Abstract
Liquiritigenin (LQ), as a dihydroflavone monomer compound extracted from Glycyrrhiza uralensis Fisch, has been demonstrated to show anti-tumor effects in multiple human cancers, including lung adenocarcinoma. Our study aimed to explore its role in lung squamous cell carcinoma (LSCC) development and the related mechanism. The effects of LQ on SK-MES-1 and NCI-H520 cell proliferation, cell cycle, and apoptosis were investigated. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and colony formation assays revealed that LQ inhibited LSCC cell viability and proliferation in a dose- and time-dependent manner. Flow cytometry analysis demonstrated that LQ promoted G2/M cell cycle arrest, cell apoptosis, and loss of mitochondrial membrane potential. In vivo assays showed that LQ administration suppressed tumor growth in nude mice. Additionally, LQ treatment reduced the levels of phosphorylated PI3K, AKT, and mTOR levels in LSCC cells. Pretreatment with the PI3K inhibitor LY294002 antagonized the LQ-mediated effects on cell proliferation, cell cycle arrest, and apoptosis in LSCC cells. Collectively, LQ induces cell cycle arrest and apoptosis in LSCC by inactivating the PI3K/AKT/mTOR pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the main cause of cancer-related death and is related to 18% of total cancer deaths worldwide [1]. Lung squamous cell carcinoma (LSCC) accounts for 30% of all the lung cancer cases and mainly originates in the bronchial epithelium [2]. LSCC is a heterogeneous malignancy characterized by a high mutational burden, which is present in the early stage of this disease [3]. Currently, chemotherapy and immune checkpoint inhibitors are the first-line treatment options for advanced LSCC, which are administered as monotherapy or combination therapy [4]. Even though these therapies have improved the clinical outcomes, only approximately 23 to 30% of patients with advanced NSCLC qualify for the use of pembrolizumab [5, 6]. Therefore, it is needed to find effective therapeutic strategies and new agents for LSCC to reduce mortality to improve the overall survival of patients.
In recent years, natural compounds, with low toxicity and high efficiency, have been demonstrated to be promising novel anticancer drugs [7]. Glycyrrhiza uralensis Fisch is a Chinese herbal medicine and has antioxidant, anti-inflammatory, antibacterial, antiviral, anti-spasmodic, anticancer, lowering blood pressure, lowering blood lipids, anti-gout, and anti-cardiovascular diseases effects [8,9,10]. Liquiritigenin (LQ; chemical structure is shown in Fig. 1A) is a dihydroflavone monomer compound extracted from Glycyrrhiza uralensis Fisch, which exerts effective anti-tumor effects in multiple human cancers [11]. Wang et al. found that LQ increased reactive oxygen species levels, induced loss of mitochondrial membrane potential (MMP), and enhanced cell apoptosis rate in hepatocellular carcinoma and xenografted mouse models [12]. Zhang et al. reported that LQ suppressed the proliferative, migratory, and invasive abilities of breast cancer cells and enhanced cell apoptosis [13]. Shi et al. reported that LQ strengthened the inhibitory effects of cisplatin on invasion and metastasis of B16F10 melanoma cells [14]. Importantly, LQ was reported to suppress lung adenocarcinoma A549 cell migration by inhibiting the PI3K/AKT pathway [15]. However, whether LQ participates in LSCC development remains largely unknown.
Effect of LQ on the growth of LSCC cells. A The chemical structure of LQ. B–D The viability of SK-MES-1, NCI-H520, and BEAS-2B cells after LQ treatment for different time points was detected by MTT assay. N = 3. E Representative pictures of tumors dissected from control and LQ or gefitinib-treated xenograft mouse models. F–G Tumor growth curve and tumor weight. N = 6. *p < 0.05, **p < 0.01
The effects of LQ on LSCC cell growth, cell cycle, and apoptosis were investigated in our study. The regulatory role of the PI3K/AKT/mTOR pathway in the LQ-mediated LSCC development was also explored. Our study might help understand the anti-tumor role of LQ in LSCC and provide more effective therapeutic strategies for LSCC.
