Abstract
In reality, people are often co-exposed to multiple heavy metals; however, current research has focused on the association between individual heavy metals and inflammation. Therefore, it is more relevant to explore the combined effects of multiple heavy metal exposure on inflammation. The study included data from the National Health and Nutrition Examination Survey (NHANES), 2011–2016. The systemic immune-inflammation index (SII) was used to reflect systemic immune-inflammation status. In this study, single variable models were used to assess the linear and non-linear relationships between single heavy metal exposures and SII. To analyze the combined effect of mixed heavy metals exposure on SII, we constructed three statistical models, including weighted quantile sum (WQS) regression, quantile-based g computation (qgcomp), and Bayesian kernel machine regression (BKMR). The single-exposure analysis found positive associations between multiple heavy metals and SII, while mercury in blood was negatively associated with SII, and U-shaped correlations were observed between blood lead, urine barium and strontium, and SII. In the WQS model, SII increased significantly with increasing concentrations of mixed heavy metals, while consistent results in the qgcomp model, but not statistically significant. In the BKMR model, exposure to heavy metal mixtures was positively associated with SII, with mercury, cadmium, and cobalt in urine contributing the most to the mixed exposure. In addition, synergistic and antagonistic effects between heavy metals on increasing SII were found in our study. In summary, our results reveal that combined exposure to multiple heavy metals is positively associated with SII in the US adults.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammation is a protective response to stimulation by adverse environmental factors, such as infection, tissue stress, and injury [1]. There is an increasing prevalence of chronic diseases, which usually involve a disruption of the body’s homeostasis and are almost universally associated with chronic inflammation [2]. Prolonged chronic immune inflammatory abnormalities are also a common causal factor in many modern diseases, including not only common diseases such as arthritis, obesity, and diabetes, but also major diseases such as cancer, coronary heart disease, and Alzheimer’s disease [3, 4]. Therefore, the immune inflammatory system is closely related to various health outcomes.
Hu et al. created a new composite index, the systemic immune-inflammation index (SII), calculated as (neutrophil × platelet)/lymphocyte count, which integrates three hematologic markers of inflammation, has been proven to be a predictor of prognosis in various cancers and cardiovascular diseases, and is considered to provide a comprehensive reflection of the balance of inflammatory and immune status of the host [5,6,7]. A higher SII value indicates a more active immune system and inflammatory state, while a lower SII indicates a relatively weak immune system and inflammatory state. Recently emerging research has found that SII is associated with a variety of autoimmune diseases, such as rheumatoid arthritis, ankylosing spondylitis, and Behçet’s disease, and that SII can be applied as a new predictive indicator of disease activity in such diseases [8,9,10].
Metals are defined as heavy metals (heavy metals) based on densities greater than 5 g/cm3 [11]. Heavy metals are a common group of persistent and harmful endocrine disruptors in daily life [12] and are widely exposed in everyday life, including metal-based industries, the use of pesticides, herbicides, and fertilizers in agriculture, and the contamination of drinking water with heavy metals [11]. Heavy metals are known to be non-biodegradable; they are mainly excreted through urine, and when exposed to excessive amounts of heavy metals, they bioaccumulate in the organism [13]. Heavy metals have been found to have different effects on the nervous, respiratory, reproductive, hematopoietic, hepatic, and renal systems [14], with altered immune inflammatory states being an important and fundamental type of damage. Moreover, systemic inflammation may be a vital intermediate link in the process of heavy metals causing various diseases, as has been demonstrated in several studies [15, 16].
