Abstract
One of the most important side effects of chemotherapy is cardiovascular complications, such as cardiotoxicity. Many factors are involved in the pathogenesis of cardiotoxicity; one of the most important of which is long non-coding RNAs (lncRNAs). lncRNA has 200–1000 nucleotides. It is involved in important processes such as cell proliferation, regeneration and apoptosis; today it is used as a prognostic and diagnostic factor. A, various drugs by acting on lncRNAs can affect cells. Therefore, by accurately identifying IncRNAs function, we can play an effective role in preventing the development of cardiotoxicity-induced chemotherapy drugs, and use them as a therapeutic strategy to improve clinical symptoms and increase patient survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is one of the most important and influential diseases in a human being [1]. Chemotherapy and related drugs are mainly used to treat this malignancy. Although chemotherapy drugs have positive effects on the treatment of cancer patients, they also have side effects such as cardiotoxicity [2]. Cardiotoxicity is a complication caused by chemotherapy drugs during or after administration; it leads to cardiovascular disease (CVD) [3].
It has recently been shown that Long noncoding RNAs (lncRNA) are involved in the development of this type of cardiotoxicity (Table 1) [4]. Different chemotherapy drugs can play effective role in drug-induced cardiotoxicity [5]. One of the most important of these drugs is doxorubicin [6]. Cardiotoxicity induced by chemotherapy drugs occurs following the aging of the heart muscle cells. Some actions occur during the process of aging, including: decrease cell proliferation, P53 and P16 proteins expression increment, decrease telomere length and telomerase activity. In these processes, lncRNA plays an important role as a regulatory molecule, so shutting down the lncRNA molecule plays a protective role against the aging of heart cells, and lead to drug-induced cardiotoxicity [7].
In addition, lncRNA can induce toxicity in cells by inhibiting proliferation, inducing apoptosis, and producing reactive oxygen species (ROS) [8, 9]. Thus, lncRNA via PI3K/AKT pathway can induce apoptosis and ROS production, and subsequently induce cell toxicity via Notch1/KLF15 pathway by inhibiting cell proliferation [10, 11]. Studies have also shown, that lncRNA can lead to CVD through toxin induction in cardiac muscle cells [12]. Thus, lncRNA Cfast can trigger the TGF-β signaling pathway by inhibiting the interaction of COLT1 with the TRAP1 molecule; subsequently, by increasing the formation of SMAD2/SMAD4 complexes, it induces fibrosis and finally toxicity in cardio myocytes, the product of which can be CVD induction [13].
So this study investigated the role of lncRNAs in the induction chemo toxicity by chemotherapy drugs, in order to obtain appropriate information in this field. The findings of the present study will help to provide appropriate treatment strategies to combat the cardiotoxicity complications.
The Role of lnRNAs in Cardio Myocyte Regeneration
Regeneration is the repair process of heart muscle cells by themselves; it leads to form a similar cell. Recent studies have indicated to the lncRNAs role in this process [20].
IL-22 is an inflammatory cytokine, which increases the expression of lncRNA H19, through Protein Kinase A (PKA) and STAT3 transcription factor. This lncRNA can be effective on inducing regeneration from different pathways. lncRNA H19 binds to P53, microRNA 34a and let 7 (cell cycle inhibitors), and inhibits them; Finally, by promoting the cell cycle and increasing the expression of genes involved in the cell regeneration, it induces this process in the cell [21].
On the other hand, lncRNA H19 encodes two microRNAs from exon number one, called miR-675-3P and miR-675-5P. miR-675-3P suppresses the Bone Morphogenetic Protein (BMP) pathway by binding to the anti-differentiation transcription factor Smad (Smad1,5). miR-675-5P also plays a role in inducing the regeneration process by lncRNA H19, by suppressing cdc6 as a DNA replication initiator [22]. lncRNA H19 together with microRNA 675 inhibits Transforming Growth Factor-β1 (TGF-β1) protein expression, and subsequently reduces the phosphorylation of Smad 3, which ultimately leads to inactivation of Histone Deacetylase 4.5 (HAD 4.5) [23]. HDAC4/5 suppresses TBX5 transcription factor by deacetylation, and inhibits the Myocyte Enhancer Factor 2 (MEF 2) gene expression. The MEF 2 gene plays an important role in the development and regeneration of heart muscle cells; by inhibiting HDAC 4/5 performance, it Increases TBX5 transcription factor activity and MEF 2 gene expression to induce myocardial cells regeneration [24, 25].
Downregulation of lncRNA H19 can induce the effect of temozolomide on cancer cells by reducing the proliferation by suppressing the Wnt/β-Catenin pathway [26]. lncRNA H19 also binds to miR 29b-3p, which has an inhibitory role in the TGF-β1 protein, thereby activating the TGF-β1 signaling pathway [27]. TGF-β1 protein along with activin-βA leads to cell regeneration, while SB-431542 molecule has a negative effect on TGF-β1 protein. On the other hand, in downstream of the TGF-β1 signaling pathway, there is a molecule called Smad3, which binds to the SMCα promoter and increases its expression; it can also induce cellular regeneration [28].
Thus, in the cell, Growth Arrest Specific 5 (GAS5) binding to Smad3 prevents its binding to the SBEDNA sequence of TGF-β1-responsive elements in the SMCα gene promoter; as a result, inhibition of TGF-β1/Smad3 signaling leads to the inhibition of cell regeneration [29, 30]. The lncRNA H19 can destroy miR-141 and miR-22, by affecting the β-catenin gene, which is common between the two microRNAs, and activates the Wnt/β- catenin-signaling pathway; the two cited mirs have inhibitory effect in the Wnt/β-Catenin-signaling pathway [31].
In this way, the Wnt molecule binds to its receptors, the Frizzled receptor and the LRP5/6 receptor, on the surface of target cell. This binding leads to the AXIN molecule phosphorylation in the β-Catenin complex and prevents its intra-cytoplasmic destruction by the proteasome complex. In the nucleus of the β-Catenin complex, it binds to the transcription factor T cell factor/lymphoid enhancing factor (TCF/LEF) and activates it, which increases the expression of Wnt 7, Fz7, DVl, Lgr5, C-myc genes. Wnt7, Fz7, DVl genes are involved in the early stages and C-myc in all stages of regeneration [31, 32].
The GSK-3β molecule of the β-Catenin complex can also activate the mTOR signaling pathway, which also plays an important role in the cellular regeneration [31]. One study has showed, that removing the PTEN, TSC1/2 and NB-3 molecules, using cytosolic HDAC5, Wnt10b, Melanopsin/GPCR, and increasing the activity of IL-6 and the PF-4708671 small molecule, activates mTOR molecule; the activation causes regeneration of cells [33]. Also in the downstream part of the signaling pathway of the mTOR molecule, the Arnt-Sim Domain Kinase (PASK) enzyme is phosphorylated and activated, which induces the cell differentiating factors production by phosphorylation of the Wrd5 molecule. In the next step, using the S6K molecule in the mTOR signaling pathway, leads to form the secondary structures; it induces cellular regeneration [34].
Activation of PI3K enzyme in the phosphorylation pathway and activation of its own downstream molecules leads to AKt/mTOR/P70S6K pathway activation; this pathway plays an important role in the regeneration of cells after injury [35]. Activation of the mTOR molecule leads to the activation of Eukaryotic Initiation Factor 4 E (elF4E) molecule, which also induces Cyclin D activation; it promotes cell cycle and recovery in cell function and ultimately leads to the cell regeneration [36]. Considering the mTOR signaling pathways in cell regeneration, it is hypothesized that such similar pathways may be effective in cardio myocyte regeneration.
Falcor is a type of lncRNA causes cell regeneration by acting on the Foxa transcription factor. Increased expression of Foxa1/a2 transcription factor by acting on Mucin2 (Muc2) and preproglucagon gene promoters plays effective role in cell differentiation and regeneration. Excessive increase in Foxa transcription factor affects Falcor lncRNA; it reduces production and disruption of Falcor-Foxa pathway in the cell regeneration [37, 38].
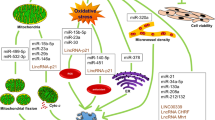
Two other lncRNAs, which are impressive in inducing cardio myocyte regeneration are ECRAR and CRRL. ECARE activates ERK1/2 enzyme, that phosphorylates and activates Cyclin D1 and Cyclin E1. Activation of these cyclins leads to the cell cycle advancement and heart muscle cells repair [39]. Cardio myocyte Regeneration related lncRNA (CRRL) acts as an (Competing endogenous) CeRNA. It binds to the miR199a3p and increases Hopx expression, which has a negative effect on cardio myocytes proliferation. CRRL lncRNA suppression can be effective in inducing cardio myocyte regeneration [40] (Fig. 1).
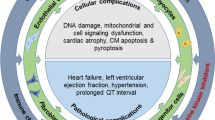
The Role of lncRNA in cardiotoxicity. lncRNAs can play a decisive role in inducing cardiotoxicity by affecting regeneration, proliferation and apoptosis processes of cardio myocytes. In general, the effect of lncRNA on reducing the process of cell regeneration and proliferation and consequently increasing the process of cell apoptosis in cardio myocytes can induce cardiotoxicity. lncRNA long non coding RNA, miR microRNA, TGF-beta transforming growth factor—beta, GAS5 growth arrest specific 5, ECRAR endogenous cardiac regeneration associated regulator, ERK extracellular signal-regulated kinase, CRRL cardiomyocyte regeneration_related lncRNA, CRNDE colorectal neoplasia differentially expressed gene, LYRM1 LYR motif Containing1, SIRT1 Sirtuin1, JAK Janus kinase, STAT3 signal transducer and activator of transcription 3, IFN interferon, IL interleukin, BAX BCL2-associated X protein
Another lncRNA called Cardiac regeneration-related lncRNA (CAREL) can inhibit cardio myocytes proliferation by inhibiting the function of miR-296 and increasing the expression of Trp53inp1 and Itm2 genes, thereby inducing a negative effect on cardiac cell regeneration [41]. In general, the identification of lncRNAs involved in the regeneration of cardio myocytes can lead to design strategies to prevent cardiotoxicity in patients.
The Role of lnRNAs in Cardio Myocyte Proliferation
In addition to regeneration, lncRNAs can also be involved in the cardio myocytes proliferation [42]. Sirt1 antisense lncRNA by forming a duplex antisense lncRNA/mRNA can protect Sirt1 mRNA against miR-34a function. It increases the translation of this mRNA by ribosomes, which results in increased production of Sirt1 protein. This protein can also induce proliferation in the cells by increasing the activity of cyclins B, D and E and promoting the cell cycle [43, 44]. On the other hand, it has been shown that C2dat1 lncRNA can also induce cell proliferation by binding and inhibiting the miR-34a function, and increasing the Sirtuin1 (SIRT1) expression.
In addition to the above mechanism, this lncRNA can also induce cell proliferation through the expression of the Proliferating Cell Nuclear Antigen (PCNA). Thus, by affecting the Cyclin D/CDK4-CDK6 and Cyclin E/CDK2 complexes, the PCNA molecule promotes cell progression from phase G1; it also prevents stop in the S phase of the mitosis by affecting P21[45, 46].
Minichromosome maintenance 3 (MMC3) is a DNA replication initiator; it can also be involved in inducing cell proliferation by advancing the cell cycle from phase G1 to S. lncRNA CPR can turn off the MMC3 promoter by using the DNMT3A enzyme by methylating the cysteine-phosphate-guanine sites; thereby it inhibits cardio myocytes proliferation [47, 48]. lncRNA MALAT1 plays an important role in cell proliferation, so lncRNA MALAT1 silencing leads to the cessation of the cell cycle in the G0/G1 phase. On the other hand, this lncRNA can also induce cell proliferation by binding and inhibiting miR-129-5p, which can inhibit cell proliferation by ZFP36L1 suppression [49, 50].