Materials and Methods
Cell Culture
The human LSCC cell lines, including SK-MES-1 (#CL-0213) and NCI-H520 (#CL-0402) were provided by Procell (Wuhan, China), and a normal lung epithelial cell line (BEAS-2B; #ml096003) were provided by Mlbio (Shanghai, China). All cells were placed in an incubator with 5% CO2 at 37 °C. RPMI 1640 medium (#LM87077C; LMAI Bio; Shanghai) containing 1% penicillin/streptomycin (#ZY90307; Zeye Biotechnology, Shanghai) and 10% fetal bovine serum (#40130ES76; Yeason, Shanghai) was used for cell culture.
Cell Viability Assay
LQ (#PHL89543) was obtained from Sigma-Aldrich (Shanghai). SK-MES-1, NCI-H520, and BEAS-2B cells were collected and seeded (5 × 103 cells/well) in 96-well plates. LQ (0, 25, 50, 100, and 200 μM) was used to treat SK-MES-1 and NCI-H520 cells for 24, 48, and 72 h and treat BEAS-2B cells for 24 h. The doses of LQ used for cell treatment were selected according to the previous literature [14]. Then, 20 μL of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL; #M8180; Solarbio, Beijing, China) was added to each well and incubated with cells for 4 h. The absorbance was detected using a microplate reader at 570 nm.
Cell Proliferation Assay
LSCC cells were seeded (500 cells/well) in a 6‐well culture dish and incubated for 2 weeks. Cells were washed twice with phosphate buffer saline (PBS; #BJ-S963625; Bangjing, Shanghai) after the supernatant was removed. Next, the cells were stained with 0.5% crystal violet (#60506ES60; Yeason) after fixation with 4% paraformaldehyde (#XY-PCR-1654; Xuanya, Shanghai). The colonies were observed and photographed microscopically. The number of colonies was calculated using ImageJ software (National Institutes of Health, MD, USA). The pictures were imported into the software, and the color and parameters were set.
Cell Cycle Assay
LSCC cells were seeded (2 × 106 cells/well) in 12-well plates and treated as described above. Then, cells were collected, digested with 0.25% trypsin (#25200-072; Reanta, Beijing, China), washed with PBS, and fixed with cold 70% ethanol at 4 °C overnight. Next, cells were incubated with 50 μL of RNase A (1 mg/mL) at 37 °C for 30 min and then with 400 μL of propidium iodide (PI) solution (50 mg/L) for 30 min in the dark. Finally, cell cycle distribution was analyzed using flow cytometry (BD FACS Calibur) equipped with Cell Quest software.
Cell Apoptosis Assay
LSCC cells were seeded (1 × 105 cells/mL) in 96-well plates. After LQ treatment for 24 h, cells were harvested using 0.25% trypsin and resuspended in ice-cold 1 × binding buffer. Then, 5 μL of Annexin V-FITC solution and 10 μL of PI solution (#C9212; Warbio, Nanjing, China) were added. Cells were incubated on ice for 15 min in the dark. Apoptosis was analyzed by flow cytometry equipped with Cell Quest software.
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine Iodide (JC-1) Staining
JC-1 assay kit (#M8650; Solarbio) was used to detect changes in MMP. LSCC cells were seeded (5 × 105 cells/well) in 6-well plates and incubated overnight. After LQ treatment for 24 h, cells were obtained by centrifugation, washed with PBS, and stained with JC-1 for 20 min at 37 °C as per the manufacturer’s instructions. Then, cells were washed in 1 × JC-1 staining buffer two times. The data were analyzed using flow cytometry.