Currently, a large number of studies have shown a correlation between heavy metal exposure and inflammation levels. A study in China found that exposure to Cd and Pb promoted inflammation throughout the body [17]. A Korean study showed that subjects in the highest quartile of blood mercury had an increased risk of increased high sensitivity C-reactive protein levels compared to subjects in the lowest quartile of blood mercury [18]. However, Balkrishna et al. described the use of arsenic or mercury preparations for the treatment of various diseases and found that an herb-metal medicine consisting of arsenic, mercury, and its oxides could act as an effective anti-asthmatic and anti-inflammatory agent by modulating various pathways [19]. The effect of mercury on inflammation is not yet clear. Furthermore, in one study, exposure to cobalt-based pigments, even at non-toxic doses, induced dysfunction in macrophages that lasted for a long time [20]. Harmon et al. found that the weighted proximity of residence to abandoned uranium mines was associated with the circulating inflammatory potential in residents [21]. Exposure to fine particulate matter PM2.5 bound to metals (such as vanadium, arsenic, selenium, cadmium, and lead) causes oxidative stress and systemic inflammatory responses [22]. Taken together, these studies suggest a complex association between heavy metals on immune inflammation; however, current studies have focused on the association between individual heavy metals and inflammation. In reality, people are often simultaneously exposed to multiple low concentrations of heavy metals through contaminated air, food, water, or soil, and these metals may exhibit additive, synergistic, or antagonistic effects in the organism [23,24,25]. Therefore, exploring the combined impact of mixed exposure to multiple heavy metals on inflammatory indicators of SII has more practical significance and is worth being explored.
The association between heavy metals and SII was investigated in this study using the National Health and Nutrition Examination Survey (NHANES) database. Specifically, the linear and non-linear associations between individual metals and SII were investigated with a generalized linear model (GLM) and restricted cubic spline regression method (RCS), and mixed models including weighted quantile sum (WQS) regression, quantile-based g computation (qgcomp), and Bayesian kernel machine regression (BKMR) were used to explore the joint associations between mixed heavy metal exposures and SII and the unknown interaction patterns within it, aiming to provide new insights into the complex associations between heavy metals and inflammation.
Methods
Study Design and Population
The NHANES is a long-term, national cross-sectional survey focused on the health and nutrition of Americans. The survey was approved by the National Centre for Health Statistics (NCHS) Ethics Review Board. Data from three cycles of NHANES from 2011 to 2016 were included in this research. A total of 8648 participants included both blood and urinary heavy metals and complete blood count data. Next, participants with missing heavy metals, urine creatinine, and complete blood count data were excluded. Pregnancy and people younger than 20 years of age were also excluded, as was the lack of covariate data. A final total of 4463 participants were entered into our study, and detailed data are presented in Fig. 1.
Assessment of Blood and Urinary Heavy Metals
The exposure factors in this study were 5 heavy metals in blood including lead, cadmium, mercury, selenium, and manganese (Pb, Cd, Hg, Se, Mn), and 13 heavy metals in urine including barium, cadmium, cobalt, cesium, molybdenum, manganese, lead, antimony, tin, strontium, thallium, tungsten, and uranium (Ba, Cd, Co, Cs, Mo, Mn, Pb, Sb, Sn, Sr, Tl, Tu, Ur). Inductively coupled plasma mass spectrometry (ICP-MS) was used to detect the concentrations of 18 heavy metals. In the case of results below the detection limit (LOD), the value of the heavy metal variables was accounted for as the LOD divided by the square root of two. Urinary Mn was excluded from this study as more than half of the final 4463 participants had urinary Mn detection values below the LOD. In summary, 17 heavy metals were entered into our study. Details of the detection rates for each heavy metal are given in Table S1. All urinary heavy metals are standardized by dividing by the gram weight of creatinine per liter of urine, thus eliminating the effect of the concentration of urine [26].
Assessment of SII
SII is the outcome indicator of interest in this study. The formula for calculating SII is the platelet count multiplied by the neutrophil count divided by the lymphocyte count and expressed as × 10®3 cells/µL. The complete blood count was performed on a quantitative, automated hematology analyzer (Coulter® DxH 800 analyzer) using the participants’ EDTA blood tubes at the NHANES Mobile Examination Centre (MEC). All quality control procedures recommended by the manufacturer were followed throughout. The counting method was based on the Coulter Principle. The results were measured in duplicate and averaged.