Drugs derived from Taxanes compounds such as Paclitaxel and Docetaxel as well as Adriamycin can reduce the proliferation of cancer cells by suppressing lncRNA MALAT1 and induce the effect of these drugs on these cells [51]. miR-138 can also inhibit cell proliferation by inhibiting Cyclin D3 and suppressing the SOX9 molecule. Brain Cytoplasmic RNA 1 (BCYRNA1) lncRNA can induce cell proliferation by suppressing this microRNA and increasing the SOX9 and Cyclin D3 molecules expression; it also promotes cell cycle [52,53,54].
One study has shown, that lncRNA LIPCAR can promote cell cycle; it increases cell proliferation by reducing P21 expression and increasing the Cyclin D2 and PCNA function [55]. Recent studies also have indicated to the lncRNA FEZF1-AS1 role in the cell proliferation, thus lncRNA FEZF1-AS1 can induce cell proliferation by inhibiting miR-4443 function and suppressing the P21 expression, through LSD1 mediated H3K4me2 demethylation [56, 57].
Another lncRNA called MT1JP can also act as a CeRNA and inhibits cell proliferation by inhibiting miR-214-3P and increasing the RUNX1 transcription factor expression; subsequently, MT1JP induces P21 and Bim molecules expression, which leads to cell proliferation inhibition [58]. MT1JP lncRNA can induce the Cisplatin effect on cancer cells by inhibiting miR-24-3P and suppressing the Wnt/Beta-Catenin pathway in these cells. Due to the inhibitory role of this lncRNA in the cell proliferation, Cisplatin can lead to cardiotoxicity by inducing this lncRNA in cardio myocytes by inhibiting the cell proliferation [59].
LUCAT1 lncRNA can affect cell proliferation by regulating miR-181a-5P function and KLF15 and KLF6 expression. Thus, the KLF molecule can induce proliferation in the cell by increasing the C-JUN gene expression and Cyclin D1 activation, which promote cell cycle [60, 61]. LncRNA PEBP1P2 (also known as lncRNA5) also reduces the expression of alpha-SMA, Calponin1 (CNN1) and Smooth Muscle Myosine Heavy Chain (SMHC) genes by reducing CDK9 activity and ultimately reduces cell proliferation [62]. Also ZEB1 antisense1 lncRNA (ZEB1-AS1) can induce cell proliferation by downregulating miR-335-5P. miR-335-5P inhibits the function of cyclin B2 and stops the cell in G0/G1 phase; this inhibitory function is blocked by ZEB1-AS1 lncRNA [63, 64].
Studies have shown that inhibition of ZEB1-AS1 lncRNA is required for the effect of Cisplatin on cancer cells. Inhibition of ZEB1-AS1 lncRNA in cancer cells leads to upregulation of miR-129-5P and subsequently inhibits cell proliferation and apoptosis induction in cancer cells through the inhibition of ZEB1 and bcl2 molecules. Considering the role of ZEB1-AS1 lncRNA in cell proliferation, including cardiac muscle cells, it is hypothesized that Cisplatin drug needs to inhibit ZEB1-AS1 lncRNA to induce its effect on cancer cells. Consequently, one of the side effects of this chemotherapy drug is probably cardiotoxicity; it happens by reducing the expression of ZEB1-AS1 lncRNA and inhibiting its function in the proliferation of cardio myocytes [65].
Azin2-SV lncRNA also increases the expression of PTEN molecule by inhibiting miR-214 function. On the other hand, this lncRNA by binding to the PTEN molecule can also lead to its stability. Increased expression and stability of PTEN molecule leads to decreased phosphorylation of AKT and Cyclin D molecules, thus it leads to cell cycle blocking. Azin2-SV can inhibit cardio-myocytes proliferation in this way [66]. CTBP1-AS2 lncRNA can also inhibit cell proliferation by inhibiting miR-216a function and increasing the expression of its target molecule, PTEN [67].
miR-216a inhibits the activity of Janus kinase 2 (JAK2) enzyme, which plays an important role in cardio myocytes proliferation by phosphorylating the transcription factor Signal Transducer and Activator of Transcription3 (STAT3). lncRNA NR-045363 activates the JAK2/STAT3 pathway by inhibiting the function of miR-216a, and ultimately induces proliferation in the heart cells [68, 69]. Another lncRNA called Cancer-associated region long non-coding RNA (CARLO-7), also promotes cell cycle and cell proliferation by regulating miR-302 and miR-182 function, and increasing the CDK1 activity. On the other hand, this lncRNA can also induce cell proliferation using the JAK2/STAT3 and Wnt/Beta-Catenin signaling pathway and increasing the MYC transcription factor activity [70].
One study showed that Tumor Necrosis Factor-α (TNF-α), OX-LDL, and Platelet-derived growth factor-BB (PDGF-BB) molecules can lead to the HIX003209 upregulation. HC003209 can induce cell proliferation by regulating miR-6089 function [71]. OXLDL and IL-6 molecules can also lead to HCG11 lncRNA upregulation in the cell. Increased expression of HCG11 lncRNA subsequently inhibits miR-144 function by increasing the FOXF1 gene expression, which plays an important role in inducing cell proliferation. HCG11 lncRNA increases cell proliferation by regulating miR-144/FOXF1 function [72]. miR-23b binding to 3/UTR –fork head box O4 (FOXO4) inhibits FOXO4 expression and subsequently reduces cell proliferation, while lncRNA XR007793 inhibits cell proliferation by inhibiting the miR-23b function [73].
MCM3AP-AS1 lncRNA also increases the expression of its target molecule, the fork head box A1 (FOXA1), by inhibiting the miR-194-5P function; it allows the cell to pass through G1 check point of the cell cycle and proliferate [74]. Another lncRNA called ADAMTS9-AS2 can inhibit proliferation in cells by inhibiting miR-27a-3P function and increasing the expression of fork head Box protein 1 (FOXO1) [75]. In another study, it was shown, that lncRNA called Nuclear Enriched Abundant Transcript 1 (NEAT1) could inhibit miR-193a function in the ischemic heart cells; it acts as a tumor suppressive in the cell. NEAT1 leads to proliferation of cardiac cells in ischemic conditions, by advancing the cell cycle. It seems that this lncRNA can induce cell proliferation under normal conditions in cardio myocytes [76].
Studies show a distinctive role for NEAT1 lncRNA to induce the effect of chemotherapy drugs. Thus, NEAT1 lncRNA can induce drug resistance in cancer cells to Sorafenib by modulating the function of miR-149-5P/AKT axis. But on the other hand, NEAT1 lncRNA can be effective in inducing apoptosis in the cancer cells by Imatinib via regulating the C-Myc gene expression; it indicates the dual role of this lncRNA to induce the effect of chemotherapy drugs [77, 78]. In addition, inhibition of NEC1 lncRNA function can be effective to inhibite cell proliferation and induce of apoptosis in the cancer cells following cisplatin administration. In other words, the effectiveness of Cisplatin on cancer cells depends on the reduced expression and inhibition of NEC1 lncRNA function [79].
Another lncRNA called Colorectal Neoplasia differentially expressed gene (CRNDE) can also induce proliferation in cardio myocytes by inhibiting the miR-181a function and increasing the expression of its target molecule, LYR motif Containing1 (LYRM1), which is involved in promoting cell [80]. Downregulation of CRNDE lncRNA can be effective in inducing the effect of fluorouracil on cancer cells by increasing the miR-33a function and reducing the HMGA2 expression. On the other hand, due to CRNDE lncRNA role in the proliferation of cardio myocytes, its reduction in the of cardiac muscle cells following the use of fluorouracil may lead to cardiotoxicity [81] (Fig. 1).
Other studies have shown, that lncRNA called small nucleolar RNA host gene (SNHG), also plays an important role in the cell proliferation. Thus, SNHG7 lncRNA can induce proliferation in cells by inhibiting the miR-122-5P function and increasing the expression of its target molecule, Ribosomal protein L4 (RPL4). SNHG5 lncRNA also induce cell proliferation by inhibiting the miR-32 function and increasing the KLF4 (Kruppel-like Factor 4) molecule and SNHG20 lncRNA expression, by inhibiting the miR-495-3P and upregulating the Zinc Finger Protein X-linked (ZFX) molecules; they are the target molecules of the listed microRNAs. Given these studies and the role of SNHG lncRNA in the cell proliferation, it is hypothesized that it may also play effective role in the cardio myocyte proliferation [82,83,84]. Expression of SNGH lncRNA may also be important in inducing resistance to chemotherapy drugs, so that SNGH12 lncRNA can induce drug resistance to Sunitinib in the cancer cells through upregulation of CDCA3 molecule [85].
YAP1 is a molecule that plays an important role in the cell proliferation. miR-29a inhibits its function by binding to YAP1, while lncRNA called CTTN-IT1 can act as a CeRNA and binds to miR-29a to stop this inhibitory pathway, and promotes functional YAP1 molecule and cell proliferation [86]. Ezrin antisense RNA 1 (EZR-AS1) lncRNA induces cell proliferation through EZR expression. In this way, this lncRNA regulates SET and MYND domain-containing protein3 (SMYD3), which is a histone H3 lysine 4 specific methyl transferase, and subsequently can affect EZR factor transcription; it is involved in the cell proliferation [87].
miR-539 has an inhibitory role in the cell cycle and is a tumor suppressor; it increases MMP-9 protein expression. Another study showed that LINC00460 lncRNA by degrading miR-539 could increase cell proliferation [88]. WEC2 antisense lncRNA (WEE2-AS1) also binds to the miR-32-5P and degrades it to increase the expression of Transducer of ERBB2 (TOB1) gene in the downstream part of the microRNA; it ultimately induces proliferation in the cell [89]. HOTAIR lncRNA can also induce cell proliferation by inhibiting miR-326 and increasing the expression of its target molecule, Phox2a [90, 91]. Inhibition of HOTAIR lncRNA can induce the PI3K/AKt/mTOR signaling pathway and thus be effective in inducing the effect of doxorubicin on the cancer cells [92]. HOTAIR lncRNA can also lead to drug resistance to Imatinib in the cancer cells via the miR-130a/ATG2B pathway [93].
miR-145 inhibits the function of the Zeb1 molecule, which plays an important role in the cell proliferation. TUG1 lncRNA acts as a CeRNA by inhibiting the miR-145 function, leading to the activation of the Zeb1 molecule and the induction of cell proliferation [94]. Studies also showed the dual role of TUG1 lncRNA in inducing the effect of chemotherapy drugs. TUG1 lncRNA can inhibit proliferation and induce apoptosis in cancer cells by suppressing miR-221 and increasing the expression of PTEN molecules; subsequently, it leads to chemo sensitivity and induces the effect of Cisplatin on cancer cells [95]. On the other hand, TUG1 lncRNA can act as a CeRNA and induce drug resistance in cancer cells against Paclitaxel by degrading miR-29b-3P [96].
Another lncRNA called HOXA transcript at the distal tip (HOTTIP) also induces proliferation in the cells by inhibiting the function of miR-490-3P; miR-490-3P inhibits the expression of high mobility group B1 (HMGB1) and inhibits cell proliferation [97]. Inhibition of HOTTIP lncRNA by increasing the expression of miR-214 and decreasing the function of KPNA3 molecule can lead to the induction of the Mitomycin effect on cancer cells; HOTTIP lncRNA can also be involved in the drug resistance of cancer cells to Cisplatin by modulating miR-137 [98, 99]. In addition, HOTTIP lncRNA can play an impressive role in inducing chemo resistance by activating the Wnt/Beta-Catenin pathway [100].