Reverse Transcriptase Quantitative Polymerase Chain Reaction (RT-qPCR)
Total cellular RNA was extracted using AG RNAex Pro RNA reagent (#AG21102; Accurate Biology, Hunan, China). Then, 2 μg of RNA was used to synthesize cDNA by the use of Evo M‐MLV reverse transcription master mix (#AG11706; Accurate Biology). RT‐qPCR was conducted by using SYBR Green Pro Taq HS premixed qPCR kit (# AG11701; Accurate Biology) with the following primers for PCNA: forward GTAATGACTCTATGTGATGCC; reverse GATAAAAGGTTACAAACGATG; Ki67: forward CTCCATCCTGGCCTCGCTGT; reverse GCTGTCACCTTCACCGTTCC; and GAPDH: forward TGTTCGTCATGGGTGTGAAC and reverse ATGGCATGGACTGTGGTCAT. GAPDH served as internal control. Data were analyzed using the 2−ΔΔCT calculation method.
Western Blotting
LSCC cells were lysed in RIPA lysis buffer (#C1053-500; APPLYGEN, Beijing) on ice for 30 min. The protein samples were electroblotted onto polyvinylidene difluoride membrane after being electrophoretically separated by 10% SDS-polyacrylamide gel. The membrane was blocked for 1 h in 5% nonfat milk (#abs952; Absin, Shanghai), and the immunoblots were probed with primary antibodies against Ki67 (#FNab09788; FineTest, Wuhan), PCNA (#FNab06216; FineTest), P21 (#FNab06067; FineTest), P27 (#FNab06068), Cyclin B1 (#FNab02122; FineTest), CDK1 (#FNab01550; FineTest), Bax (#FNab00810; FineTest), Bak (#FNab00796; FineTest), Cleaved caspase 3 (#FNab10013; FineTest), Cleaved PARP (#abs132006; Absin), Bcl-2 (#FNab00839; FineTest), Bcl-xl (#abs131907; Absin), Mcl-1 (#FNab05052; FineTest), p-PI3K p85α (#AP0854; ABclonal, Wuhan), PI3K p85α (#A11177; ABclonal), p-AKT (#AP1259; ABclonal), AKT (#A24477; ABclonal), p-mTOR (#FNab10006; FineTest), mTOR (#FNab10318; FineTest), and GAPDH (#FNab03342; FineTest) at a dilution of 1:1000 at 4°C overnight. The membrane was further incubated with the secondary antibody (#FNSA-0004; FineTest) at a dilution of 1:5000 at room temperature for 1 h. Finally, the reactive proteins were visualized with an enhanced chemiluminescence detection kit (#LM1012; LMAl Bio), and quantified using ImageJ software
Tumor Xenograft Experiment
The animal experiment was approved by the Animal Care and Use Committees of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology. Six-week-old male BALB/c nude mice were subcutaneously injected with 2 × 106 SK-MES-1 cells. The mice were then randomly divided into the control (n = 6), the LQ group (n = 6), and the gefitinib group (n = 6). After three days, the mice in the LQ group and the gefitinib group were given LQ (20 mg/kg/day) [14, 16] and gefitinib (2 mg/kg/day) [17] by oral gavage. The control mice were given 5% dimethyl sulfoxide. The tumor volume was calculated using the formula: Volume=Length × Width2/2 every three days post-injection. After 24 days of LQ administration, the mice were sacrificed through cervical dislocation. Tumors were excised, photographed, and weighed.
Statistical Analysis
Three independent biological replicates were conducted for each in vitro assay. GraphPad 6.0 software was employed for statistical analysis. Data are presented as mean ± standard deviation. Student’s t test or one-way analysis of variance was performed to analyze the differences among groups. p < 0.05 was deemed as statistically significant.
Results
LQ Inhibits the Growth of LSCC Cells
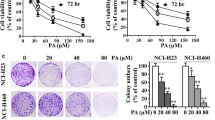
First, the cytotoxicity of LQ was determined through MTT assay. The results revealed that LQ at the concentrations of 25 μM, 50 μM, 100 μM, and 200 μM reduced the viability of LSCC cells (SK-MES-1, NCI-H520) in a dose- and time-dependent manner (Fig. 1B-C). Interestingly, LQ at 25 μM, 50 μM, and 100 μM exerted no significant cytotoxicity to normal lung epithelial cells (BEAS-2B) (Fig. 1D). Furthermore, in vivo assays showed that LQ (20 mg/kg) administration significantly suppressed tumor growth in nude mice, as evidenced decrease in tumor weight and volume (Fig. 1E-1G). Moreover, there was no significant difference on tumor weight and volume between the LQ group and gefitinib group.
