Abstract
This study was designed to investigate whether the pretreatment with curcumin, a yellow pigment from turmeric (Curcuma longa) known for its potent antioxidant capacity, was able to protect against the oxidant damage and mitochondrial dysfunction induced by reperfusion injury in isolated hearts. Rats were treated with a daily intragastric dose of curcumin (200 mg/kg) for 7 days prior to experimental ischemia (30 min) and reperfusion (60 min) (I/R). Cardiac mechanical work was measured during periods of stabilization, ischemia, and reperfusion. Oxidant stress and activity of antioxidant enzymes were measured in both homogenates of cardiac tissue and in isolated mitochondria. In addition, oxygen consumption was measured in isolated mitochondria. It was found that curcumin pretreatment attenuates the I/R injury as evidenced by (a) loss of cardiac mechanical work, (b) oxidant stress (increase in lipid peroxidation and decrease in reduced glutathione content) and (c) decrease in the activity of the antioxidant enzymes superoxide dismutase and glutathione reductase in both cardiac tissue and isolated mitochondria, and (d) decrease in mitochondrial respiratory capacity. In conclusion, the protective effect of curcumin was associated with the attenuation of oxidant stress and mitochondrial dysfunction secondary to I/R injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Curcumin (diferuloylmethane) is a naturally occurring phenolic compound isolated as a yellow pigment from turmeric (Curcuma longa), which is commonly used as a spice, additive, and food colorant [1]. Curcumin is well documented for its medicinal properties in traditional Indian medicine [2]. The compound has been reported to possess a variety of biological and pharmacological activities including antioxidant [3, 4], anti-inflammatory [5], antimicrobial [6], and anticancer activities [7] and thus has a potential effect against various malignant diseases [1]. Curcumin exhibits strong antioxidant activity and has been shown to be a potent scavenger of a variety of reactive oxygen species (ROS) including superoxide anion, hydroxyl radicals [8], and nitrogen dioxide radicals [9, 10]. Phase II enzymes including glutathione peroxidase, glutathione reductase (GR), glutathione S-transferase (GST), γ-glutamate cysteine ligase, and NADPH quinone oxidoreductase 1 protect cells from stress by diminishing oxidant stress or detoxifying carcinogens [11], and curcumin has shown to increase the expression of these enzymes by inducing nuclear translocation of the transcription factor Nrf2 (NF-E2-related factor-2) [12, 13]. Several studies have shown that curcumin is able to ameliorate mitochondria-induced ROS production and lipid peroxidation in different models of oxidant damage [14–16], including that occurring in cardiac tissue [17, 18]. In this respect, several reports have shown that mitochondria are both targets and sources of myocardial damage during ischemia and reperfusion [19, 20]. Indeed, ischemia–reperfusion (I/R) results in damage to the respiratory chain with impairment of oxidative phosphorylation [21]. There is substantial evidence that ROS are generated during I/R in the heart. In this condition, damage to mitochondria favors oxidative stress via ROS production by complex I and III [22]; xanthine oxidase [23] and NADPH oxidase [24] are also involved. This study explored whether curcumin pretreatment was able to attenuate the mechanical injury, mitochondrial dysfunction, and oxidative stress induced by I/R injury in the isolated rat heart.
Materials and Methods
Chemical Reagents
Curcumin (Cat. No. C1386, batch # 079K1756), glutathione reduced form (GSH), glutathione oxidized form (GSSG), nitroblue tetrazolium (NBT), tetramethoxypropane (TMPO), GR, GST, 1-chloro-2,4-dinitrobenzene (CDNB), 4 ethylenediaminetetraacetic acid (EDTA), 1-methyl-2-phenylindole, xanthine, xanthine oxidase, β-nicotinamide adenine dinucleotide phosphate reduced form (NADPH), nicotinamide adenine dinucleotide reduced form (NADH), aprotinin, leupeptin, pepstatin, N-(2-hydroxyethyl)piperazine-N-(2-ethanesulfonic acid) (HEPES), butylated hydroxytoluene (BHT), bovine serum albumin (BSA), adenosine diphosphate (ADP), carbonyl cyanide m-chlorophenylhydrazone (CCCP), rotenone, succinate, malate, glutamate, sodium octanoate, subtilisin A, and guanidine hydrochloride were purchased from Sigma-Aldrich (St. Louis MO, USA). Monochlorobimane was purchased from Fluka (Schnelldorf, Germany). Hydrogen peroxide (H2O2) was purchased from J.T. Baker (Xalostoc Edo. Mex, México). All other reagents and chemicals used were of the highest grade of purity commercially available.