LINC00668 lncRNA can also act as a CeRNA and induce cell proliferation by binding and inhibiting the miR-532-5P function; it also increases YY1 gene expression [101]. Another lncRNA called Antisense non-coding RNA in the INK4 locus (ANRIL) can also be involved in the cell proliferation. ANRIL Knockdown can inhibit cell proliferation by increasing miR-122-5p expression. Therefore, ANRIL lncRNA acts as a CeRNA, and can lead to cell proliferation by inhibiting the miR-122-5P function [102]. ANRIL lncRNA can also induce the effect of Cisplatin on cancer cells by regulating the let-7a/HMGA2 pathway [103].
Another study showed, that MIR31HG lncRNA could inhibit cell proliferation by degrading miR-575 and regulating the function of the Suppression of 7-like tumorigenicity (ST7L) molecule [104]. On the other hand, AGAP2-AS1 lncRNA increases the ANXA11 molecule expression through miR-16-5P degradation, which can also lead to cell proliferation by activating the AKT pathway [105]. Musculin antisense RNA1 (MSC-AS1) lncRNA can also be effective in inducing cell proliferation by regulating the expression of 6-Phosphofructo-2-Kinase/fructose-2,6 biphosphatase 3 (PFKFB3) [106]. lncRNA Materially expressed gene 3 (MEG3) can inhibit cell proliferation by inhibiting miR-96-5P function and increasing the expression of its target molecule, Metastasis Suppressor1 (MTSS1) [107]. On the other hand, BC032469 lncRNA plays an effective role in inducing cell proliferation by degrading miR-1207-5P and increasing the hTERT expression [108].
PDZK1 is the target molecule of miR-15b. PENG lncRNA also increases its expression, by inhibiting miR-15b function; as a result, PENG lncRNA can inhibit cell proliferation in this way [109]. Another study also showed, that ZNFX1 lncRNA antisense RNA1 (ZFAS1) could induce cell proliferation by inhibiting miR-19a function and activating the SKA1 signaling pathway [110]. Evaluating the involved lncRNAs in cardio myocyte proliferation can be effective to induce repair in the chemotherapy damaged cells. In addition, some drugs can affect cell proliferation by affecting lncRNAs in the downstream of their mechanism; they may be used as therapeutic targets for cardiotoxicity and cardio myocyte damage (Table 2).
The Role of lncRNAs in Cardio Myocyte Apoptosis
Apoptosis is referred to as programmed cell death. In fact, apoptosis is the physiological cell death, that naturally removes old, damaged, excess and harmful cells; it is essential for tissue homeostasis [123]. Recent studies show, that lncRNAs also play a role in cardio myocyte apoptosis [124]. MIRF lncRNA can induce apoptosis in cardio myocytes by regulating the miR-26a function and increasing the expression of the target molecule, Bak1; it is a key molecule in promoting the mitochondrial pathway of apoptosis. On the other hand, this lncRNA reduces the content of ATP and MMP (mitochondrial membrane potential) by regulating the miR-26a function; miR-26a also plays an important role in apoptosis induction in the heart muscle cells [125].
lncRNA BANCR also induces IFN-β-STAT1 signaling pathway through interaction with the STAT1 enzyme, which ultimately leads to increased expression of interferon-family pro-apoptotic genes. Therefore, this lncRNA using STAT1 enzyme can lead to interferon beta-induced apoptosis in cardio-myocytes [126]. BANCR lncRNA can also attenuate the effect of Cisplatin on cancer cells by inducing the ERK1/2 pathway, thereby creating drug resistance to Cisplatin in the cancer cells [127].
lncRNA RP11-468E2.5 upregulation leads to cell proliferation inhibition and apoptosis induction in cells, by inhibiting the JAK/STAT signaling and suppressing STAT5 and STAT6 [128]. Β-type—natriuretic peptide (BNP) molecule up regulates the lncRNA called LSINCT5, which can induce cell apoptosis by activating caspase 1/IL-1β signaling pathway; so BNP also expresses lncRNA LSINCT5 in cardio myocytes. The use of Caspase 1/interleukin 1—β-signaling pathway can lead to myocardial cells apoptosis [129] (Fig. 1).
Taurine-upregulated lncRNA1 (TUG1) induces epigenetic changes and silences the BAX pro-apoptotic protein expression by interacting with EZH2 domain of the pre-apoptotic BAX protein; it ultimately leads to cellular apoptosis suppression [130]. Downregulation of GASL1 lncRNA increases TGF-β molecule expression. This molecule leads to Bax and Bak1 molecules oligomerization in the outer membrane of the mitochondria, due to BH3-containing proteins such as Bim; it leads to cytochrome C release from the mitochondria and apoptosis in cardio-myocytes through the internal apoptotic pathway [131, 132].
MORT lncRNA can also induce apoptosis in cardio-myocytes through miR-93 downregulation; miR-93 inhibits cellular apoptosis by inhibiting RUNX3 and subsequently promoting the TGF-β/Smad signaling pathway [133, 134]. lncRNA-ATB overexpression can also induce apoptosis in the cell by activating the TGF-β/SMAD2/3 signaling pathway [135]. DANCR lncRNA can affect cell apoptosis by regulating miR-758-3P function and PAX6 molecule expression, so that downregulation of this lncRNA leads to increased expression of Caspase 3, Caspase9 and Bax molecules. It indicates apoptosis induction in the cell, as the mentioned molecules (Caspase3, Caspase9 and Bax) play an important role in promoting cellular apoptosis [136].
DANCR lncRNA downregulation can increase the effect of Docetaxel on the cancer cells by increasing miR-34a-5P expression and suppressing the JAG1 pathway [137]. DANCR lncRNA downregulation can also be effective to induce the effect of Sorafenib in cancer cells by suppressing the IL6/STAT3 pathway [138]. In addition, downregulation of this lncRNA by increasing the function of miR-874-3P and suppressing the ATG16L1 molecule can also lead to the induction of the Cytarabine drug effects on cancer cells [139]. Therefore, downregulation of DNCR lncRNA by the mentioned chemotherapy drugs can lead to cardiotoxicity due to its role in cardio myocyte apoptosis induction.
LINC00152 lncRNA knocking down increases the miR-125b expression, which can induce apoptosis in the cells by inhibiting the MCL1 molecule function. The MCL1 molecule binds to the BAK and BAX molecules; it prevents the permeability of the outer membrane of the mitochondria and the entry of cytochrome C into the cytosol, which induces the mitochondrial pathway apoptosis. By inhibiting this function, apoptosis occurs in cell [140].
lncRNA CEBPA-AS 1 can induce apoptosis in cells by using the Notch signaling pathway. In this pathway, NICD, HIF-1α, NF-Kβ, MAPK (P38) and JNK/C-JUN molecules can cause apoptosis using apoptotic stimulants, such as BAX and Caspase3 and 9 [141]. In hypoxia, an lncRNA called FOXD3-AS1 can increase Caspase3, Caspase7, and pro-apoptotic molecules expression in myocardial cells by modulating miR-150-5P function; it ultimately induces apoptosis in cardio myocytes. Therefore, it is possible to prevent cardiomyocyte apoptosis by using the FOXD3-AS1 antagonists in hypoxic conditions [142]. On the other hand, microRNA 150 inhibits apoptosis induction in cell by inhibiting the PDCD4 function, while FOXD3-AS1 lncRNA can induce apoptosis in cell by inhibiting miR-150 and increasing the PDCD4 expression in another pathway [143]. In addition, FOXD3-AS1 lncRNA can induce the phenomenon of Cisplatin chemo resistance by suppressing the miR-127-3P and increasing the MDM2 molecule expression [144].
Another lncRNA called RNA-Steroid receptor RNA activator 1 (SRA1) can induce apoptosis in cells by regulating the miR-208a function and increasing the expression of its target molecule, programmed cell death4 (PDCD4) [145]. TNF-α induces the expression of lncRNA called Hypoxy-inducible factor 1 alpha antisens RNA 1 (HIF1α-AS1) in cell. This lncRNA can cause apoptosis in cell through caspase 3 upregulation [146]. On the other hand, androgens induce SOCS2-AS1 lncRNA, which can inhibit cellular apoptosis by suppressing the TNFSF10 gene expression (a member of the TNF family) [147]. NORAD lncRNA can also prevent apoptosis in cells by inhibiting the miR-214 function and increasing the activation of the AKT/mTOR pathway. This lncRNA can also affect cell apoptosis by regulating the caspase 3 function [148].
ACART lncRNA increases the expression of BCL2 molecule and suppresses the Bax and cytochrome C molecules production by activating the PPAR-γ molecule. Considering the anti-apoptotic role of BCL2 and the pro-apoptotic role of Bax and cytochrome C, it can be said that ACART lncRNA can prevent apoptosis in cardio-myocytes by activating the PPAR-γ molecule, and subsequently using the BCL2 pathway [149].
OX-LDL in the cell can also lead to lncRNA 00,152 upregulation; this lncRNA inhibits apoptosis in cells by inhibiting the miR-4767 function, and increasing the BCL2L12 and EGFR proteins expression [150]. Due to the miR-143 role in inhibiting the expression of BCL2 anti-apoptotic protein and inducing apoptosis in cells, LOXL1-AS1 lncRNA can affect cell apoptosis by regulating the function of this microRNA [151, 152]. A substance called Berberine leads to CASC2 lncRNA upregulation in cell; it can cause cell apoptosis through this lncRNA. CASC2 lncRNA activates by binding to the AUF1 molecule; after activation, this lncRNA inhibits translation of BCL2 by binding to the mRNA of this molecule, and induces apoptosis in the cell [153]. In addition, CASC2 lncRNA can increase the antitumor activity of Cisplatin by inhibiting the AKT pathway through inhibiting miR-181a [154].
ASNR lncRNA can also regulate cellular apoptosis through a similar mechanism to CASC2 lncRNA, via interaction with AUF1 molecule and BCL2 mRNA degradation [155]. PANDAR lncRNA affects BCL2 protein transcription by interaction with NF-YA molecule, and thus can regulate cellular apoptosis [156]. PANDAR lncRNA can also induce drug resistance to Cisplatin in the cancer cells by regulating SFRS2 mediated P53 phosphorylation [157]. Considering the role of the mentioned lncRNAs in regulating the function of the BCL2 anti-apoptotic protein, it is hypothesized that these lncRNAs can also induce apoptosis in cardio myocytes by regulating BCL2.
lncRNA FGD antisense RNA1 (FGD-AS1) can also inhibit apoptosis in myocardial cells by inhibiting the miR-195 function and RORA Upregulation [158]. In addition, downregulation of FGD-AS1 lncRNA could induce the effect of Cisplatin on cancer cells via the miR-142/PD-L1 pathway [159]. The LPS molecule leads to LUADT1 lncRNA downregulation, which can increase miR-195 expression; subsequently miR-195 binds to its target molecule, Pim1, to inhibit it and induce apoptosis in cardiac endothelial cells. By upregulating the LUADT1 lncRNA, it can be used as a CeRNA to prevent apoptosis induction in the cardiac endothelial cells via inhibiting miR-195 function and increasing Pim1 expression [160].
lncRNA LNC-000898 can also prevent apoptosis in cardio myocytes by inhibiting miR-375 function and increasing the expression of the target molecule of this microRNA, PDK1 [161]. Studies have shown that overexpression of lncRNAs Gm43050, Gm15621 through the regulation of miR-640 and miR-133a function, leads to Increased expression of the target molecules of these microRNAs, ZFP1 and SOX4, respectively; they can subsequently lead to cellular apoptosis reduction [162, 163]. The TGP molecule also increases miR-124-3P function by inhibiting the XIST lncRNA, which inhibits cellular apoptosis via ITGB1 molecule inhibition [164]. Thus, lncRNA XIST can play an impressive role in inducing cellular apoptosis.