LQ Suppresses the Proliferation of LSCC Cells
As shown in colony formation assay, treatment with 50 μM or 100 μM LQ caused a significant reduction in the number of colonies formed by LSCC cells (Fig. 2A). The mRNA levels of cell proliferation markers, Ki67 and PCNA, were measured by RT-qPCR. We found that Ki67 and PCNA mRNA levels were markedly reduced in SK-MES-1 and NCI-H520 cells after LQ treatment (Fig. 2B). Since the inhibitory effects of LQ at the concentrations of 50 μM and 100 μM were relatively significant, these two concentrations were used for the subsequent assays. The western blotting results revealed that LQ treatment decreased Ki67 and PCNA protein levels in LSCC cells (Fig. 2C).
Effect of LQ on LSCC cell proliferation. A LSCC cell proliferation after treatment with LQ (25, 50, or 100 μM) was detected by colony formation assay. B Ki67 and PCNA mRNA expression in control and LQ-treated LSCC cells was measured by RT-qPCR. C Ki67 and PCNA protein levels in LSCC cells after LQ treatment were measured using western blotting. N = 3. **p < 0.01
LQ Promotes Cell Cycle Arrest of LSCC Cells
Next, flow cytometry was used for cell cycle analysis following LQ treatment. As revealed in Fig. 3A, cell cycle percentage at the G2/M phase were significantly increased after LQ treatment. Western blotting was conducted to analyze the levels of cell cycle markers. It was shown that LQ treatment upregulated P21 and P27 protein levels but downregulated Cyclin B1 and CDK1 protein levels in LSCC cells (Fig. 3B), suggesting that LQ induces cell cycle arrest of LSCC cells.
Effect of LQ on the cell cycle distribution of LSCC cells. A Cell cycle distribution of LQ-treated LSCC cells was analyzed using PI staining and flow cytometry assay. B The levels of cell-cycle-related proteins in control and LQ-treated LSCC cells were measured using western blotting. N = 3.*p < 0.05, **p < 0.01
LQ Induces the Apoptosis of LSCC Cells
Meanwhile, whether LSCC cell apoptosis is affected by LQ treatment was investigated. Flow cytometry assay manifested that the apoptosis rate in LQ-treated cells was notably higher than in untreated cells (Fig. 4A). The decrease in MMP is considered to be the earliest biological event that occurs during apoptosis. JC-1 staining showed that LQ treatment caused significant loss of MMP in LSCC cells (Fig. 4B). Additionally, western blotting revealed that Bak, Bax, Cleaved caspase 3, and Cleaved PARP levels were enhanced whereas Bcl-2, Bcl-xl, and Mcl-1 levels were decreased in LSCC cells after LQ treatment (Fig. 4C, D).
Effect of LQ on LSCC cell apoptosis. A Control and LQ-treated LSCC cells were double stained with Annexin V-FITC/PI and the percentage of Annexin V-positive cells was analyzed by flow cytometry. B Mitochondrial membrane potential (MMP) in LQ-treated LSCC cells was detected using JC-1 staining and flow cytometry. C, D Evaluation of the expression of pro-apoptotic proteins (Bak, Bax, Cleaved caspase 3, and Cleaved PARP) and anti-apoptotic proteins (Bcl-2, Bcl-xl, and Mcl-1) in LQ-treated LSCC cells. N = 3. **p < 0.01
LQ Inhibits LSCC Through Inactivating the PI3K/AKT/mTOR Pathway
Finally, the molecular mechanism by which LQ exerts regulatory effects on LSCC cell proliferation, cell cycle, and apoptosis were explored. As indicated in Fig. 5A, LQ treatment markedly reduced p-PI3K, p-AKT, and p-mTOR protein levels in LSCC cells (Fig. 5A), demonstrating the inhibition of LQ on the activation of the PI3K/AKT/mTOR pathway. The PI3K inhibitor LY294002 was used to verify the role of this signaling pathway in the LQ-mediated LSCC development. LSCC cells were pretreated with LY294002 (10 μM) before LQ (100 μM) treatment, and we observed that LY294002 pretreatment reversed the LQ-induced decrease in PCNA, Ki67, and CDK1 levels and increase in Bax and Cleaved caspase 3 levels in LSCC cells (Fig. 5B).