Animal Treatment
All animal experiments were conducted in accordance with the NIH guide for the care and use of laboratory animals. Fours groups of male Wistar rats (270–300 g) were studied (n = 4–7/group): Control (CT), I/R, I/R + curcumin (I/R + CUR), and curcumin (CUR). Rats from CT group were treated daily via oral gavage with carboxymethylcellulose 0.05% for 7 days, and then the hearts of the animals were used for the Langendorff perfusion experiments. Rats from CUR groups received curcumin (200 mg/kg, body weight), dissolved in carboxymethylcellulose 0.05%, that was given daily via oral gavage for 7 days before the Langendorff perfusion experiments.
Langendorff Perfusion Experiments
After treatment, the animals were anesthetized with sodium pentobarbital (60 mg/kg) and anticoagulated with sodium heparin (1,000 U/kg). Five minutes after the heparin injection, a midstream thoracotomy was performed and the heart was rapidly excised and placed in ice-cold Krebs–Henseleit buffer solution, consisting of 118 mM NaCl, 4.75 mM KCl, 1.18 mM KH2PO4, 1.18 mM MgSO4 7H2O, 2.5 mM CaCl2, 25 mM NaHCO3, 5 mM glucose, and 100 μM sodium octanoate, pH 7.4. The heart was quickly fixed onto a Langendorff heart perfusion system and perfused retrogradely via the aorta at a constant flux of 12 ml/min with Krebs–Henseleit solution that was continuously bubbled with 95% O2 and 5% CO2, at 37°C. Mechanical work was measured at a left ventricular end-diastolic pressure of 10 mm Hg, using a latex balloon inserted into the left ventricle and connected to a pressure transducer. All variables were recorded using a computer acquisition data system designed by the Instrumentation and Technical Development Department of the National Institute of Cardiology (México, D.F., México) [25].
Experimental Protocols
I/R Protocol
Krebs–Henseleit buffer was perfused for 20 min to stabilize the hearts of CT, I/R, I/R + CUR, and CUR groups. I/R and I/R + CUR groups were generated as follows. The hearts were subjected to global ischemia for 30 min, by turning off the pumping system, and then to reperfusion for additional 60 min (I/R hearts) [25]. Hearts from CT and CUR groups were continuously perfused as long as I/R hearts. Hearts that developed arrhythmias before the ischemia were discarded and replaced.
Isolation of Heart Mitochondria
At the end of the perfusion protocol (see above), the hearts were dismounted from the Langendorff system and placed in cold buffer solution, which contained 250 mM sucrose, 10 mM Tris/HCl, and 1 mM EDTA (pH 7.4). The hearts were minced and incubated for 10 min with the same buffer, plus 2 mg/ml subtilisin A, in an ice bath. The tissue was then washed and suspended in the same buffer without the enzyme. Heart tissue was homogenized and mitochondria were obtained by differential centrifugation as described previously [26].
Determination of Oxygen Consumption
Mitochondrial oxygen consumption was measured using a Clark-type oxygen electrode (Yellow Springs Instruments, Yellow Spring, OH, USA). The experiments were carried out in 1.5 ml of basic medium containing 125 mM KCl, 10 mM HEPES, and 3 mM inorganic phosphate, pH 7.3. State 4 respiration was evaluated in the presence of 10 mM sodium glutamate and 10 mM sodium malate or 10 mM succinate plus 1 μg/ml rotenone. State 3 respiration was stimulated by the addition of 200 μM ADP. Respiratory rates are expressed as nanograms atoms of oxygen/min/mg protein (ngAO/min/mg). Respiratory control index (RC) was calculated as the ratio of state 3/state 4. Uncoupled respiration was measured by adding 1 μM CCCP. Phosphorylation efficiency (ADP/O ratio) was calculated from the added amount of ADP and total amount of oxygen consumed during state 3 [25].