Covariates
Based on previous studies, several foundational biological characteristics including age, gender, and race, and sociodemographic characteristics including education, marriage, and household economic level were screened as covariates [27]. Other covariates associated with inflammation including body mass index (BMI, m/kg2), smoking, hypertension, and diabetes status were also included [28,29,30]. Cotinine, the main metabolite of nicotine in the body, can be considered a biomarker of tobacco exposure in both active and passive smoking [31]. Smoking status is classified as high exposure (≥ 0.052 ng/mL) and low exposure (< 0.052 ng/mL) according to serum cotinine level [32]. Household economic status is expressed in terms of the household poverty-income ratio (PIR). The household economic situation is divided into three levels according to the level of PIR (< 1.30, 1.30–2.99, and ≥ 3.00) [33]. As shown in Table 1, age and BMI are continuous variables, and the rest are categorical.
Statistical Analysis
In the original description, continuous variables are expressed as medians (interquartile range), and categorical variables are expressed as frequencies (percentages). In this study, we first described the associations between covariates and exposure variables with SII. Since both SII and the heavy metal concentrations were right-skewed distributions, we applied the logarithmic transformation (ln-transformed) to approximate the normal distribution for subsequent analysis. Spearman correlation coefficients (r) between the ln-transformed heavy metals were also calculated in this study.
Our study consists of three parts: firstly, to explore the linear association between single heavy metal exposure variables and SII, secondly, to analyze the non-linear association between single heavy metal exposure variables and SII, and thirdly, to analyze the joint effect of the mixture of heavy metals on SII.
In this study, the GLM model was constructed to evaluate the linear association between each heavy metal variable and SII. Based on the included covariates, we applied three GLM models. Model 1 was not corrected for any covariates, model 2 was corrected only for foundational biological characteristics (age, gender, and race), and model 3 added covariates of education, marriage, PIR, BMI, cotinine, hypertension, and diabetes on the basis of model 2. In addition, we performed gender-stratified analyses. The estimates and 95% confidence intervals (β (95%CI)) are provided to express the effect of heavy metals on SII. In this study, the RCS method with 3 knots was utilized to fit a smooth curve to assess the non-linear association between single heavy metal exposure and SII. The three knots of this method were the 10th, 50th, and 90th percentiles of the heavy metal variables.
To evaluate the combined effects of mixed heavy metal exposure on SII, three statistical models were constructed, including WQS regression, qgcomp, and BKMR models. Simultaneously, we applied blood, urine, and total mixed heavy metal exposure to the three models. Moreover, we also performed gender-stratified analyses.
The WQS regression model is able to estimate the effect of mixture exposure on the outcome and assess the degree of importance of each exposure variable on the outcome by assigning weights to each exposure variable [34]. As the direction of the association between heavy metals mixtures and SII is not clear, both positive and negative models were constructed. The WQS regression model has an obvious drawback, which is that it assumes that the correlation between exposure and outcome is linear, additive, and in the same direction. Therefore, we developed the qgcomp model, which is based on the WQS model with a flexible combination of g-computation and is not limited by the homogeneity of direction.
Finally, we built the BKMR model. The model allows for non-linear effects between exposures and outcomes and the existence of interactions between exposures, which is its advantage over the two models above. The exposure variables were standardized before the BKMR model was developed. This study investigates the combined effects of mixed heavy metal exposure on SII by fixing heavy metal mixtures at different percentile levels compared to the median level. To obtain the relative importance of each heavy metal variable on SII, we present posterior inclusion probabilities (PIPs) from the BKMR. The BKMR model includes the ability to visualize different cross-sections of the exposure–response surface to observe the non-linear association between univariate and SII. Binary exposure–response functions were applied to further explore the interactions between heavy metal variables that have an effect on SII in this BKMR model. In this research, the model was run for 10,000 iterations using the Markov Chain Monte Carlo method.
All statistical analysis was performed using R software (version 4.2.2, www.r-project.org) with R packages “stats,” “rms,” “gWQS,” “qgcomp,” and “bkmr.” P values less than 0.05 were considered statistically significant (two-tailed).
Results
Basic Characteristics of Participants
As shown in Table 1, a total of 4463 participants were included in our study with a median SII level of 445.0 × 1000 cells/µL, 2244 (50.3%) for males and 2219 (49.7%) for females, with a median age of approximately 48 years. The covariates associated with SII were gender, age, race, marriage, BMI, hypertension, and diabetes status. Cd, Hg, and Mn in blood and Ba, Cd, Co, Cs, Sb, Sn, Sr, Tu, and Ur in urine were significantly associated with SII. The Spearman correlation matrix plot between the heavy metals suggested that the correlation coefficients between the metal variables were all below 0.8 (coefficients ranged from –0.07 to 0.78), there were no strongly correlated metals, and most of the metals were positively correlated with each other (Fig. S1).