Effect of LQ on the PI3K/AKT/mTOR pathway in LSCC cells. A PI3K, p-PI3K p85α, AKT, p-AKT (Thr308), mTOR, and p-mTOR (S2448) protein levels in LSCC cells after LQ treatment were measured using western blotting. B PCNA, Ki67, CDK1, Bax, and Cleaved caspase 3 protein levels in LSCC cells after LY294002 plus LQ treatment were measured using western blotting. N = 3. **p < 0.01; ##p < 0.01
Discussion
The occurrence and development of malignancies are usually accompanied by a serious imbalance between tumor cell growth and apoptosis. Inhibiting proliferation and enhancing apoptosis have become promising anti-tumor strategies. Compared with traditional chemical drugs, natural plant-derived agents have the advantages of fewer side effects and lower toxicity, showing strong anti-tumor efficacy [18]. Our study disclosed the anti-tumor efficacy of LQ in LSCC and the related mechanism.
The uncontrolled proliferation of cancer cells is a key issue in the development of cancer. This is due to fast division speed, short life cycle, and abnormal cell cycle of cancer cells [19]. Prolonged retention of cancer cells at the G2/M phase can inhibit cell growth and trigger apoptosis by accumulating DNA damage [20]. Maturation-promoting factor [21], a complex composed of CDK1 and Cyclin B, is required for the transition from the G2 to the M phase during mitosis [22]. CDK1 is stably expressed throughout the cell cycle, whereas Cyclin B is specifically expressed at the G2/M phase [23]. When CDK1 binds to Cyclin B, the conformational change of CDK1 leads to the activation of Thr161 by CDK kinase and the dephosphorylation of Thr14 and Tyt1 by CDC25, thereby forming the active MPF complex [24]. P21 can not only inhibit CDK1 activity by inhibiting the phosphorylation of CDK1 (Thr161) but also mediates Cyclin B degradation in the presence of DNA damage and maintains the cellular G2/M arrest [25, 26]. P27 not only attenuates MPF activity by inactivating MPF and sequestering it in the nucleus but also inhibits Cyclin B and CDK1 [27, 28]. Previously, many studies have clarified that the development of LSCC can be impeded through inducing G2/M cell cycle arrest and suppressing abnormal proliferation of tumor cells [29, 30]. Here, our results showed that LQ treatment markedly suppressed LSCC cell proliferation, as evidenced by reduced levels of proliferation-related proteins Ki67 and PCNA. Moreover, we observed G2/M cell cycle arrest after LQ treatment, accompanied by upregulated levels of P21 and P27 and downregulated levels of Cyclin B1 and CDK1 in LSCC cells.
Apoptosis is a type of programmed cell death [31]. Cancer cells are able to escape from apoptotic signaling, thus retaining their ability to survive and continue to grow and spread [32]. It is known that apoptosis occurs through both intracellular and extracellular pathways [33]. Mitochondria are the regulatory center of apoptosis [34]. The pro-apoptotic proteins such as Bax and Bak have the function of promoting the mitochondrial permeability pore. When cells are stimulated by external harsh signals, these proteins will be activated or translocated to the mitochondrial membrane to form an oligomeric complex, which leads to loss of transmembrane potential loss and release of cytochrome c from the mitochondria to the cytoplasm [35]. Cytochrome c will bind to Apaf-1 to form an apoptotic complex to trigger a cascade of caspase reactions and apoptosis [36]. PARP, the main substrate of caspase 3, can be cleaved by caspase 3 when apoptosis is initiated [37]. Previously, LQ was revealed to induce human cervical carcinoma (HeLa) cell apoptosis through the mitochondrial apoptotic pathway [38]. Furthermore, LQ promoted pituitary adenoma cell apoptosis through the reactive oxygen species-dependent mitochondrial pathway [39]. Here, we reported that LQ induced loss of MMP, elevated Bak, Bax, Cleaved caspase 3, and Cleaved PARP levels, and reduced Bcl-2, Bcl-xl, and Mcl-1 levels in LSCC cells, thereby enhancing the apoptosis of LSCC cells.
