findings thus support idea of the complexity of the mechanisms of MSC-mediated immunosuppression.
Immunoregulatory mechanisms activated in MSCs by IL-4 or IFN-γ. Untreated MSCs spontaneously express genes for TGF-β, IL-6, HGF, Fas and FasL, but do not inhibit IL-10 production by LPS-stimulated B cells. In the presence of IL-4, an upregulation of Fas gene can be detected and MSCs inhibit IL-10 production. In the presence of IFN-γ MSCs strongly upregulate genes for IDO, Cox-2 and PD-L1 molecules and inhibit IL-10 production. The inhibition of IL-10 production by IFN-γ-activated MSCs involves the Cox-2 pathway, while the suppression of IL-10 production by IL-4-activated MSCs is Cox-2 independent
References
Di Nicola, M., Carlo-Stella, C., Magni, M., Milanesi, M., Longoni, P. D., Matteucci, P., et al. (2012). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood, 99(10), 3838–3843.
Le Blanc, K., & Ringdén, O. (2007). Immunomodulation by mesenchymal stem cells and clinical experience. Journal of Internal Medicine, 262(5), 509–525.
Aggarwal, S., & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105(4), 1815–1822.
Abumaree, M., Al Jumah, M., Pace, R. A., & Kalionis, B. (2012). Immunosuppressive properties of mesenchymal stem cells. Stem Cell Reviews, 8(2), 375–392.
English, K., Barry, F. P., & Mahon, B. P. (2008). Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunology Letters, 115(1), 50–58.
Cho, D. I., Kim, M. R., Jeong, H. Y., Jeong, H. C., Jeong, M. H., & Yoon, S. H. (2014). Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Experimental and Molecular Medicine, 46, e70. doi:10.1038/emm.2013.135.
Angoulvant, D., Clerc, A., Benchalal, S., Galambrun, C., Farre, A., Bertrand, Y., et al. (2004). Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology, 41(3–4), 469–476.
Spaggiari, G. M., Capobianco, A., Abdelrazik, H., Becchetti, F., Mingari, M. C., & Moretta, L. (2008). Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood, 111(3), 1327–1333.
Bartholomew, A., Sturgeon, C., Siatskas, M., Ferrer, K., McIntosh, K., Patil, S., et al. (2002). Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology, 30(1), 42–48.
Le Blanc, K., Rasmusson, I., Sundberg, B., Götherströmm, C., Hassan, M., Uzumel, M., et al. (2004). Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet, 363(9419), 1439–1441.
Zappia, E., Casazza, S., Pedemonte, E., Benvenuto, F., Bonanni, I., Gerdoni, E., et al. (2005). Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood, 106(5), 1755–1761.
English, K. (2013). Mechanisms of mesenchymal stromal cell immunomodulation. Immunology and Cell Biology, 91(1), 19–26.
Oh, J. Y., Kim, M. K., Shin, M. S., Wee, W. R., & Lee, J. H. (2009). Cytokine secretion by human mesenchymal stem cells cocultured with damaged corneal epithelial cells. Cytokine, 46(1), 100–103.
Holan, V., Trosan, P., Cejka, C., Javorkova, E., Zajicova, A., Hermankova, B., et al. (2015). A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells and Translational Medicine, 4(9), 1052–1063.
Rasmusson, I., Ringdén, O., Sundberg, B., & Le Blanc, K. (2005). Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Experimental Cell Research, 305(1), 33–41.
Franquesa, M., Hoogduijn, M. J., Bestard, O., & Grinyó, J. M. (2012). Immunomodulatory effect of mesenchymal stem cells on B cells. Frontiers in Immunology, 2012;doi: 10.3389/fimmu.2012.00212.
Corcione, A., Benvenuto, F., Ferretti, E., Giunti, D., Cappiello, V., Cazzanti, F., et al. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood, 107(1), 367–372.