The Single Effect of Heavy Metal Exposure on SII
Of the three GLM models, there was a high degree of confidence that individual metals were significantly correlated with SII when all three models were significant. When unstratified for gender, all three GLM model results showed positive correlations between blood Cd and urine Cd, Co, Tu, and Ur, and SII and negative correlations with blood Hg (Table 2). After stratification by gender, the GLM model for males showed that blood Pb and Cd and urine Cd and Tu were positively correlated with SII and negatively correlated with blood Hg (Table S2), while the GLM model for females showed that urine Co was positively correlated with SII and negatively correlated with blood Hg and Pb (Table S3). From this, we can find that blood Pb showed a very different behavior by gender, with a positive correlation in males and a negative correlation in females, and therefore did not show an association with SII in the total population. Blood Cd and urine Cd and Tu were similar, with a significant association with SII only in males, but not in females.
The curves fitted by the RCS model revealed a non-linear correlation between blood Pb and urine Ba and Sr and SII (all P-nonlinear < 0.05, Fig. S2), and all were U-shaped correlations. The inverse-ln transformation of the lowest point of the curve showed that the lowest SII levels were found at blood Pb and urine Ba and Sr concentrations of 1.03 µg/dL, 1.16 µg/g creatinine, and 73.60 µg/g creatinine, respectively.
The Combined Effect of the Mixture of Heavy Metal Exposure on SII
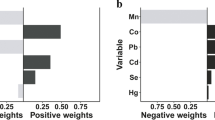
Total metal co-exposure significantly increased SII in the positive WQS model (0.087 (0.042, 0.132), Fig. 2A), with urinary Cd and Sn contributing the highest weighted index (Fig. S3). Mixed urinary metal exposure had a significant effect on SII (0.081 (0.042, 0.120), Fig. 2A), with urinary Cd and Sn being the most important (Fig. S3). In the gender stratification, the metals found to contribute more in males were blood Mn and urine Cd, and in females, the more important metals were urine Sb and Tu (Fig. S3). No statistically significant effects on SII were found in the WQS model for blood (0.021 (− 0.014, 0.056)) and its gender stratification (males: − 0.016 (− 0.053, 0.021), females: 0.020 (− 0.025, 0.065)). In the negative WQS model, there was a statistically significant association for SII in the model for total as well as for blood, and this association was more pronounced in females (Fig. S5). Based on the weighting plots (Fig. S6), we were able to identify blood Hg as the main driver of the negative model. In the qgcomp model, results showed a significant positive correlation between metals in urine and its gender stratification (Fig. 2B), while in total metals, results showed a positive trend but not statistically significant (0.040 (− 0.001, 0.081), with blood Hg and urine Mo and Cd occupying the top three positions (Fig. S4).
A Associations of total, blood, and urinary heavy metal exposure with SII levels and their gender-stratified subgroups by the positive WQS model. B Associations of total, blood, and urinary heavy metals exposure with SII levels and their gender-stratified subgroups by the qgcomp model. These models were corrected for age, gender, race, education, marriage, PIR, BMI, cotinine, hypertension, and diabetes
In the BKMR model, co-exposure to mixed metals in total or urine significantly increased the level of SII, no such effect was found in blood (Fig. 3). The effect of mixed metal exposure on SII was unchanged in gender stratification compared with non-stratification (Fig. 3). We further analyzed the effects of the individual metal variable on SII. Trends in the association between individual metal variables and SII were observed through univariate exposure–response function curves, and the relative importance of each metal variable was reflected through PIPs. In the model for total metals, blood Hg and urinary Cd and Co were the most important (Table S4), with blood Hg significantly decreased SII levels and urine Cd and Co significantly increased SII levels (Fig. 4A). In the effect of single metal exposure stratified by gender, it can be seen that blood Hg is consistently significant in both males and females (Fig. 4B, C); however, there are some differences in that urinary Sr and Cd are more influential in males and urinary Co and Ur are more pronounced in females (Table S4).