Preparation of Heart and Mitochondrial Homogenates
At the end of the perfusion protocol (see Sect. “I/R Protocol”), the hearts were dismounted from the Langendorff system and heart tissue was frozen in liquid nitrogen until the determinations of lipid peroxidation, GSH content, and activity of antioxidant enzymes (catalase, GR, GPx, GST, and SOD) were performed. Heart tissue and isolated mitochondria were homogenized in a Polytron (Model PT 2000, Brinkmann, Westbury, NY, USA) for 10 s in cold potassium phosphate buffer (50 mM) with 0.1% Triton X-100, pH 7.0 [27]. The homogenates were centrifuged at 19,000× g at 4°C for 30 min, and the supernatant was separated to measure total protein, activity of antioxidant enzymes (CAT, GR, GPx, GST, and SOD), and GSH content. To measure lipid peroxidation, a oxidative stress marker, butylated hydroxytoluene (0.5 M), leupeptin (5 μg/ml), pepstatin (7 μg/ml), and aprotinin (5 μg/ml) were added to the potassium phosphate buffer (50 mM), pH 7.0 and samples were homogenized as described above. Total protein was measured by the method of Lowry et al. [28].
Evaluation of Oxidant Damage in Heart and Mitochondrial Homogenates
GSH Content
GSH levels were measured in heart and mitochondrial homogenates using monochlorobimane as previously described by Fernández-Checa and Kaplowitz [29]. The method is based on the appearance of fluorescent adducts of monochlorobimane with GSH in a reaction catalyzed by the enzyme GST. The fluorescence was measured using excitation and emission wavelengths at 385 and 478 nm, respectively, using a Synergy HT multi-mode microplate reader (Biotek Instruments Inc., Winooski, VT, USA). Data were expressed as μmol GSH/mg protein.
Lipid Peroxidation
Malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) were measured using a standard curve of TMPO. A solution of 1-methyl-2-phenylindole was added to the heart and mitochondrial homogenates and the reaction was started by adding 37% HCl. The method is based on the formation of a colored complex between MDA and 4-HNE with 1-methyl-2-phenylindole. Optical density was measured at 586 nm after 1 h of incubation at 45°C [30]. Data were expressed as nmol MDA and 4-HNE/mg protein.
Activity of Antioxidant Enzymes in Heart and Mitochondrial Homogenates
CAT activity was assayed in heart and mitochondrial homogenates by a method based on the disappearance of 30 mM H2O2 at 240 nm [31]. The data were expressed as k/mg protein, where k (first-order reaction) can be used as a direct measure of the catalase concentration as described by Aebi [32].
GR activity was assayed in heart and mitochondrial homogenates using oxidized glutathione as substrate and measuring the disappearance of NADPH at 340 nm [31]. One unit of GR was defined as the amount of enzyme that oxidizes 1 μmol of NADPH per minute. Data were expressed as U/mg protein.
GPx activity was measured in heart and mitochondrial homogenates using GR and NADPH in a coupled reaction [31]. The disappearance of NADPH was monitored at 340 nm. One unit of GPx was defined as the amount of enzyme that oxidizes 1 μmol of NADPH per minute. Data were expressed as U/mg protein.
GST activity was assayed in heart and mitochondrial homogenates in a mixture containing GSH and CDNB as previously described [33]. The method is based on the formation of a complex between GSH and CDNB, which has a maximum absorbance at 340 nm. One unit of GST was defined as the amount of enzyme that conjugates 1 μmol of CDNB with GSH per minute. Data were expressed as U/mg protein.