Mizoguchi, A., Mizoguchi, E., Smith, R. N., Preffer, F. I., & Bhan, A. K. (1977). Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. Journal of Experimental Medicine, 186(10), 1749–1756.
Mauri, C., & Bosma, A. (2012). Immune regulatory function of B cells. Annual Review of Immunology, 30, 221–241.
Bouaziz, J. D., Le Buanec, H., Saussine, A., Bensussan, A., & Bagot, M. (2012). IL-10 producing regulatory B cells in mice and humans: state of the art. Current Molecular Medicine, 12(5), 519–527.
Yoshizaki, A., Miyagaki, T., DiLillo, D. J., Matsushita, T., Horikawa, M., Kountikov, E., et al. (2012). Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature, 491(7423), 264–268.
Holan, V., Zajicova, A., Javorkova, E., Trosan, P., Chudickova, M., Pavlikova, M., et al. (2014). Distinct cytokines balance the development of regulatory T cells and interleukin-10-producing regulatory B cells. Immunology, 141(4), 577–586.
Hermankova, B., Zajicova, A., Javorkova, E., Chudickova, M., Trosan, P., Hajkova, M., et al. (2016). Suppression of IL-10 production by activated B cells via a cell contact-dependent cyclooxygenase-2 pathway upregulated in IFN-γ-treated mesenchymal stem cells. Immunobiology, 221(12), 129–136.
Javorkova, E., Trosan, P., Zajicova, A., Krulova, M., Hajkova, M., & Holan, V. (2014). Modulation of the early inflammatory microenvironment in alkali-burned eye by systemically administered interferon-γ treated mesenchymal stem cells. Stem Cells and Development, 23(20), 2490–2500.
Asari, S., Itakura, S., Ferreri, K., Liu, C. P., Kuroda, Y., Kandeel, F., et al. (2009). Mesenchymal stem cells suppress B-cell terminal differentiation. Experimental Hematology, 37(5), 604–615.
Auletta, J. J., Deans, R. J., & Bartholomew, A. M. (2012). Emerging roles for multipotent, bone marrow-derived stromal cells in host defense. Blood, 119(8), 1801–1809.
Waterman, R. S., Tomchuck, S. L., Henkle, S. L., & Betancourt, A. M. (2010). A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE, 5(4), e10088. doi:10.1371/journal.pone.0010088.
Najar, M., Raicevic, G., Fayyad-Kazan, H., De Bruyn, C., Bron, D., Toungouz, M., et al. (2012). Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Reviews, 8(4), 1188–1198.
Ren, G., Zhang, L., Zhao, X., Xu, G., Zhang, Y., Roberts, A. I., et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell, 2(2), 141–150.
English, K., Barry, F. P., Field-Corbett, C. P., & Mahon, B. P. (2007). IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunology Letters, 110(2), 91–100.
Patel, S. P., & Kurzrock, R. (2015). PD-L1 expression as a predictive biomarker in cancer immunotherapy. Molecular Cancer Therapeutics, 14(4), 847–856.
Muenst, S., Soysal, S. D., Tzankov, A., & Hoeller, S. (2015). The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opinions on Therapeutic Targets, 19(2), 201–211.
Akiyama, K., Chen, C., Wang, D., Xu, X., Qu, C., Yamaza, T., et al. (2012). Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell, 10(5), 544–555.
Acknowledgments
This work was supported by grant 14-12580S from the Grant Agency of the Czech Republic, project 80815 from the Grant Agency of Charles University, and by the projects SVV 260206, CZ.1.05/1.1.00/02.0109, CZ.2.16/3.1.00/21528, UNCE 204013, NPU-I:LO1508 and NPUI: LO1309.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest
Rights and permissions
About this article
Cite this article
Holan, V., Hermankova, B., Bohacova, P. et al. Distinct Immunoregulatory Mechanisms in Mesenchymal Stem Cells: Role of the Cytokine Environment. Stem Cell Rev and Rep 12, 654–663 (2016). https://doi.org/10.1007/s12015-016-9688-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-016-9688-y