Univariate exposure–response function curves for the association of single heavy metal exposure with SII levels and their gender-stratified subgroups by the BKMR model. The models were corrected for age, gender, race, education, marriage, PIR, BMI, cotinine, hypertension, and diabetes. h(exposure) represents the value of the dose effect on SII produced by each exposure
Finally, the interaction between metals was analyzed by the bivariate exposure–response function from the BKMR model. In the total model, there was some interaction between blood Hg and urinary Sr, Co, and Cd (Fig. S7A), with stronger associations of urinary Co and Sr with SII, and weaker associations of urinary Cd with SII observed as blood Hg concentrations increased, suggesting synergistic effects of blood Hg with urinary Co and Sr and antagonistic effects with urinary Cd. At lower urinary Cd levels (from the 10th to the 50th percentile), there was an antagonistic effect between urinary Co and Cd (Fig. S7A). In the blood model, there was an interaction between blood Hg and Cd, with a stronger negative effect of Hg on SII when Cd increased and a weaker positive effect of Cd on SII when Hg increased (Fig. S7B), suggesting an antagonistic effect between the two metals, which is consistent with the results found in the total model. In the urinary model, the effect of Tu on SII was weaker as Co increased (Fig. S7C), suggesting an antagonistic effect between the metals. At lower levels of Cd, there was an antagonistic effect between Co and Cd (Fig. S7C), similarly, which is consistent with the results found in the total model.
Discussions
This cross-sectional study is the first to comprehensively analyze the association between blood and urine metal concentrations and SII. In the analysis of individual metals and SII, Cd in blood and Cd, Co, Tu, and Ur in urine were found to be positively correlated with SII, while Hg in blood was negatively correlated with SII. Interestingly, there was a U-shaped correlation between blood Pb and urine Ba and Sr and SII. In the WQS and BKMR model, results suggest that SII increases significantly with increasing concentrations of mixed metals, with blood Hg and urinary Cd and Co contributing the most in mixed exposure metals. In the qgcomp model, total metals showed a positive but not statistically significant association with SII, possibly due to the inability of this model to identify metal interactions. In the BKMR model, Hg remained the main driver of the negative correlation with SII, while among the metals positively correlated with SII, Cd and Sr were more important in males, and Co and Ur were more important in females. Taken together, the results show that co-exposure to multiple heavy metals is associated with SII levels.
Although the mechanisms of action of different metals on inflammation are often varied, there are similar ways of acting. There are several potential mechanisms to explain the results of this study. Heavy metal ions are covalently bound to the sulfhydryl group (-SH) of proteins, which is the active group of many important enzymes in cellular metabolism, and, when bound to the sulfhydryl group of some endogenous antioxidant enzymes such as superoxide dismutase and glutathione oxidase, can interfere with their activity or even inactivate them, thus affecting inflammation levels [35,36,37]. Another classical pathway is that when metal ions accumulate in the body, they can produce large amounts of free radicals that disrupt the antioxidant system causing oxidative stress damage and leading to altered levels of inflammation [38]. An increasing number of studies have found that heavy metals affect the metabolic health of the host by altering the composition and function of the intestinal flora [39, 40]. When there is an imbalance in the intestinal flora, systemic chronic inflammation often occurs along with it [41].