Abnormal activation of the PI3K/AKT/mTOR pathway is a key event in the development of multiple malignant cancers, including lung, breast, colon, uroepithelial, ovarian, prostate, and endometrial cancers, etc, and this signaling pathway is involved in the regulation of cell cycle, growth, apoptosis, metabolism, adhesion, and metastasis [40, 41]. Clinical research has demonstrated that inhibitors targeting the PI3K/AKT/mTOR pathway have positive effects on tumor treatment [42]. Many flavonoids have been elucidated to hinder lung cancer progression by inhibiting the PI3K/AKT/mTOR signaling [43,44,45]. Importantly, LQ was previously confirmed to exert its anti-tumor effects by repressing the PI3K/AKT/mTOR pathway. For example, LQ inhibited colorectal cancer HCT116 cell growth and invasion through inactivating the PI3K/AKT pathway [46]. LQ repressed the PI3K/AKT pathway to reduce lung adenocarcinoma A549 cell migration [15]. Here, we found that LQ treatment considerably inhibited PI3K, AKT, and mTOR phosphorylation in LSCC cells, confirming the suppression of LQ on the PI3K/AKT/mTOR pathway. Additionally, pretreatment with the PI3K inhibitor LY294002 antagonized the LQ-induced anti-tumor effects in LSCC cells, which demonstrated that LQ suppressed LSCC cell growth and facilitated apoptosis via inhibition of the PI3K/AKT/mTOR pathway.
Limitations in the current understanding of the drug’s mechanism of action hinders the identification of appropriate methods to observe a specific result in drug research. Network pharmacology gives a new idea for drug research. Through the analysis and integration of big data, potential drug targets and signaling pathways can be predicted more comprehensively [47]. We will conduct the network pharmacology method to predict the signaling pathways of LQ for LSCC and comprehensively reveal the mechanism of action of LQ. Additionally, identification of early diagnostic biomarkers and prognostic biomarkers is important for the prevention and treatment of cancer [48]. More drug targets related to LQ in LSCC will be elucidated to improve the outcome of LSCC.
In summary, our research reports the anti-tumor role of LQ in LSCC. Our findings show that LQ treatment reduced LSCC cell growth, induced G2/M cell cycle arrest, and enhanced apoptosis by suppressing the PI3K/AKT/mTOR pathway (Fig. 6). This finding suggests that LQ might be an effective therapeutic agent for LSCC.
References
Sung, H., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249.
Duma, N., Santana-Davila, R. & Molina, J. R. (2019). Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clinic Proceedings, 94(8), 1623–1640.
Choi, M., et al. (2017). Mutation profiles in early-stage lung squamous cell carcinoma with clinical follow-up and correlation with markers of immune function. Annals of Oncology, 28(1), 83–89.
Reck, M., Remon, J. & Hellmann, M. D. (2022). First-line immunotherapy for non-small-cell lung cancer. Journal of Clinical Oncology, 40(6), 586–597.
Reck, M., et al. (2016). Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. The New England Journal of Medicine, 375(19), 1823–1833.
Garon, E. B., et al. (2015). Pembrolizumab for the treatment of non-small-cell lung cancer. The New England Journal of Medicine, 372(21), 2018–28.
Naeem, A., et al. (2022). Natural products as anticancer agents: Current status and future perspectives. Molecules, 27(23), 8367.