Studies have shown, that Carboplatin can inhibit the growth of cancer cells through the lncRNA XIST/miR-200a-3P/NRP1 pathway, thereby inducing its effect on cancer cells [165]. Downregulation of PVT1 lncRNA also results in increased miR-145 expression; miR-145 binds to the target gene, FSC1, and inhibits its expression and induces apoptosis in cells [166]. Studies have shown that Cisplatin can induce apoptosis in cancer cells by inhibiting the function of PVC1 lncRNA, and subsequently induces the expression of apoptotic proteins such as Bax and Caspase3 [167].
Inhibition of SBF2-AS1 lncRNA through miR-30a upregulation and FOXA1 expression inhibition can be effective in cellular apoptosis induction [168]. In addition, SBF2-AS1 lncRNA is effective in Temozolomide resistance induction by inhibiting the miR-151A-3P and XRCC4 molecule expression. Therefore, SBF2-AS1 lncRNA inhibition can be effective to induce the effect of this drug on cancer cells, and SBF2-AS1 lncRNA function inhibition can induce cellular apoptosis; so one of the possible side effects of cited lncRNA inhibition is cardiotoxicity due to apoptosis in the cardio myocytes The cited process is the Temozolomide mechanism of action [169]. UBE2R2-AS1 lncRNA can also act as a CeRNA; it induces cellular apoptosis by degrading miR-877-3P and increasing TLR4 mRNA expression. Considering the TLR4 role in apoptosis induction in cardio myocytes through SIRT2 dependent p53 deacetylation, it is hypothesized that UBE2R2-AS1 lncRNA can also induce apoptosis in cardio myocytes through miR-877-3P/TLR4 pathway [170, 171]. Another study also showed, that Lnc10 could affect cell apoptosis by interacting with QKI5 molecule and regulating the P38MAPK signaling pathway [172]. lncRNA00312 can also induce apoptosis by inhibiting miR-34a-5P function and ASS1 overexpression [173].
lncRNA LINC00472 also induces apoptosis by inhibiting miR-24-3P function and increasing the expression of Death Effector Domain containing DNA binding protein (DEDD) [174]. TP73-AS1 lncRNA can also prevent apoptosis in cells by inhibiting the KISS1 molecule expression and inactivating the PI3K/AKT/mTOR signaling pathway [175]. lncRNA HITTERS acts as a scaffold RNA, and forms the MRE11-RAD50-NBS1 complex to protect cells against induced apoptosis as a result of DNA damage by Endoplasmic Reticulum Stress [176]. TNRC6C-AS1 lncRNA with increased STK4 gene promoter methylation has led to suppress the expression of this molecule and subsequently can inhibit cell apoptosis by activating the Hippo Signaling pathway [177].
PCAT6 lncRNA also increases the transcription of the Apoptosis Repressor with Caspase recruitment (ARC) molecule by forming a complex with EZH2, and increasing methylation of H3K4 (H3K4me3) gene promoter regions ARC; Therefore, PCAT6 lncRNA can prevent apoptosis induction by increasing the activity and expression of the ARC molecule [178]. PCAT6 lncRNA can induce Fluorouracil resistance in cancer cells by inhibiting the function of miR-204 and inducing the HMGA2/PI3K signaling pathway. Therefore, downregulation of PCAT6 lncRNA can lead to miR-204 overexpression, which can also be impressive to induce the effects of Fluorouracil by targeting the HMGA2/PI3K pathway. However, since PCAT6 lncRNA has an inhibitory role in cellular apoptosis, function inhibition of this lncRNA can induce cellular apoptosis in cardio myocytes and subsequently induce the cardiotoxicity effect of Fluorouracil [179].
lncRNA—UCA1 Knocking down increases the miR-193a activity, which induces apoptosis in the cell by CDK6 downregulation and blocking the PI3K/AKT, MAPK and Notch signaling pathways [180]. In addition, downregulation of UCA1 lncRNA can be effective in reducing drug resistance and inducing the effect of Cetuximab on cancer cells. Therefore, since inhibition of UCA1 lncRNA function can induce cellular apoptosis, one of the possible side effects of decreased UCA1 lncRNA expression can be cardiotoxicity; it can induce apoptotic process in cardio myocyte by inhibiting UCA1 lncRNA function [181].
TTN-AS1 lncRNA can regulate cellular apoptosis by increasing the MBTD1 expression via inhibiting the miR-134-5P function. miR-134-5P is the target microRNA of this molecule [182]. Studies have also shown, that SOX2-OT lncRNA can intensify the induction of cellular apoptosis by Doxorubicin in cardio myocytes by targeting the miR-942-5P/DP5 pathway. Unlike SOX2-OT lncRNA, NEAT1 lncRNA by degrading miR-221-3P can prevent Doxorubicin-induced cardiac senescence in cardio myocytes; thereby it inhibits cardiotoxicity, whereas lncRNA SOX2-OT could be due to the effect of Doxorubicin inducing apoptosis in cardio myocytes [183, 184].
Conclusion
lncRNAs play important roles in the apoptosis, proliferation, and regeneration of cardio myocytes; identifying the signaling pathways associated with them, as well as evaluating the upstream and downstream molecules of these pathways can be effective to design chemo toxic induced prevention strategies. In addition, targeting these pathways due to their role can provide some suitable therapeutic strategies, which require more studies in the future.
References
Norouzirad, R., Khazaei, Z., Mousavi, M., Adineh, H. A., Hoghooghi, M., Khabazkhoob, M., et al. (2017). Epidemiology of common cancers in Dezful County, Southwest of Iran. Immunopathologia Persa, 4(1), e10.
Štěrba, M., Popelová, O., Vávrová, A., Jirkovský, E., Kovaříková, P., Geršl, V., et al. (2013). Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxidants & Redox Signaling, 18(8), 899–929.
Braña, I., Zamora, E., Oristrell, G., & Tabernero, J. (2018). Cardiotoxicity. Side effects of medical cancer therapy (pp. 367–406). Springer.
Lotfian, S., Farmanara, H., Naderi, N., Solaymani-Dodran, M., & Shekarchizadeh, M. (2019). Association of body composition with quality of life, cardiovascular risk factors, and physical activity in patients with chronic heart failure. Journal of Preventive Epidemiology, 4(1), e09.
Haybar, H., Shahrabi, S., Rezaeeyan, H., Jodat, H., & Saki, N. (2019). Strategies to inhibit arsenic trioxide-induced cardiotoxicity in acute promyelocytic leukemia. Journal of Cellular Physiology, 234(9), 14500–14506.
Mancilla, T. R., Iskra, B., & Aune, G. J. (2011). Doxorubicin-induced cardiomyopathy in children. Comprehensive Physiology, 9(3), 905–931.
Xie, Z., Xia, W., & Hou, M. (2018). Long intergenic non-coding RNA-p21 mediates cardiac senescence via the Wnt/β-catenin signaling pathway in doxorubicin-induced cardiotoxicity. Molecular Medicine Reports, 17(2), 2695–2704.
Wang, Q., Liu, L., Zhang, S., Ming, Y., Liu, S., Cheng, K., et al. (2020). Long noncoding RNA NEAT1 suppresses hepatocyte proliferation in fulminant hepatic failure through increased recruitment of EZH2 to the LATS2 promoter region and promotion of H3K27me3 methylation. Experimental & Molecular Medicine, 52(3), 461–472.
Zadeh, F. J., Mohammadtaghizadeh, M., Bahadori, H., Saki, N., & Rezaeeyan, H. (2020). The role of exogenous Fibrinogen in cardiac surgery: Stop bleeding or induce cardiovascular disease. Molecular Biology Reports, 47, 8189.
Cai, L., Tu, L., Li, T., Yang, X., Ren, Y., Gu, R., et al. (2019). Downregulation of lncRNA UCA1 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson’s disease through the inhibition of the PI3K/Akt signaling pathway. International Immunopharmacology, 75, 105734.
Sun, P., Wang, J., Guo, X., Chen, Y., Xing, C., & Gao, A. (2017). Benzene and its metabolite decreases cell proliferation via LncRNA-OBFC2A-mediated anti-proliferation effect involving NOTCH1 and KLF15. Oncotarget, 8(25), 40857.
Haybar, H., Rezaeeyan, H., Shahjahani, M., Shirzad, R., & Saki, N. (2019). T-bet transcription factor in cardiovascular disease: Attenuation or inflammation factor? Journal of Cellular Physiology, 234(6), 7915–7922.
Zhang, F., Fu, X., Kataoka, M., Liu, N., Wang, Y., Gao, F., et al. (2021). Long noncoding RNA Cfast regulates cardiac fibrosis. Molecular Therapy-Nucleic Acids, 23, 377–392.
Lu, Q., Huo, J., Liu, P., Bai, L., & Ma, A. (2020). lncRNA HOXB-AS3 protects doxorubicin-induced cardiotoxicity by targeting miRNA-875-3p. Experimental and Therapeutic Medicine, 19(2), 1388–1392.
Chen, S., Wang, J., & Zhou, Y. (2019). Long non-coding RNA SNHG1 protects human AC16 cardiomyocytes from doxorubicin toxicity by regulating miR-195/Bcl-2 axis. Bioscience Reports, 39(7), BSR20191050.
Zhang, S., Yuan, Y., Zhang, Z., Guo, J., Li, J., Zhao, K., et al. (2019). LncRNA FOXC2-AS1 protects cardiomyocytes from doxorubicin-induced cardiotoxicity through activation of WNT1-inducible signaling pathway protein-1. Bioscience, Biotechnology, and Biochemistry, 83(4), 653–658.
Li, J., Li, L., Li, X., & Wu, S. (2018). Long noncoding RNA LINC00339 aggravates doxorubicin-induced cardiomyocyte apoptosis by targeting MiR-484. Biochemical and Biophysical Research Communications, 503(4), 3038–3043.
Zhan, J., Hu, P., & Wang, Y. (2020). lncRNA PVT1 aggravates doxorubicin-induced cardiomyocyte apoptosis by targeting the miR-187–3p/AGO1 axis. Molecular and Cellular Probes, 49, 101490.
Aung, L.-H.-H., Chen, X., Liu, Z., Li, Z., & Li, P. (2020). Cardiac mitochondrial dynamic-related LncRNA (CMDL)-1 protects cardiomyocytes against apoptosis in doxorubicin-induced cardiotoxicity. Circulation, 142(Suppl_3), A16442.
King, B. L., Rosenstein, M. C., Smith, A. M., Dykeman, C. A., Smith, G. A., & Yin, V. P. (2018). RegenDbase: A comparative database of noncoding RNA regulation of tissue regeneration circuits across multiple taxa. NPJ Regenerative Medicine, 3(1), 1–13.
Geng, H., Bu, H.-F., Liu, F., Wu, L., Pfeifer, K., Chou, P. M., et al. (2018). In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology, 155(1), 144–155.
Dey, B. K., Pfeifer, K., & Dutta, A. (2014). The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes & Development, 28(5), 491–501.
Huang, Y., Zheng, Y., Jia, L., & Li, W. (2015). Long noncoding RNA H 19 promotes osteoblast differentiation Via TGF-β1/S mad3/HDAC signaling pathway by deriving mi R-675. Stem Cells, 33(12), 3481–3492.