In this study, multiple heavy metals were associated with SII, both in the single metal and mixed metal analyses. In a study by Liu et al., heavy metal Hg was found to be negatively correlated with four immunoinflammatory biomarkers, and cadmium was positively correlated with four immunoinflammatory biomarkers [42], which is consistent with the findings of this study and adds stability to the conclusions of this study. In the present study, a significant positive association was found between Cd and SII, and this association was more pronounced in males. The heavy metal Cd is known to be an immunotoxin inhibitor, which can induce apoptosis or oxidative stress by competitively displacing essential metals in proteases, leading to the production of large amounts of inflammatory factors [43]. In addition, a study has also found that metallic cadmium accumulation leads to electron leakage and the production of reactive oxygen species in mitochondria, resulting in altered levels of inflammation [44]. This is consistent with our findings. The effect of the heavy metal Hg on the immune-inflammatory state of the organism remains controversial, with Hg found to promote an inflammatory response in most previous studies [45, 46], but mercuric chloride also indicated to weaken the macrophage pro-inflammatory response [47, 48]. However, in this study, metal Hg showed a remarkable negative correlation in both single variable analysis and mixed model analysis. It has been reported that low doses of Hg may be beneficial against inflammation [49], and a study has shown that methylmercury has an immunosuppressive effect in animal models [50]. Therefore, it is speculated that the change in concentration as well as the chemical form of Hg may have different effects on the immune inflammatory system and deserve further exploration. Besides Cd and Hg, the effect of other heavy metals on SII cannot be overlooked. In a study among the elderly population, tumor necrosis factor-alpha levels were found to elevate with rising serum Co concentration [51], and also, Harmon et al. found that the heavy metal Ur was able to promote circulating inflammation [21], both of which are consistent with our findings. Furthermore, Scammell et al. observed that upper tertile urinary Ba, Cs, Pb, Sr, and Tu were associated with autoimmune biomarker (antinuclear antibodies) positivity at 1:160 dilution [52], and similarly, Cs and Tu were generally consistent with our result. However, in our study, there was a U-shaped correlation between urinary Ba, Sr, and SII, and this difference in results was found to be mainly due to the fact that urinary metal concentrations in the Scammell investigation were generally higher than those in our study, with mean urinary Ba and Sr concentrations in the Scammell study being 2.03 and 151.29 µg/g creatinine, compared to 1.01 and 94.90 µg/g creatinine in our study, respectively.
In the present study, a significant positive association was found between heavy metal mixtures and SII, while the effect of the heavy metals Cd and Sr on SII was more pronounced in males, and the effects of the heavy metals Co and Ur on SII were more pronounced in females. Among US adults, the prevalence of smoking is higher in males than in females [53], and Cd is the major heavy metal in tobacco [54], and the exposure rate of Cd is much higher in males than in females, which may be one of the reasons why Cd is more prominent in males. In addition, one study found that exposure patterns to metal mixtures were associated with sex hormone imbalance in children aged 12–19 years in a sexually dimorphic pattern [55] and that sex hormones mediate the balance of immune inflammation [41, 56], suggesting that differences in sex hormone levels in different genders may impact the association between metals and inflammation.
Potential interactions between heavy metals on increasing SII were identified in our study, with Hg being antagonistic with Cd, synergistic with Co and Sr, Co antagonistic with Tu, and at low concentrations of Cd, Co antagonistic with Cd. These heavy metals interact in the organism, possibly by competing for target protein sites causing metal-to-metal interactions [57], or some metals are able to regulate the damage caused by others in key pathways [58], or by gradually reaching a limit on the immune-inflammatory state into a stable plateau as more and more heavy metals are exposed and are not merely additive. The mechanism of action has not yet been elucidated in the literature, and future studies should explore the above-mentioned antagonistic and synergistic effects in depth.
Among the 17 heavy metals in this study, they have a cumulative effect on the immune-inflammatory state of the population through simple additive effects and potential interactions. Most metals are positively correlated with SII, a few are negatively correlated with SII, and some metals interact with each other, but the overall trend of heavy metals to SII is increasing. However, it is necessary to mention that part of the heavy metals is not associated with SII, probably due to the concentrations of these metals at safe doses in the US adult population, which does not represent a real lack of effect on inflammation.
This study has the following advantages. This study is the first to explore the association between a variety of heavy metals and SII and specifically analyze the association between each metal and SII as well as the interactions between metals, filling in the gaps. Secondly, three models were employed to analyze the association between mixed heavy metal exposure and SII, increasing the stability of the results. Finally, multiple confounding factors were corrected, and there was still a significant positive association between mixed heavy metal exposure and SII. However, there are some limitations in this research. First, this study is cross-sectional and cannot determine the causal association of mixed heavy metals exposure to SII. Second, NHANES applied a complex multi-stage probability sampling strategy, whereas the sample was not weighted in this study. However, the weighted analysis method may introduce excessive adjustment bias when the model control includes variables that calculate sampling weights [59]. In addition, sampling weights should not be considered when the purpose of this study is to explore the association between pollutant exposure and health outcomes. Third, this article only used a single indicator to reflect the systemic immune-inflammatory status, and repeated analysis should be combined with multiple indicators in future studies.