Deutch, M. R., et al. (2019). Bioactive candy: Effects of licorice on the cardiovascular system. Foods, 8(10), 495.
Maria Pia, G. D., et al. (2019). Biological effects of licochalcones. Mini-Reviews in Medicinal Chemistry, 19(8), 647–656.
Yang, R., et al. (2017). The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharmaceutical Biology, 55(1), 5–18.
Zhou, M., Higo, H. & Cai, Y. (2010). Inhibition of hepatoma 22 tumor by Liquiritigenin. Phytotherapy Research, 24(6), 827–33.
Wang, D., et al. (2014). Liquiritigenin induces tumor cell death through mitogen-activated protein kinase- (MPAKs-) mediated pathway in hepatocellular carcinoma cells. BioMed Research International, 2014, 965316
Zhang, Z., et al. (2022). Liquiritigenin blocks breast cancer progression by inhibiting connective tissue growth factor expression via up-regulating miR-383-5p. International Journal of Toxicology, 41(1), 5–15.
Shi, H., et al. (2015). Liquiritigenin potentiates the inhibitory effects of cisplatin on invasion and metastasis via downregulation MMP-2/9 and PI3 K/AKT signaling pathway in B16F10 melanoma cells and mice model. Nutrition and Cancer, 67(5), 761–70.
Wang, Y., et al.(2012). Inhibitory effect of liquiritigenin on migration via downregulation proMMP-2 and PI3K/Akt signaling pathway in human lung adenocarcinoma A549 cells. Nutrition and Cancer, 64(4), 627–34.
Ji, Y., et al. (2021). Liquiritigenin exerts the anti-cancer role in oral cancer via inducing autophagy-related apoptosis through PI3K/AKT/mTOR pathway inhibition in vitro and in vivo. Bioengineered, 12(1), 6070–6082.
Gao, F., et al. (2020). Deguelin suppresses non-small cell lung cancer by inhibiting EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1 destabilization. Cell Death and Disease, 11(2), 143
Xiang, Y., et al. (2019). Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Medicine, 8(5), 1958–1975.
Kubicka, A., Matczak, K. & Łabieniec-Watała, M. (2021). More than meets the eye regarding cancer metabolism. International Journal of Molecular Sciences, 22(17), 9507
Chen, H., et al. (2013). Enhancement of cisplatin-mediated apoptosis in ovarian cancer cells through potentiating G2/M arrest and p21 upregulation by combinatorial epigallocatechin gallate and sulforaphane. Journal of Clinical Oncology, 2013, 872957
Kempf-Bielack, B., et al. (2005). Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). Journal of Clinical Oncology, 23(3), 559–68.
Satyanarayana, A., & Kaldis, P. (2009). Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene, 28(33), 2925–39.
Ding, L., et al. (2020). The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. International Journal of Molecular Sciences, 21(6), 1960
Lim, S., & Kaldis, P. (2013). Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development, 140(15), 3079–93.
Gillis, L. D., et al. (2009). p21Cip1/WAF1 mediates cyclin B1 degradation in response to DNA damage. Cell Cycle, 8(2), 253–6.
Smits, V. A., et al. (2000). p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. Journal of Biological Chemistry, 275(39), 30638–43.
Foijer, F., et al. (2005). Mitogen requirement for cell cycle progression in the absence of pocket protein activity. Cancer Cell, 8(6), 455–66.
Pagano, M. (2004). Control of DNA synthesis and mitosis by the Skp2-p27-Cdk1/2 axis. Molecular Cell, 14(4), 414–6.
Liu, Z., et al. (2022). Isovalerylspiramycin I suppresses non-small cell lung carcinoma growth through ROS-mediated inhibition of PI3K/AKT signaling pathway. International Journal of Biological Sciences, 18(9), 3714–3730.
Yang, C. J., et al. (2009). Pyrogallol induces G2-M arrest in human lung cancer cells and inhibits tumor growth in an animal model. Lung Cancer, 66(2), 162–8.