Ghosh, T. K., Aparicio-Sánchez, J. J., Buxton, S., & Brook, J. D. (2019). HDAC4 and 5 repression of TBX5 is relieved by protein kinase D1. Scientific Reports, 9(1), 1–10.
Zhang, L. X., DeNicola, M., Qin, X., Du, J., Ma, J., Tina Zhao, Y., et al. (2014). Specific inhibition of HDAC4 in cardiac progenitor cells enhances myocardial repairs. American Journal of Physiology-Cell Physiology, 307(4), C358–C372.
Jia, L., Tian, Y., Chen, Y., & Zhang, G. (2018). The silencing of LncRNA-H19 decreases chemoresistance of human glioma cells to temozolomide by suppressing epithelial-mesenchymal transition via the Wnt/β-Catenin pathway. OncoTargets and Therapy, 11, 313.
Lu, Y.-F., Liu, Y., Fu, W.-M., Xu, J., Wang, B., Sun, Y.-X., et al. (2017). Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-β1 signaling. The FASEB Journal, 31(3), 954–964.
Gilbert, R. W., Vickaryous, M. K., & Viloria-Petit, A. M. (2016). Signalling by transforming growth factor beta isoforms in wound healing and tissue regeneration. Journal of Developmental Biology, 4(2), 21.
Tang, R., Zhang, G., Wang, Y.-C., Mei, X., & Chen, S.-Y. (2017). The long non-coding RNA GAS5 regulates transforming growth factor β (TGF-β)–induced smooth muscle cell differentiation via RNA Smad–binding elements. Journal of Biological Chemistry, 292(34), 14270–14278.
Liang, W.-C., Fu, W.-M., Wang, Y.-B., Sun, Y.-X., Xu, L.-L., Wong, C.-W., et al. (2016). H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Scientific Reports, 6, 20121.
Bastakoty, D., & Young, P. P. (2016). Wnt/β-catenin pathway in tissue injury: Roles in pathology and therapeutic opportunities for regeneration. The FASEB Journal, 30(10), 3271–3284.
Yuan, J., Gao, Y., Sun, L., Jin, S., Zhang, X., Liu, C., et al. (2019). Wnt signaling pathway linked to intestinal regeneration via evolutionary patterns and gene expression in the sea cucumber Apostichopus japonicus. Frontiers in Genetics, 10, 112.
Ren, L., Chen, S., Liu, W., Hou, P., Sun, W., & Yan, H. (2019). Downregulation of long non-coding RNA nuclear enriched abundant transcript 1 promotes cell proliferation and inhibits cell apoptosis by targeting miR-193a in myocardial ischemia/reperfusion injury. BMC Cardiovascular Disorders, 19(1), 192.
Kikani, C. K., Wu, X., Fogarty, S., Kang, S. A. W., Dephoure, N., Gygi, S. P., et al. (2019). Activation of PASK by mTORC1 is required for the onset of the terminal differentiation program. Proceedings of the National Academy of Sciences USA, 116(21), 10382–10391.
Zanou, N., Schakman, O., Louis, P., Ruegg, U. T., Dietrich, A., Birnbaumer, L., et al. (2012). Trpc1 ion channel modulates phosphatidylinositol 3-kinase/Akt pathway during myoblast differentiation and muscle regeneration. Journal of Biological Chemistry, 287(18), 14524–14534.
Lund-Ricard, Y., Cormier, P., Morales, J., & Boutet, A. (2020). mTOR signaling at the crossroad between metazoan regeneration and human diseases. International Journal of Molecular Sciences, 21(8), 2718.
Diana, Z. Y., & Kaestner, K. H. (2009). Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L-and D-cells in mice. Gastroenterology, 137(6), 2052–2062.
Swarr, D. T., Herriges, M., Li, S., Morley, M., Fernandes, S., Sridharan, A., et al. (2019). The long noncoding RNA Falcor regulates Foxa2 expression to maintain lung epithelial homeostasis and promote regeneration. Genes & Development, 33(11–12), 656–668.
Chen, Y., Li, X., Li, B., Wang, H., Li, M., Huang, S., et al. (2019). Long non-coding RNA ECRAR triggers post-natal myocardial regeneration by activating ERK1/2 signaling. Molecular Therapy, 27(1), 29–45.
Chen, G., Li, H., Li, X., Li, B., Zhong, L., Huang, S., et al. (2018). Loss of long non-coding RNA CRRL promotes cardiomyocyte regeneration and improves cardiac repair by functioning as a competing endogenous RNA. Journal of Molecular and Cellular Cardiology, 122, 152–164.
Cai, B., Ma, W., Ding, F., Zhang, L., Huang, Q., Wang, X., et al. (2018). The long noncoding RNA CAREL controls cardiac regeneration. Journal of the American College of Cardiology, 72(5), 534–550.
Wang, J., Geng, Z., Weng, J., Shen, L., Li, M., Cai, X., et al. (2016). Microarray analysis reveals a potential role of LncRNAs expression in cardiac cell proliferation. BMC Developmental Biology, 16(1), 1–9.
Li, B., Hu, Y., Li, X., Jin, G., Chen, X., Chen, G., et al. (2018). Sirt1 antisense long noncoding RNA promotes cardiomyocyte proliferation by enhancing the stability of Sirt1. Journal of the American Heart Association, 7(21), e009700.
Wang, G.-Q., Wang, Y., Xiong, Y., Chen, X.-C., Ma, M.-L., Cai, R., et al. (2016). Sirt1 AS lncRNA interacts with its mRNA to inhibit muscle formation by attenuating function of miR-34a. Scientific Reports, 6(1), 1–13.
Strzalka, W., & Ziemienowicz, A. (2011). Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Annals of Botany, 107(7), 1127–1140.
Wang, H., Jin, Z., Pei, T., Song, W., Gong, Y., Chen, D., et al. (2019). Long noncoding RNAs C2dat1 enhances vascular smooth muscle cell proliferation and migration by targeting MiR-34a-5p. Journal of Cellular Biochemistry, 120(3), 3001–3008.
Ponnusamy, M., Liu, F., Zhang, Y.H., Li, R.B., Zhai, M., Liu, F., et al. (2019). Long noncoding RNA CPR (cardiomyocyte proliferation regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation, 139(23), 2668–2684.
Zhou, H., Xiong, Y., Zhang, G., Liu, Z., Li, L., Hou, S., et al. (2020). Elevated expression of minichromosome maintenance 3 indicates poor outcomes and promotes G1/S cell cycle progression, proliferation, migration and invasion in colorectal cancer. Bioscience Reports. https://doi.org/10.1042/BSR20201503
Guo, X., Piao, H., Zhang, Y., Sun, P., & Yao, B. (2020). Overexpression of microRNA-129-5p in glioblastoma inhibits cell proliferation, migration, and colony-forming ability by targeting ZFP36L1. Bosnian Journal of Basic Medical Sciences, 20(4), 459.
Zuo, Y., Li, Y., Zhou, Z., Ma, M., & Fu, K. (2017). Long non-coding RNA MALAT1 promotes proliferation and invasion via targeting miR-129-5p in triple-negative breast cancer. Biomedicine & Pharmacotherapy, 95, 922–928.
Yu, J., Jin, T., & Zhang, T. (2020). Suppression of long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) potentiates cell apoptosis and drug sensitivity to taxanes and adriamycin in breast cancer. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 26, e922672–e922681.
Liu, Y., Zhang, W., Liu, K., Liu, S., Ji, B., & Wang, Y. (2016). miR-138 suppresses cell proliferation and invasion by inhibiting SOX9 in hepatocellular carcinoma. American Journal of Translational Research, 8(5), 2159.
Wang, W., Zhao, L.-J., Tan, Y.-X., Ren, H., & Qi, Z.-T. (2012). MiR-138 induces cell cycle arrest by targeting cyclin D3 in hepatocellular carcinoma. Carcinogenesis, 33(5), 1113–1120.
Haybar, H., Shahrabi, S., Rezaeeyan, H., Shirzad, R., & Saki, N. (2019). Endothelial cells: From dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovascular Toxicology, 19(1), 13–22.
Wang, X., Li, D., Chen, H., Wei, X., & Xu, X. (2019). Expression of long noncoding RNA LIPCAR promotes cell proliferation, cell migration, and change in phenotype of vascular smooth muscle cells. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 25, 7645.
Gong, J., Wang, J., Liu, T., Hu, J., & Zheng, J. (2018). lncRNA FEZF1-AS1 contributes to cell proliferation, migration and invasion by sponging miR-4443 in hepatocellular carcinoma. Molecular Medicine Reports, 18(6), 5614–5620.
Liu, Y.-W., Xia, R., Lu, K., Xie, M., Yang, F., Sun, M., et al. (2017). LincRNA FEZF1-AS1 represses p21 expression to promote gastric cancer proliferation through LSD1-Mediated H3K4me2 demethylation. Molecular Cancer, 16(1), 1–16.
Xu, Y., Zhang, G., Zou, C., Zhang, H., Gong, Z., Wang, W., et al. (2018). LncRNA MT1JP suppresses gastric cancer cell proliferation and migration through MT1JP/MiR-214-3p/RUNX3 axis. Cellular Physiology and Biochemistry, 46(6), 2445–2459.
Zhu, D., Zhang, X., Lin, Y., Liang, S., Song, Z., & Dong, C. (2019). MT1JP inhibits tumorigenesis and enhances cisplatin sensitivity of breast cancer cells through competitively binding to miR-24-3p. American Journal of Translational Research, 11(1), 245.
Liu, Y., Cheng, T., Du, Y., Hu, X., & Xia, W. (2020). LncRNA LUCAT1/miR-181a-5p axis promotes proliferation and invasion of breast cancer via targeting KLF6 and KLF15. BMC Molecular and Cell Biology, 21(1), 1–11.
Liu, Y., Wen, J.-K., Dong, L.-H., Zheng, B., & Han, M. (2010). Krüppel-like factor (KLF) 5 mediates cyclin D1 expression and cell proliferation via interaction with c-Jun in Ang II-induced VSMCs. Acta Pharmacologica Sinica, 31(1), 10–18.
He, X., Lian, Z., Yang, Y., Wang, Z., Fu, X., Liu, Y., et al. (2020). Long non-coding RNA PEBP1P2 suppresses proliferative VSMCs phenotypic switching and proliferation in atherosclerosis. Molecular Therapy-Nucleic Acids, 22, 84–98.
Wang, X., Xiao, H., Wu, D., Zhang, D., & Zhang, Z. (2020). miR-335-5p regulates cell cycle and metastasis in lung adenocarcinoma by targeting CCNB2. OncoTargets and Therapy, 13, 6255.
Zhang, L.-L., Zhang, L.-F., Guo, X.-H., Zhang, D.-Z., Yang, F., & Fan, Y.-Y. (2018). Downregulation of miR-335–5p by long noncoding RNA ZEB1-AS1 in gastric cancer promotes tumor proliferation and invasion. DNA and Cell Biology, 37(1), 46–52.
Gao, J., Yuan, Y., Zhang, L., Yu, S., Lu, J., Feng, J., et al. (2020). Inhibition of ZEB1-AS1 confers cisplatin sensitivity in breast cancer by promoting microRNA-129–5p-dependent ZEB1 downregulation. Cancer Cell International. https://doi.org/10.1186/s12935-020-1164-8
Abbas, N., Perbellini, F., & Thum, T. (2020). Non-coding RNAs: Emerging players in cardiomyocyte proliferation and cardiac regeneration. Basic Research in Cardiology, 115(5), 1–20.
Cui, K., & Zhu, G. (2020). LncRNA CTBP1-AS2 regulates miR-216a/PTEN to suppress ovarian cancer cell proliferation. Journal of Ovarian Research, 13(1), 1–6.