SOD activity in heart and mitochondrial homogenates was assayed spectrophotometrically at 560 nm by a previously reported method using NBT as the indicator reagent [31]. The method is based on the oxidation of NBT to formazan by superoxide anion, which is generated by the reaction system xanthine/xanthine oxidase; in the presence of SOD, the oxidation of NBT is inhibited. The amount of protein that inhibited NBT reduction to 50% of maximum was defined as one unit of SOD activity. Results were expressed as U/mg protein.
Statistical Analysis
Results were expressed as mean ± SEM. Data were analyzed by two-way ANOVA followed by Bonferroni’s multiple comparisons test using software Prism 5.0 (GraphPad, San Diego, CA, USA). A P value < 0.05 was considered statistically significant.
Results
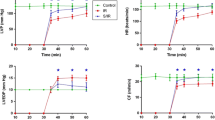
The effect of curcumin administration on contractile function was evaluated in hearts subjected to I/R. Figure 1 shows that hearts from I/R + CUR group rapidly recovered from the ischemic insult, reaching mechanical work levels comparable to the CT group in remarkable contrast to reperfused (I/R) hearts, which early during reperfusion loose mechanical function. Curcumin administration to the rats had no effect on the mechanical work in the isolated system (CUR group) (Fig. 1).
Effect of curcumin (CUR) pretreatment on the mechanical work of postischemic hearts. In the I/R group, hearts were subjected to 20 min of stabilization and then to 30 min of ischemia, after that, the pump system was restored and each heart was reperfused for 60 min. In the CUR group, curcumin (200 mg/kg, body weight) was given daily via oral gavage for 7 days before the Langendorff perfusion experiments. Hearts of CT, I/R, I/R + CUR, and CUR groups were perfused for 20 min. The hearts from I/R and I/R + CUR groups were subjected to global ischemia for 30 min and then to reperfusion for additional 60 min. Hearts from CT and CUR groups were continuously perfused as long as I/R hearts. CT control, I/R ischemia and reperfusion. Data are mean ± SEM, n = 6. * P < 0.05 versus CT, # P < 0.05 versus I/R
Tables 1 and 2 show that I/R injury was associated with enhanced oxidant stress (lipid peroxidation) and decreased GSH content as well as a decrease in the activity of SOD and GR both in heart (Table 1) and mitochondrial homogenates (Table 2). These changes were effectively prevented by CUR pretreatment in the I/R + CUR group. The changes in the activity of GPx, GST, and CAT induced by I/R were not significant. Interestingly, in heart homogenates, the activity of catalase in the I/R + CUR group was significantly higher than that in the I/R group. In addition, both in heart homogenates (Table 1) as well as in isolated mitochondria (Table 2), the activity of GST increased in the CUR group suggesting that this enzyme was induced by CUR treatment.
Respiratory activities of heart mitochondria isolated from CT, CUR, I/R, and I/R + CUR groups were measured in the presence of malate/glutamate (Fig. 2) or succinate plus rotenone (Fig. 3) as substrates. A diminution in state 3 respiration rate was observed in mitochondria from the I/R groups when using NADH-linked substrates (15 ± 1 ngAO/min/mg vs. 82 ± 10 ngAO/min/mg in the CT group) or succinate (68 ± 10 ngAO/min/mg vs. 100 ± 10 ngAO/min/mg in the CT group), but state 4 rates remained unchanged (Figs. 2, 3). State 3 respiration was significantly recovered in the I/R + CUR group when using malate-glutamate (Fig. 2), but not when mitochondria oxidized succinate (Fig. 3). RC was calculated to determine coupling between mitochondrial respiration and oxidative phosphorylation. In the I/R group, the ability to synthesize ATP was clearly compromised (RC = 1), whereas RC values of I/R + CUR group were similar to those obtained in the CT group (Figs. 2 and 3). To evaluate the mitochondrial electron transfer rate and the integrity of respiratory complexes, respiration in the presence of the uncoupler CCCP was measured. Uncoupled respiration decreased to 17% in control levels at the end of I/R injury (16 ± 3 vs. 93 ± 30 ngAO/min/mg), while uncoupled respiration in the I/R + CUR group increased to 46 ± 13 ngAO/min/mg (50% of the control) with complex I-linked substrates (Fig. 2). When using succinate as substrate (Fig. 3), curcumin treatment did not improve maximal electron transfer in the I/R + CUR group (60 ± 13 ngAO/min/mg) as compared with mitochondria from reperfused hearts (68 ± 14 ngAO/min/mg).