Xu, X., Lai, Y. & Hua, Z. C. (2019). Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Bioscience Reports, 39(1), BSR20180992
Pistritto, G., et al. (2016). Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY), 8(4), 603–19.
Wong, R. S. (2011). Apoptosis in cancer: from pathogenesis to treatment. Journal of Experimental & Clinical Cancer Research, 30(1), 87
Picca, A., et al. (2021). Cell death and inflammation: The role of mitochondria in health and disease. Cells, 10(3), 537.
Yang, Z., et al. (2016). Equol induces mitochondria-dependent apoptosis in human gastric cancer cells via the sustained activation of ERK1/2 pathway. Molecular Cell, 39(10), 742–749.
Losuwannarak, N., Sritularak, B., & Chanvorachote, P. (2018). Cycloartobiloxanthone induces human lung cancer cell apoptosis via mitochondria-dependent apoptotic pathway. In Vivo, 32(1), 71–78.
Affar, E. B., et al. (2001). Caspase-3-mediated processing of poly(ADP-ribose) glycohydrolase during apoptosis. Journal of Biological Chemistry, 276(4), 2935–42.
Liu, C., et al. (2011). Liquiritigenin induces mitochondria-mediated apoptosis via cytochrome c release and caspases activation in HeLa Cells. Phytotherapy Research, 25(2), 277–83.
Wang, D., et al. (2014). Liquiritigenin exhibits antitumour action in pituitary adenoma cells via Ras/ERKs and ROS-dependent mitochondrial signalling pathways. Journal of Pharmacy and Pharmacology, 66(3), 408–17.
Glaviano, A., et al. (2023). PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Molecular Cancer, 22(1), 138
Yu, L., Wei, J. & Liu, P. (2022). Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Seminars in Cancer Biology, 85, 69–94.
Alzahrani, A. S. (2019). PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Seminars in Cancer Biology, 59, 125–132.
Gong, G., et al. (2022). Antitumor effects of ononin by modulation of apoptosis in non-small-cell lung cancer through inhibiting PI3K/Akt/mTOR pathway. Oxidative Medicine and Cellular Longevity, 2022, 5122448
Liu, X., et al. (2019). Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biological Research, 52(1), 7
Wang, S., et al. (2019). Sotetsuflavone induces autophagy in non-small cell lung cancer through blocking PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Biological Research, 10, 1460
Meng, F. C. & Lin, J. K. (2019). Liquiritigenin inhibits colorectal cancer proliferation, invasion, and epithelial-to-mesenchymal transition by decreasing expression of runt-related transcription factor 2. Oncology Research, 27(2), 139–146.
Gao, S., Tan, H. & Li, D. (2023). Oridonin suppresses gastric cancer SGC-7901 cell proliferation by targeting the TNF-alpha/androgen receptor/TGF-beta signalling pathway axis. Journal of Cellular and Molecular Medicine, 27(18), 2661–2674.
Gao, S., et al. (2021). Computational analysis for identification of early diagnostic biomarkers and prognostic biomarkers of liver cancer based on GEO and TCGA databases and studies on pathways and biological functions affecting the survival time of liver cancer. BMC Cancer, 21(1), 791.
Acknowledgements
We appreciate all the participants providing supports for this study.
Funding
The work was supported by Wuhan Traditional Chinese Medicine Scientific Research Project (W22Q47).
Author information
Authors and Affiliations
Contributions
Yaqi Liu was the main designer of this study. Yaqi Liu, Yixiao Wang, Yiran Yang and Mingxing Guo performed the experiments and analyzed the data. Yaqi Liu, Yixiao Wang, Yihong Quan and Mingxing Guo drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The xenograft tumor model protocols were ratified by the Animal Care and Use Committees of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Wang, Y., Yang, Y. et al. Liquiritigenin Induces Cell Cycle Arrest and Apoptosis in Lung Squamous Cell Carcinoma. Cell Biochem Biophys 82, 1397–1407 (2024). https://doi.org/10.1007/s12013-024-01294-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-024-01294-w