Hou, B.-H., Jian, Z.-X., Cui, P., Li, S.-J., Tian, R.-Q., & Ou, J.-R. (2015). miR-216a may inhibit pancreatic tumor growth by targeting JAK2. FEBS Letters, 589(17), 2224–2232.
Wang, J., Chen, X., Shen, D., Ge, D., Chen, J., Pei, J., et al. (2019). A long noncoding RNA NR_045363 controls cardiomyocyte proliferation and cardiac repair. Journal of Molecular and Cellular Cardiology, 127, 105–114.
Huang, H., Fan, X., Zhang, X., Xie, Y., & Ji, Z. (2020). LncRNA CARLo-7 facilitates proliferation, migration, invasion, and EMT of bladder cancer cells by regulating Wnt/β-catenin and JAK2/STAT3 signaling pathways. Translational Andrology and Urology, 9(5), 2251.
Shi, X., Pan, S., Li, L., Li, Y., Ma, W., Wang, H., et al. (2020). HIX003209 promotes vascular smooth muscle cell migration and proliferation through modulating miR-6089. Aging (Albany NY), 12(10), 8913.
Liu, Y., Cui, X., Wang, C., & Zhao, S. (2020). LncRNA HCG11 regulates proliferation and apoptosis of vascular smooth muscle cell through targeting miR-144–3p/FOXF1 axis in atherosclerosis. Biological Research. https://doi.org/10.1186/s40659-020-00306-2
Wu, Y.-X., Zhang, S.-H., Cui, J., & Liu, F.-T. (2018). Long noncoding RNA XR007793 regulates proliferation and migration of vascular smooth muscle cell via suppressing miR-23b. Medical science monitor: International Medical Journal of Experimental and Clinical Research, 24, 5895.
Wang, Y., Yang, L., Chen, T., Liu, X., Guo, Y., Zhu, Q., et al. (2019). A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Molecular Cancer, 18(1), 1–16.
Song, E.-L., Xing, L., Wang, L., Song, W.-T., Li, D.-B., Wang, Y., et al. (2019). LncRNA ADAMTS9-AS2 inhibits cell proliferation and decreases chemoresistance in clear cell renal cell carcinoma via the miR-27a-3p/FOXO1 axis. Aging (Albany NY), 11(15), 5705.
Ren, L., Chen, S., Liu, W., Hou, P., Sun, W., & Yan, H. (2019). Downregulation of long non-coding RNA nuclear enriched abundant transcript 1 promotes cell proliferation and inhibits cell apoptosis by targeting miR-193a in myocardial ischemia/reperfusion injury. BMC Cardiovascular Disorders, 19(1), 1–8.
Niu, Y., Tang, G., Wu, X., & Wu, C. (2020). LncRNA NEAT1 modulates sorafenib resistance in hepatocellular carcinoma through regulating the miR-149-5p/AKT1 axis. Saudi Journal of Gastroenterology: Official Journal of the Saudi Gastroenterology Association, 26(4), 194.
Zeng, C., Liu, S., Lu, S., Yu, X., Lai, J., Wu, Y., et al. (2018). The c-Myc-regulated lncRNA NEAT1 and paraspeckles modulate imatinib-induced apoptosis in CML cells. Molecular Cancer, 17(1), 1–6.
Zhao, W., Li, W., Jin, X., Niu, T., Cao, Y., Zhou, P., et al. (2019). Silencing long non-coding RNA NEAT1 enhances the suppression of cell growth, invasion, and apoptosis of bladder cancer cells under cisplatin chemotherapy. International Journal of Clinical and Experimental Pathology, 12(2), 549.
Li, C., Zhang, Y., Tang, Y., Xiao, J., Gao, F., Ouyang, Y., et al. (2020). LncRNA CRNDE modulates cardiac progenitor cells’ proliferation and migration via the miR-181a/LYRM1 axis in hypoxia. Journal of Thoracic Disease, 12(5), 2614.
Han, S., Han, B., Li, Z., & Sun, D. (2019). Downregulation of long noncoding RNA CRNDE suppresses drug resistance of liver cancer cells by increasing microRNA-33a expression and decreasing HMGA2 expression. Cell Cycle, 18(19), 2524–2537.
Cui, N., Liu, J., Xia, H., & Xu, D. (2019). LncRNA SNHG20 contributes to cell proliferation and invasion by upregulating ZFX expression sponging miR-495-3p in gastric cancer. Journal of Cellular Biochemistry, 120(3), 3114–3123.
Yang, X., Sun, L., Wang, L., Yao, B., Mo, H., & Yang, W. (2019). LncRNA SNHG7 accelerates the proliferation, migration and invasion of hepatocellular carcinoma cells via regulating miR-122–5p and RPL4. Biomedicine & Pharmacotherapy, 118, 109386.
Zhao, L., Han, T., Li, Y., Sun, J., Zhang, S., Liu, Y., et al. (2017). The IncRNA SNHG5/miR-32 axis regulates gastric cancer cell proliferation and migration by targeting KLF4. The FASEB Journal, 31(3), 893–903.
Liu, Y., Cheng, G., Huang, Z., Bao, L., Liu, J., Wang, C., et al. (2020). Long noncoding RNA SNHG12 promotes tumour progression and sunitinib resistance by upregulating CDCA3 in renal cell carcinoma. Cell Death & Disease, 11(7), 1–17.
Wu, T., Wang, S., Wang, L., Zhang, W., Chen, W., Lv, X., et al. (2020). Long Noncoding RNA (lncRNA) CTTN-IT1 elevates skeletal muscle satellite cell proliferation and differentiation by acting as ceRNA for YAP1 through absorbing miR-29a in Hu sheep. Frontiers in Genetics, 11, 843.
You, G., Long, X., Song, F., Huang, J., Tian, M., Xiao, Y., et al. (2020). Long noncoding RNA EZR-AS1 regulates the proliferation, migration, and apoptosis of human venous endothelial cells via SMYD3. BioMed Research International, 2020, 1–11.
Zou, X., Guo, Z. H., Li, Q., & Wang, P. S. (2020). Long noncoding RNA LINC00460 modulates MMP-9 to promote cell proliferation, invasion and apoptosis by targeting miR-539 in papillary thyroid cancer. Cancer Management and Research, 12, 199.
Wang, R., Huang, Z., Qian, C., Wang, M., Zheng, Y., Jiang, R., et al. (2020). LncRNA WEE2-AS1 promotes proliferation and inhibits apoptosis in triple negative breast cancer cells via regulating miR-32-5p/TOB1 axis. Biochemical and Biophysical Research Communications, 526(4), 1005–1012.
Wang, R., Chen, X., Xu, T., Xia, R., Han, L., Chen, W., et al. (2016). MiR-326 regulates cell proliferation and migration in lung cancer by targeting phox2a and is regulated by HOTAIR. American Journal of Cancer Research, 6(2), 173.
Wu, X., Cao, X., & Chen, F. (2017). WITHDRAWN: LncRNA-HOTAIR activates tumor cell proliferation and migration by suppressing MiR-326 in cervical cancer. Oncology Research. https://doi.org/10.3727/096504017x15037515496840
Li, Z., Qian, J., Li, J., & Zhu, C. (2019). Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Experimental and Therapeutic Medicine, 18(1), 435–442.
Zhang, J., Chen, K., Tang, Y., Luan, X., Zheng, X., Lu, X., et al. (2021). LncRNA-HOTAIR activates autophagy and promotes the imatinib resistance of gastrointestinal stromal tumor cells through a mechanism involving the miR-130a/ATG2B pathway. Cell Death & Disease, 12(4), 1–14.
Lei, H., Gao, Y., & Xu, X. (2017). LncRNA TUG1 influences papillary thyroid cancer cell proliferation, migration and EMT formation through targeting miR-145. Acta Biochimica et Biophysica Sinica, 49(7), 588–597.
Guo, S., Zhang, L., Zhang, Y., Wu, Z., He, D., Li, X., et al. (2019). Long non-coding RNA TUG1 enhances chemosensitivity in non-small cell lung cancer by impairing microRNA-221-dependent PTEN inhibition. Aging (Albany NY), 11(18), 7553.
Gu, L., Li, Q., Liu, H., Lu, X., & Zhu, M. (2020). Long noncoding RNA TUG1 promotes autophagy-associated paclitaxel resistance by sponging miR-29b-3p in ovarian cancer cells. OncoTargets and Therapy, 13, 2007.
Guo, X., Liu, Y., Zheng, X., Han, Y., & Cheng, J. (2020). HOTTIP knockdown inhibits cell proliferation and migration via regulating miR-490–3p/HMGB1 axis and PI3K-AKT signaling pathway in ox-LDL-induced VSMCs. Life Sciences, 248, 117445.
Chen, X., Liu, Y., Zhang, Q., Liu, B., Cheng, Y., Zhang, Y., et al. (2020). Exosomal long noncoding RNA HOTTIP increases resistance of colorectal cancer cells to mitomycin via impairing miR-214-mediated degradation of KPNA3. Frontiers in Cell and Developmental Biology, 8, 1492.
Yin, F., Zhang, Q., Dong, Z., Hu, J., & Ma, Z. (2020). LncRNA HOTTIP participates in cisplatin resistance of tumor cells by regulating miR-137 expression in pancreatic Cancer. OncoTargets and Therapy, 13, 2689.
Li, Z., Zhao, L., & Wang, Q. (2016). Overexpression of long non-coding RNA HOTTIP increases chemoresistance of osteosarcoma cell by activating the Wnt/β-catenin pathway. American Journal of Translational Research, 8(5), 2385.
Xuan, W., Zhou, C., & You, G. (2020). LncRNA LINC00668 promotes cell proliferation, migration, invasion ability and EMT process in hepatocellular carcinoma by targeting miR-532–5p/YY1 axis. Bioscience Reports, 40(5), BSR20192697.
Ma, J., Li, T., Han, X., & Yuan, H. (2018). Knockdown of LncRNA ANRIL suppresses cell proliferation, metastasis, and invasion via regulating miR-122-5p expression in hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology, 144(2), 205–214.
Miao, J.-T., Gao, J.-H., Chen, Y.-Q., Chen, H., Meng, H.-Y., & Lou, G. (2019). LncRNA ANRIL affects the sensitivity of ovarian cancer to cisplatin via regulation of let-7a/HMGA2 axis. Bioscience Reports, 39(7), 20182101.
Yan, S., Tang, Z., Chen, K., Liu, Y., Yu, G., Chen, Q., et al. (2018). Long noncoding RNA MIR31HG inhibits hepatocellular carcinoma proliferation and metastasis by sponging microRNA-575 to modulate ST7L expression. Journal of Experimental & Clinical Cancer Research, 37(1), 1–16.
Liu, Z., Wang, Y., Wang, L., Yao, B., Sun, L., Liu, R., et al. (2019). Long non-coding RNA AGAP2-AS1, functioning as a competitive endogenous RNA, upregulates ANXA11 expression by sponging miR-16-5p and promotes proliferation and metastasis in hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research, 38(1), 1–15.
Jin, X., Qiao, L., Fan, H., Liao, C., Zheng, J., Wang, W., et al. (2021). Long non-coding RNA MSC-AS1 facilitates the proliferation and glycolysis of gastric cancer cells by regulating PFKFB3 expression. International Journal of Medical Sciences, 18(2), 546.
Zhang, S., & Guo, W. (2019). Long non-coding RNA MEG3 suppresses the growth of glioma cells by regulating the miR-96-5p/MTSS1 signaling pathway. Molecular Medicine Reports, 20(5), 4215–4225.