Effect of curcumin pretreatment on I/R-induced alterations in mitochondrial oxygen consumption using malate (10 mM)/glutamate (10 mM) as substrate. a State 4 and state 3 respiration; b Respiratory control; c Uncoupled respiration; d Adenosine diphosphate/oxygen (ADP/O) ratio. State 3 was stimulated by adding 200 μM ADP. Uncoupled respiration was measured by adding 1 μM CCCP. Curcumin (200 mg/kg, body weight) was given daily via oral gavage for 7 days before the Langendorff perfusion experiments. Hearts of CT, I/R, I/R + CUR, and CUR groups were perfused for 20 min. The hearts from I/R and I/R + CUR groups were subjected to global ischemia for 30 min and then to reperfusion for additional 60 min. Hearts from CT and CUR groups were continuously perfused as long as I/R hearts. CT mitochondria isolated from control rat hearts, I/R mitochondria isolated from rat hearts subjected to ischemia and reperfusion; I/R + CUR heart mitochondria from curcumin pretreated rats. ngAO/min/mg nanograms atoms of oxygen/minute/milligram of protein. Data are mean ± SEM, n = 4–6. # P < 0.05 versus State 4, * P < 0.05 versus CT, ** P < 0.05 versus I/R
Effect of curcumin (CUR) pretreatment on I/R-induced alterations in mitochondrial oxygen consumption using succinate (10 mM) as substrate. a State 4 respiration; state 3 respiration (b); Respiratory control; c Uncoupled respiration; d adenosine diphosphate/oxygen (ADP/O) ratio. State 3 was stimulated by adding 200 μM ADP. Uncoupled respiration was measured by adding 1 μM CCCP. Curcumin (200 mg/kg, body weight) was given daily via oral gavage for 7 days before the Langendorff perfusion experiments. Hearts of CT, I/R, I/R + CUR, and CUR groups were perfused for 20 min. The hearts from I/R and I/R + CUR groups were subjected to global ischemia for 30 min and then to reperfusion for additional 60 min. Hearts from CT and CUR groups were continuously perfused as long as I/R hearts. CT mitochondria isolated from control rat hearts, I/R mitochondria isolated from rat hearts subjected to ischemia and reperfusion; I/R + CUR heart mitochondria from curcumin pretreated rats. ngAO/min/mg nanograms atoms of oxygen/minute/milligram of protein. Data are mean ± SEM, n = 4–6. # P < 0.05 versus State 4, * P < 0.05 versus CT, ** P < 0.05 versus I/R
Mitochondria obtained from I/R + CUR group maintained similar ADP/O values than those observed in the CT group (Figs. 2, 3).
Discussion
In the present study, we show that curcumin pretreatment (200 mg/kg by 7 days) significantly reduces I/R-induced mechanical injury in isolated rat hearts. The mechanical work in the I/R + CUR groups was indistinguishable from that obtained in the CT and CUR groups (see Fig. 1). The protective effect of curcumin was associated with attenuation of mitochondrial lipid peroxidation and alterations in oxygen consumption (Decrease in state 3 respiration, RC, uncoupled respiration, and ADP/O using malate/glutamate or succinate as substrates). Interestingly, state 3 respiration was significantly recovered in the I/R + CUR group when using only malate/glutamate as substrate. Because state 3 respiration linked to complex II was not restored by curcumin, we concluded that complex III is more sensitive to I/R oxidative damage than complex I, although we did not discard that a diminution in the availability of ubiquinone, the carrier of electrons between complex I or II and complex III, could account for this result. In addition, curcumin pretreatment was able to prevent the decrease in GSH content and in the activity of SOD and GR in both heart and mitochondrial homogenates suggesting that the preservation of antioxidant enzymes and GSH content may contribute to the attenuation of I/R-induced oxidant stress. Cardiac damage induced by reperfusion interrupts biochemical and physiological processes in the heart that compromises ATP production. The increase in ROS during reperfusion has been pointed out as a main factor in such injury [34]. Electronic paramagnetic resonance studies have demonstrated a rapid increase in ROS production after reperfusion of ischemic myocardium. Besides, it has been demonstrated that O ·−2 is the predominant specie produced and that endothelial cells represent an important source of ROS [35]. On the other hand, the inhibition of xanthine oxidase by allopurinol and tungsten is capable of preventing O ·−2 generation, favoring cardioprotection in guinea pig hearts [36]. Among the main targets of ROS attack are polyunsaturated fatty acids of the lipid membrane that causes lipid peroxidation and thereby alteration in structure and in cellular function. MDA and 4-HNE are products of decomposition of the chain reaction, which leads to oxidation of polyunsaturated fatty acids; therefore, both are reliable markers of oxidative stress produced by I/R. GSH has a critical role in cellular defense against agents that cause oxidative stress, thus low levels of GSH implies cellular alteration produced by oxidative stress. Under this situation, stable products like carbonyl groups are produced by ROS action on proteins, also making protein carbonylation a reliable marker of oxidative stress.
In normal conditions, the large amount of ATP necessary to maintain contractile function and basal metabolism in heart is generated primarily by the mitochondrial oxidative metabolism. During ischemia, the ability of mitochondria to sustain ATP synthesis is highly compromised; in such condition, cardiac cells maintain ATP levels by activating the glycolytic pathway until oxidative metabolism is restored during reperfusion [37]. However, if ischemia is prolonged, the cells accumulate glycolytic by-products, like lactate and H+, which causes cardiac cell irreversible damage [38]. Paradoxically, reintroduction of oxygen during reperfusion enhances damage to ischemic cells, typically by membrane rupture, followed by cell death. ROS overproduction during reperfusion has been pointed out as a major contributor to irreversible damage of mitochondrial function and impaired recovery of physiological function in heart [39].
Curcumin is the active ingredient of turmeric and is a polyphenolic compound that is known for its potent antioxidant capacity [3, 4]. It has been shown that curcumin exerts a protective effect in several models of oxidant damage [14–16] including isoproterenol-induced cardiac injury [17] and I/R injury in rabbits [40]. Our data are consistent with these previous findings pointing to the attenuation of oxidant stress, which is a key finding in the protective effect of curcumin. In addition, our data suggest that attenuation by curcumin of mitochondrial dysfunction including oxidant stress, decrease in antioxidant enzymes, and alterations in oxygen consumption is also as a major player in the protective effect against cardiac I/R injury.
Our data suggest that the ROS scavenging ability of curcumin [8–10] is involved in the cardioprotective effect observed in our model. However, we can not discard that protective effects observed may also be mediated by induction of cytoprotective proteins [12, 13]. In fact, GST activity was induced in both heart and mitochondrial homogenates in the CUR group.
We can conclude that the attenuation of oxidant stress and mitochondrial dysfunction play a key role in the protective effect of curcumin against heart I/R injury.
References
Aggarwal, B. B., Sundaram, C., Malani, N., & Ichikawa, H. (2007). Curcumin: the Indian solid gold. Advances in Experimental Medicine and Biology, 595, 1–75.
Krishnaswamy, K. (2008). Traditional Indian spices and their health significance. Asia Pacific Journal of Clinical Nutrition, 17, 265–268.
Sharma, O. P. (1976). Antioxidant activity of curcumin and related compounds. Biochemical Pharmacology, 25, 1811–1812.
Toda, S., Miyase, T., Arichi, H., Tanizawa, H., & Takino, Y. (1985). Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chemical and Pharmaceutical Bulletin (Tokyo), 33, 1725–1728.