Lü, M., Tang, B., Zeng, S., Hu, C., Xie, R., Wu, Y., et al. (2016). Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR-1207-5p and promotes proliferation in gastric cancer. Oncogene, 35(27), 3524–3534.
Qi, Y., Ma, Y., Peng, Z., Wang, L., Li, L., Tang, Y., et al. (2020). Long noncoding RNA PENG upregulates PDZK1 expression by sponging miR-15b to suppress clear cell renal cell carcinoma cell proliferation. Oncogene, 39(22), 4404–4420.
Dong, D., Mu, Z., Wei, N., Sun, M., Wang, W., Xin, N., et al. (2019). Long non-coding RNA ZFAS1 promotes proliferation and metastasis of clear cell renal cell carcinoma via targeting miR-10a/SKA1 pathway. Biomedicine & Pharmacotherapy, 111, 917–925.
Sun, X.-H., Fan, W.-J., An, Z.-J., & Sun, Y. (2020). Inhibition of long noncoding RNA CRNDE increases chemosensitivity of medulloblastoma cells by targeting miR-29c-3p. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics, 28(1), 95–102.
Zheng, D., Zhang, Y., Hu, Y., Guan, J., Xu, L., Xiao, W., et al. (2019). Long noncoding RNA Crnde attenuates cardiac fibrosis via Smad3-Crnde negative feedback in diabetic cardiomyopathy. The FEBS Journal, 286(9), 1645–1655.
Wang, P., Chen, D., Ma, H., & Li, Y. (2017). LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axis. OncoTargets and Therapy, 10, 5137.
Murugavel Ponnusamy, F. L., Zhang, Y.-H., Li, R.-B., Zhai, M., Liu, F., Zhou, L.-Y., Liu, C.-Y. et al. (2019). The long non-coding RNA CPR regulates cardiomyocyte proliferation and cardiac repair.
Liu, E., Liu, Z., & Zhou, Y. (2015). Carboplatin-docetaxel-induced activity against ovarian cancer is dependent on up-regulated lncRNA PVT1. International Journal of Clinical and Experimental Pathology, 8(4), 3803.
Xu, J.-J., Zheng, W.-H., Wang, J., & Chen, Y.-Y. (2020). Long non-coding RNA plasmacytoma variant translocation 1 linked to hypoxia-induced cardiomyocyte injury of H9c2 cells by targeting miR-135a-5p/forkhead box O1 axis. Chinese Medical Journal, 133(24), 2953.
Liu, L., Pang, X., Shang, W., Xie, H., Feng, Y., & Feng, G. (2019). Long non-coding RNA GAS5 sensitizes renal cell carcinoma to sorafenib via miR-21/SOX5 pathway. Cell Cycle, 18(3), 257–263.
Zhou, X.-H., Chai, H.-X., Bai, M., & Zhang, Z. (2020). LncRNA-GAS5 regulates PDCD4 expression and mediates myocardial infarction-induced cardiomyocytes apoptosis via targeting MiR-21. Cell Cycle, 19(11), 1363–1377.
Wang, X., Jiang, G., Ren, W., Wang, B., Yang, C., & Li, M. (2020). LncRNA NEAT1 regulates 5-Fu sensitivity, apoptosis and invasion in colorectal cancer through the MiR-150-5p/CPSF4 axis. OncoTargets and Therapy, 13, 6373.
Chen, H., Xia, W., & Hou, M. (2020). LncRNA-NEAT1 from the competing endogenous RNA network promotes cardioprotective efficacy of mesenchymal stem cell-derived exosomes induced by macrophage migration inhibitory factor via the miR-142-3p/FOXO1 signaling pathway. Stem Cell Research & Therapy, 11(1), 31.
Chen, T., Liu, Z., Zeng, W., & Huang, T. (2019). Down-regulation of long non-coding RNA HOTAIR sensitizes breast cancer to trastuzumab. Scientific Reports, 9(1), 1–12.
Chen, J., Li, X., Zhao, F., & Hu, Y. (2021). HOTAIR/miR-17-5p axis is involved in the propofol-mediated cardioprotection against ischemia/reperfusion injury. Clinical Interventions in Aging, 16, 621.
Sigaroudi, A. E., Salari, A., Poursadeghi, M., Moaddab, F., Mirrazeghi, S. F., & Mirbolouk, F. (2019). Safety and efficacy of high-dose versus low-dose aspirin in individuals with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention: A randomized clinical trial. Immunopathologia Persa, 6(1), e05.
Zhang, J., Gao, C., Meng, M., & Tang, H. (2016). Long noncoding RNA MHRT protects cardiomyocytes against H2O2-induced apoptosis. Biomolecules & Therapeutics, 24(1), 19.
Su, X., Lv, L., Li, Y., Fang, R., Yang, R., Li, C., et al. (2020). lncRNA MIRF promotes cardiac apoptosis through the miR-26a-Bak1 axis. Molecular Therapy-Nucleic Acids, 20, 841–850.
Wang, S., He, F., Li, Z., Hu, Y., Huangfu, N., & Xie, D. (2020). Long non-coding RNA BANCR promotes interferon-β-induced cardiomyocyte apoptosis by targeting signal transducer and activator of transcription 1 in vitro. International Journal of Clinical and Experimental Pathology, 13(11), 2840.
Miao, X., Liu, Y., Fan, Y., Wang, G., & Zhu, H. (2021). LncRNA BANCR attenuates the killing capacity of cisplatin on gastric cancer cell through the ERK1/2 pathway. Cancer Management and Research, 13, 287.
Jiang, L., Zhao, X.-H., Mao, Y.-L., Wang, J.-F., Zheng, H.-J., & You, Q.-S. (2019). Long non-coding RNA RP11–468E2. 5 curtails colorectal cancer cell proliferation and stimulates apoptosis via the JAK/STAT signaling pathway by targeting STAT5 and STAT6. Journal of Experimental & Clinical Cancer Research, 38(1), 1–16.
Zhang, X., Sha, M., Yao, Y., Da, J., & Jing, D. (2015). Increased B-type-natriuretic peptide promotes myocardial cell apoptosis via the B-type-natriuretic peptide/long non-coding RNA LSINCT5/caspase-1/interleukin 1β signaling pathway. Molecular Medicine Reports, 12(5), 6761–6767.
Liu, H., Zhou, G., Fu, X., Cui, H., Pu, G., Xiao, Y., et al. (2017). Long noncoding RNA TUG1 is a diagnostic factor in lung adenocarcinoma and suppresses apoptosis via epigenetic silencing of BAX. Oncotarget, 8(60), 101899.
Deng, H., Ouyang, W., Zhang, L., Xiao, X., Huang, Z., & Zhu, W. (2019). LncRNA GASL1 is downregulated in chronic heart failure and regulates cardiomyocyte apoptosis. Cellular & Molecular Biology Letters, 24(1), 1–7.
Zadeh, F. J., Akbari, T., Samimi, A., Davari, N., & Rezaeeyan, H. (2020). The role of molecular mechanism of Ten-Eleven Translocation2 (TET2) family proteins in pathogenesis of cardiovascular diseases (CVDs). Molecular Biology Reports, 47, 5503.
Lv, J., Zhu, Y., & Yao, S. (2020). LncRNAMORT is upregulated in myocardial infarction and promotes the apoptosis of cardiomyocyte by downregulating miR-93. BMC Cardiovascular Disorders, 20, 1–7.
Haybar, H., Shahrabi, S., Rezaeeyan, H., Shirzad, R., & Saki, N. (2019). Protective role of heat shock transcription factor 1 in heart failure: A diagnostic approach. Journal of Cellular Physiology, 234(6), 7764–7770.
Sun, H., Ke, C., Zhang, L., Tian, C., Zhang, Z., & Wu, S. (2020). Long non-coding RNA (LncRNA)-ATB promotes inflammation, cell apoptosis and senescence in transforming growth factor-β1 (TGF-β1) induced human kidney 2 (HK-2) cells via TGFβ/SMAD2/3 signaling pathway. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 26, e922029–e922031.
Zhang, X. H., Li, B. F., Ding, J., Shi, L., Ren, H. M., Liu, K., et al. (2020). LncRNA DANCR-miR-758-3p-PAX6 molecular network regulates apoptosis and autophagy of breast cancer cells. Cancer Management and Research, 12, 4073.
Ma, Y., Fan, B., Ren, Z., Liu, B., & Wang, Y. (2019). Long noncoding RNA DANCR contributes to docetaxel resistance in prostate cancer through targeting the miR-34a-5p/JAG1 pathway. OncoTargets and Therapy, 12, 5485.
Liu, Y., Chen, L., Yuan, H., Guo, S., & Wu, G. (2020). LncRNA DANCR promotes sorafenib resistance via activation of IL-6/STAT3 signaling in hepatocellular carcinoma cells. OncoTargets and Therapy, 13, 1145.
Zhang, H., Liu, L., Chen, L., Liu, H., Ren, S., & Tao, Y. (2021). Long noncoding RNA DANCR confers cytarabine resistance in acute myeloid leukemia by activating autophagy via the miR-874-3P/ATG16L1 axis. Molecular Oncology, 15(4), 1203–1216.
Chen, P., Fang, X., Xia, B., Zhao, Y., Li, Q., & Wu, X. (2018). Long noncoding RNA LINC00152 promotes cell proliferation through competitively binding endogenous miR-125b with MCL-1 by regulating mitochondrial apoptosis pathways in ovarian cancer. Cancer Medicine, 7(9), 4530–4541.
Xia, P., Gu, R., Zhang, W., & Sun, Y.-F. (2020). lncRNA CEBPA-AS1 overexpression inhibits proliferation and migration and stimulates apoptosis of OS cells via notch signaling. Molecular Therapy-Nucleic Acids, 19, 1470–1481.
Zheng, J., Peng, B., Zhang, Y., Ai, F., & Hu, X. (2020). FOXD3-AS1 knockdown suppresses hypoxia-induced cardiomyocyte injury by increasing cell survival and inhibiting apoptosis via upregulating cardioprotective molecule miR-150-5p In Vitro. Frontiers in Pharmacology, 11, 1284.
Zhang, D., Lee, H., Haspel, J. A., & Jin, Y. (2017). Long noncoding RNA FOXD3-AS1 regulates oxidative stress-induced apoptosis via sponging microRNA-150. The FASEB Journal, 31(10), 4472–4481.
Zeng, Z., Zhao, G., Zhu, H., Nie, L., He, L., Liu, J., et al. (2020). LncRNA FOXD3-AS1 promoted chemo-resistance of NSCLC cells via directly acting on miR-127-3p/MDM2 axis. Cancer Cell International, 20(1), 1–12.
Guo, W., Jiang, H., Li, H., Li, F., Yu, Q., Liu, Y., et al. (2019). LncRNA-SRA1 suppresses osteosarcoma cell proliferation while promoting cell apoptosis. Technology in Cancer Research & Treatment, 18, 1533033819841438.
Wu, Y., Ding, J., Sun, Q., Zhou, K., Zhang, W., Du, Q., et al. (2018). Long noncoding RNA hypoxia-inducible factor 1 alpha-antisense RNA 1 promotes tumor necrosis factor-α-induced apoptosis through caspase 3 in Kupffer cells. Medicine, 97(4), e9483.
Misawa, A., Takayama, K.-I., Urano, T., & Inoue, S. (2016). Androgen-induced long noncoding RNA (lncRNA) SOCS2-AS1 promotes cell growth and inhibits apoptosis in prostate cancer cells. Journal of Biological Chemistry, 291(34), 17861–17880.