Satoskar, R. R., Shah, S. J., & Shenoy, S. G. (1986). Evaluation of anti-inflammatory property of curcumin (diferuloylmethane) in patients with postoperative inflammation. International Journal of Clinical Pharmacology, Therapy and Toxicology, 24, 651–654.
Negi, P. S., Jayaprakasha, G. K., Jagan Mohan Rao, L., & Sakariah, K. K. (1999). Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. Journal of Agriculture and Food Chemistry, 47, 4297–4300.
Kuttan, R., Bhanumathy, P., Nirmala, K., & George, M. C. (1985). Potential anticancer activity of turmeric (Curcuma longa). Cancer Letters, 129, 197–202.
Reddy, A. C., & Lokesh, B. R. (1994). Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Molecular and Cellular Biochemistry, 137, 1–8.
Unnikrishnan, M. K., & Rao, M. N. (1995). Curcumin inhibits nitrogen dioxide induced oxidation of hemoglobin. Molecular and Cellular Biochemistry, 146, 35–37.
Sreejayan, N., & Rao, M. N. (1997). Nitric oxide scavenging by curcuminoids. Journal of Pharmacy and Pharmacology, 49, 105–107.
Prestera, T., & Talalay, P. (1995). Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proceedings of the National Academy of Sciences of the United States of America, 92, 8965–8969.
Susan, M., & Rao, M. N. (1992). Induction of glutathione S-transferase activity by curcumin in mice. Arzneimittelforschung, 42, 962–964.
Dinkova-Kostova, A. T., & Talalay, P. (1999). Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis, 20, 911–914.
Mythri, R. B., Jagatha, B., Pradhan, N., Andersen, J., & Bharath, M. M. (2007). Mitochondrial complex I inhibition in Parkinson’s disease: How can curcumin protect mitochondria? Antioxidants & Redox Signaling, 3, 399–408.
Wei, Q., Chen, W., Zhou, B., Yang, L., & Liu, Z. (2006). Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochimica et Biophysica Acta, 1760, 70–77.
Rastogi, M., Rudra Ojha, P., Rajamanickam, G. V., Agrawal, A., Aggarwal, A., & Dubey, G. P. (2008). Curcuminoids modulates oxidative damage and mitochondrial dysfunction in diabetic rat brain. Free Radical Research, 42, 999–1005.
Nazam, A. M., Thakare, V. N., Bhandari, U., & Pillai, K. K. (2007). Protective role of curcumin in myocardial oxidative damage induced by isoproterenol in rats. Human and Experimental Toxicology, 26, 933–938.
Naik, S. R., & Patil, S. R. (2011). Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: Evidence of its antioxidant property. Experimental and Toxicologic Pathology, 63, 419–431.
Chen, Q., Camara, A. K., Stowe, D. F., Hoppel, C. L., & Lesnefsky, E. J. (2007). Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. American Journal of Physiology. Cell Physiology, 292, C137–C147.
Di Lisa, F., Canton, M., Menabo, R., Kaludercic, N., & Bernardi, P. (2007). Mitochondria and cardioprotection. Heart Failure Reviews, 12, 249–260.
Lesnefsky, E. J., Slabe, T. J., Stoll, M. S., Minkler, P. E., & Hoppel, C. L. (2001). Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. American Journal of Physiology. Heart and Circulatory Physiology, 280, H2770–H2778.
Chen, Q., Vazquez, E. J., Moghaddas, S., Hoppel, C. L., & Lesnefsky, E. J. (2003). Production of reactive oxygen species by mitochondria: central role of complex III. Journal of Biological Chemistry, 278, 36027–36031.
Chambers, D. E., Parks, D. A., Patterson, G., Roy, R., McCord, J. M., Yoshida, S., et al. (1985). Xanthine oxidase as a source of free radical damage in myocardial ischemia. Journal of Molecular and Cellular Cardiology, 17, 145–152.
Misra, M. K., Sarwat, M., Bhakuni, P., Tuteja, R., & Tuteja, N. (2009). Oxidative stress and ischemic myocardial syndromes. Medical Science Monitor, 15, 209–219.
Correa, F., Garcia, N., Robles, C., Martinez-Abundis, E., & Zazueta, C. (2008). Relationship between oxidative stress and mitochondrial function in the post-conditioned heart. Journal of Bioenergetics and Biomembranes, 40, 599–606.
Correa, F., Soto, V., & Zazueta, C. (2007). Mitochondrial permeability transition relevance for apoptotic triggering in the post-ischemic heart. International Journal of Biochemistry and Cell Biology, 39, 787–798.
Maldonado, P. D., Barrera, D., Medina-Campos, O. N., Hernández-Pando, R., Ibarra-Rubio, M. E., & Pedraza-Chaverrí, J. (2003). Aged garlic extract attenuates gentamicin induced renal damage and oxidative stress in rats. Life Sciences, 73, 2543–2556.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Fernández-Checa, J. C., & Kaplowitz, N. (1990). The use of monochlorobimane to determine hepatic GSH levels and synthesis. Analytical Biochemistry, 190, 212–219.
Guerrero-Beltrán, C. E., Calderón-Oliver, M., Tapia, E., Medina-Campos, O. N., Sánchez-González, D. J., Martínez-Martínez, C. M., et al. (2010). Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicology Letters, 192, 278–285.
Barrera, D., Maldonado, P. D., Medina-Campos, O. N., Hernández-Pando, R., Ibarra-Rubio, M. E., & Pedraza-Chaverri, J. (2003). HO-1 induction attenuates renal damage and oxidative stress induced by K2Cr2O7. Free Radical Biology and Medicine, 34, 1390–1398.
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Pedraza-Chaverri, J., Yam-Canul, P., Chirino, Y. I., Sánchez-González, D. J., Martínez-Martínez, C. M., Cruz, C., et al. (2008). Protective effects of garlic powder against potassium dichromate-induced oxidative stress and nephrotoxicity. Food and Chemical Toxicology, 46, 619–627.
Chávez, E., Briones, R., Michel, B., Bravo, C., & Jay, D. (1985). Evidence for the involvement of dithiol groups in mitochondrial calcium transport: Studies with cadmium. Archives of Biochemistry and Biophysics, 242, 293–397.
Zweier, J. L., Flaherty, J. T., & Weisfeldt, M. L. (1987). Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proceedings of the National Academy of Science, 84, 1404–1407.
Duda, M., Konior, A., Klemenska, E., & Beręsewicz, A. (2007). Preconditioning protects endothelium by preventing ET-1-induced activation of NADPH oxidase and xanthine oxidase in post-ischemic heart. Journal of Molecular and Cellular Cardiology, 42, 400–410.
Cross, H., Opie, L., Radda, G., & Clarke, K. (1996). Is high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circulation Research, 78, 482–491.
Dennis, S., Gevers, W., & Opie, L. (1991). Protons in ischemia: where do they come from: Where do they go? Journal of Molecular and Cellular Cardiology, 23, 1077–1086.
Ambrosio, G., Zweier, J., Duilio, C., Kuppusamy, P., Sanoro, G., Elia, P., et al. (1993). Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. Journal of Biological Chemisty, 268, 18532–18541.
Yeh, C. H., Wu, Y. C., & Jing, L. P. (2005). Inhibition of NFkappaB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. Journal of Surgical Research, 125, 109–116.
Acknowledgments
This work was supported by PAPIIT IN201910 and CONACYT 129838 (to JP) and CONACYT 80791 (to CZ).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Alfredo González-Salazar and Eduardo Molina-Jijón contributed equally to this work.
Rights and permissions
About this article
Cite this article
González-Salazar, A., Molina-Jijón, E., Correa, F. et al. Curcumin Protects from Cardiac Reperfusion Damage by Attenuation of Oxidant Stress and Mitochondrial Dysfunction. Cardiovasc Toxicol 11, 357–364 (2011). https://doi.org/10.1007/s12012-011-9128-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9128-9