Tao, W., Li, Y., Zhu, M., Li, C., & Li, P. (2019). LncRNA NORAD promotes proliferation and inhibits apoptosis of gastric cancer by regulating miR-214/Akt/mTOR axis. OncoTargets and Therapy, 12, 8841.
Wu, H., Zhu, H., Zhuang, Y., Zhang, J., Ding, X., Zhan, L., et al. (2020). LncRNA ACART protects cardiomyocytes from apoptosis by activating PPAR-γ/Bcl-2 pathway. Journal of Cellular and Molecular Medicine, 24(1), 737–746.
Teng, W., Qiu, C., He, Z., Wang, G., Xue, Y., & Hui, X. (2017). Linc00152 suppresses apoptosis and promotes migration by sponging miR-4767 in vascular endothelial cells. Oncotarget, 8(49), 85014.
Li, G., Yu, J., Yang, B., Gong, F., & Zhang, K. (2019). LncRNA LOXL1-AS1 inhibited cell proliferation, migration and invasion as well as induced apoptosis in breast cancer via regulating miR-143-3p. European Review for Medical and Pharmacological Sciences, 23(23), 10400–10409.
Ma, Z., Luo, Y., & Qiu, M. (2017). miR-143 induces the apoptosis of prostate cancer LNCap cells by suppressing Bcl-2 expression. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 23, 359.
Dai, W., Mu, L., Cui, Y., Li, Y., Chen, P., Xie, H., et al. (2019). Berberine promotes apoptosis of colorectal cancer via regulation of the long non-coding RNA (lncRNA) cancer susceptibility candidate 2 (CASC2)/AU-binding factor 1 (AUF1)/B-cell CLL/lymphoma 2 (Bcl-2) axis. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 25, 730.
Zhu, D., Yu, Y., Qi, Y., Wu, K., Liu, D., Yang, Y., et al. (2019). Long non-coding RNA CASC2 enhances the antitumor activity of cisplatin through suppressing the Akt pathway by inhibition of miR-181a in esophageal squamous cell carcinoma cells. Frontiers in Oncology, 9, 350.
Chen, J., Liu, L., Wei, G., Wu, W., Luo, H., Yuan, J., et al. (2016). The long noncoding RNA ASNR regulates degradation of Bcl-2 mRNA through its interaction with AUF1. Scientific Reports, 6(1), 1–11.
Han, L., Zhang, E., Yin, D., Kong, R., Xu, T., Chen, W., et al. (2015). Low expression of long noncoding RNA PANDAR predicts a poor prognosis of non-small cell lung cancer and affects cell apoptosis by regulating Bcl-2. Cell Death & Disease, 6(2), e1665.
Wang, H., Fang, L., Jiang, J., Kuang, Y., Wang, B., Shang, X., et al. (2018). The cisplatin-induced lncRNA PANDAR dictates the chemoresistance of ovarian cancer via regulating SFRS2-mediated p53 phosphorylation. Cell Death & Disease, 9(11), 1–15.
Cai, X., Zhang, P., Wang, S., Hong, L., Yu, S., Li, B., et al. (2020). lncRNA FGD5 antisense RNA 1 upregulates RORA to suppress hypoxic injury of human cardiomyocyte cells by inhibiting oxidative stress and apoptosis via miR-195. Molecular Medicine Reports, 22(6), 4579–4588.
Zhu, F., Niu, R., Shao, X., & Shao, X. (2021). FGD5-AS1 promotes cisplatin resistance of human lung adenocarcinoma cell via the miR-142-5p/PD-L1 axis. International Journal of Molecular Medicine, 47(2), 523–532.
Zhang, Z., Lv, M., Wang, X., Zhao, Z., Jiang, D., & Wang, L. (2020). LncRNA LUADT1 sponges miR-195 to prevent cardiac endothelial cell apoptosis in sepsis. Molecular Medicine, 26(1), 1–8.
Yan, M., Liu, Q., Jiang, Y., Wang, B., Ji, Y., Liu, H., et al. (2020). Long noncoding RNA LNC_000898 alleviates cardiomyocyte apoptosis and promotes cardiac repair after myocardial infarction through modulating the miR-375/PDK1 axis. Journal of Cardiovascular Pharmacology, 76(1), 77–85.
Xu, W., Zhao, Y., & Ai, Y. (2020). Overexpression of lncRNA Gm43050 alleviates apoptosis and inflammation response induced by sevoflurane treatment by regulating miR-640/ZFP91. American Journal of Translational Research, 12(8), 4337.
Zhao, Y., & Ai, Y. (2020). Overexpression of lncRNA Gm15621 alleviates apoptosis and inflammation response resulting from sevoflurane treatment through inhibiting miR-133a/Sox4. Journal of Cellular Physiology, 235(2), 957–965.
Chen, F., Hu, Y., Xie, Y., Zhao, Z., Ma, L., Li, Z., et al. (2020). Total glucosides of paeony alleviate cell apoptosis and inflammation by targeting the long noncoding RNA XIST/MicroRNA-124–3p/ITGB1 axis in renal ischemia/reperfusion injury. Mediators of Inflammation, 2020, 1–13.
Zhao, H., Wan, J., & Zhu, Y. (2020). Carboplatin inhibits the progression of retinoblastoma through IncRNA XIST/miR-200a-3p/NRP1 Axis. Drug Design, Development and Therapy, 14, 3417.
Shen, S. N., Li, K., Liu, Y., Yang, C. L., He, C. Y., & Wang, H. R. (2019). Down-regulation of long noncoding RNA PVT 1 inhibits esophageal carcinoma cell migration and invasion and promotes cell apoptosis via micro RNA-145-mediated inhibition of FSCN 1. Molecular Oncology, 13(12), 2554–2573.
Ping, G., Xiong, W., Zhang, L., Li, Y., Zhang, Y., & Zhao, Y. (2018). Silencing long noncoding RNA PVT1 inhibits tumorigenesis and cisplatin resistance of colorectal cancer. American Journal of Translational Research, 10(1), 138.
Dai, J.-H., Huang, W.-Z., Li, C., Deng, J., Lin, S.-J., & Luo, J. (2019). Silencing of long noncoding RNA SBF2-AS1 inhibits proliferation, migration and invasion and contributes to apoptosis in osteosarcoma cells by upregulating microRNA-30a to suppress FOXA1 expression. Cell Cycle, 18(20), 2727–2741.
Zhang, Z., Yin, J., Lu, C., Wei, Y., Zeng, A., & You, Y. (2019). Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. Journal of Experimental & Clinical Cancer Research, 38(1), 1–16.
Katare, P. B., Nizami, H. L., Paramesha, B., Dinda, A. K., & Banerjee, S. K. (2020). Activation of toll like receptor 4 (TLR4) promotes cardiomyocyte apoptosis through SIRT2 dependent p53 deacetylation. Scientific Reports, 10(1), 1–15.
Xu, W., Hu, G.-Q., Da Costa, C., Tang, J.-H., Li, Q.-R., Du, L., et al. (2019). Long noncoding RNA UBE2R2-AS1 promotes glioma cell apoptosis via targeting the miR-877-3p/TLR4 axis. OncoTargets and Therapy, 12, 3467.
Li, K., Zhong, S., Luo, Y., Zou, D., Li, M., Li, Y., et al. (2019). A long noncoding RNA binding to QKI-5 regulates germ cell apoptosis via p38 MAPK signaling pathway. Cell Death & Disease, 10(10), 1–14.
Zeng, J., Li, Y., Wang, Y., Xie, G., Feng, Q., Yang, Y., et al. (2020). lncRNA 00312 attenuates cell proliferation and invasion and promotes apoptosis in renal cell carcinoma via miR-34a-5p/ASS1 axis. Oxidative Medicine and Cellular Longevity, 2020, 1–16.
Su, C., Shi, K., Cheng, X., Han, Y., Li, Y., Yu, D., et al. (2018). Long noncoding RNA LINC00472 inhibits proliferation and promotes apoptosis of lung adenocarcinoma cells via regulating miR-24-3p/DEDD. Technology in Cancer Research & Treatment, 17, 1533033818790490.
Yan, S., Tang, Z., Chen, K., Liu, Y., Yu, G., Chen, Q., et al. (2018). Long noncoding RNA MIR31HG inhibits hepatocellular carcinoma proliferation and metastasis by sponging microRNA-575 to modulate ST7L expression. Journal of Experimental & Clinical Cancer Research, 37(1), 214.
Wu, C., Chen, W., Yu, F., Yuan, Y., Chen, Y., Hurst, D. R., et al. (2020). Long noncoding RNA HITTERS protects oral squamous cell carcinoma cells from endoplasmic reticulum stress-induced apoptosis via promoting MRE11-RAD50-NBS1 complex formation. Advanced Science, 7(22), 2002747.
Peng, X., Ji, C., Tan, L., Lin, S., Zhu, Y., Long, M., et al. (2020). Long non-coding RNA TNRC6C-AS1 promotes methylation of STK4 to inhibit thyroid carcinoma cell apoptosis and autophagy via Hippo signalling pathway. Journal of Cellular and Molecular Medicine, 24(1), 304–316.
Huang, W., Su, G., Huang, X., Zou, A., Wu, J., Yang, Y., et al. (2019). Long noncoding RNA PCAT6 inhibits colon cancer cell apoptosis by regulating anti-apoptotic protein ARC expression via EZH2. Cell Cycle, 18(1), 69–83.
Wu, H., Zou, Q., He, H., Liang, Y., Lei, M., Zhou, Q., et al. (2019). Long non-coding RNA PCAT6 targets miR-204 to modulate the chemoresistance of colorectal cancer cells to 5-fluorouracil-based treatment through HMGA2 signaling. Cancer Medicine, 8(5), 2484–2495.
Xin, H., Liu, N., Xu, X., Zhang, J., Li, Y., Ma, Y., et al. (2019). Knockdown of lncRNA-UCA1 inhibits cell viability and migration of human glioma cells by miR-193a-mediated downregulation of CDK6. Journal of Cellular Biochemistry, 120(9), 15157–15169.
Yang, Y.-N., Zhang, R., Du, J.-W., Yuan, H.-H., Li, Y.-J., Wei, X.-L., et al. (2018). Predictive role of UCA1-containing exosomes in cetuximab-resistant colorectal cancer. Cancer Cell International, 18(1), 1–11.
Fu, D., Lu, C., Qu, X., Li, P., Chen, K., Shan, L., et al. (2019). LncRNA TTN-AS1 regulates osteosarcoma cell apoptosis and drug resistance via the miR-134-5p/MBTD1 axis. Aging (Albany NY), 11(19), 8374.
Zhuang, L., Xia, W., Chen, D., Ye, Y., Hu, T., Li, S., et al. (2020). Exosomal LncRNA–NEAT1 derived from MIF-treated mesenchymal stem cells protected against doxorubicin-induced cardiac senescence through sponging miR-221-3p. Journal of Nanobiotechnology, 18(1), 1–16.
Wang, H., Lin, X., Li, J., Zeng, G., & Xu, T. (2021). Long noncoding RNA SOX2-OT aggravates doxorubicin-induced apoptosis of cardiomyocyte by targeting miR-942-5p/DP5. Drug Design, Development and Therapy, 15, 481.
Acknowledgements
We wish to thank all our colleagues in Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
MS has conceived the manuscript and revised it. MA and MM wrote the manuscript. MKA and AA design the tables.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals.
Additional information
Handling Editor: Mitzi C. Glover.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amrovani, M., Mohammadtaghizadeh, M., Aghaali, M.K. et al. Long Non-coding RNAs: Potential Players in Cardiotoxicity Induced by Chemotherapy Drugs. Cardiovasc Toxicol 22, 191–206 (2022). https://doi.org/10.1007/s12012-021-09681-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09681-y