Conclusions
In conclusion, our results show that combined exposure to multiple heavy metals is positively associated with SII levels in the US adult population, with the heavy metals Hg, Cd, and Co being the main drivers of altered SII levels. In addition, synergistic and antagonistic effects between heavy metals were found to increase SII levels. Further studies are needed to confirm our findings, and deeper studies are needed to explore the mechanisms involved.
References
Medzhitov R (2021) The spectrum of inflammatory responses. Science (New York, NY) 374(6571):1070–1075
Kotas ME, Medzhitov R (2015) Homeostasis, inflammation, and disease susceptibility. Cell 160(5):816–827
Langworth-Green C, Patel S, Jaunmuktane Z, Jabbari E, Morris H, Thom M, Lees A, Hardy J, Zandi M, Duff K (2023) Chronic effects of inflammation on tauopathies. The Lancet Neurology 22(5):430–442
Xiao S, Wang Z, Zuo R, Zhou Y, Yang Y, Chen T, Liu N (2023) Association of systemic immune inflammation index with all-cause, cardiovascular disease, and cancer-related mortality in patients with cardiovascular disease: a cross-sectional study. J Inflamm Res 16:941–961
Meng L, Yang Y, Hu X, Zhang R, Li X (2023) Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J Transl Med 21(1):79
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J et al (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 20(23):6212–6222
Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J (2023) Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med 12(3):1128
Liu B, Wang J, Li YY, Li KP, Zhang Q (2023) The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999–2018. Arthritis Res Ther 25(1):34
Wu J, Yan L, Chai K (2021) Systemic immune-inflammation index is associated with disease activity in patients with ankylosing spondylitis. J Clin Lab Anal 35(9):e23964
Tanacan E, Dincer D, Erdogan FG, Gurler A (2021) A cutoff value for the systemic immune-inflammation index in determining activity of Behçet disease. Clin Exp Dermatol 46(2):286–291
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691
López-Botella A, Velasco I, Acién M, Sáez-Espinosa P, Todolí-Torró JL, Sánchez-Romero R, Gómez-Torres MJ (2021) Impact of heavy metals on human male fertility-an overview. Antioxidants 10(9):1473
Esteves-Aguilar J, Mussali-Galante P, Valencia-Cuevas L, García-Cigarrero AA, Rodríguez A, Castrejón-Godínez ML, Tovar-Sánchez E (2023) Ecotoxicological effects of heavy metal bioaccumulation in two trophic levels. Environ Sci Pollut Res Int 30(17):49840–49855
Filipoiu DC, Bungau SG, Endres L, Negru PA, Bungau AF, Pasca B, Radu AF, Tarce AG, Bogdan MA, Behl T et al (2022) Characterization of the toxicological impact of heavy metals on human health in conjunction with modern analytical methods. Toxics 10(12):716
Wang X, Bin W, Zhou M, Xiao L, Xu T, Yang S, Nie X, Xie L, Yu L, Mu G et al (2021) Systemic inflammation mediates the association of heavy metal exposures with liver injury: a study in general Chinese urban adults. J Hazard Mater 419:126497
Ma J, Zhou Y, Wang D, Guo Y, Wang B, Xu Y, Chen W (2020) Associations between essential metals exposure and metabolic syndrome (MetS): exploring the mediating role of systemic inflammation in a general Chinese population. Environ Int 140:105802
Xiong L, Fan C, Song J, Wan Y, Lin X, Su Z, Qiu J, Wu W, He Z, Wu Y et al (2022) Associations of long-term cadmium exposure with peripheral white blood cell subtype counts and indices in residents of cadmium-polluted areas. Chemosphere 308(Pt 1):135946
Kim K, Park H (2023) Association of mercury exposure with the serum high-sensitivity C-reactive protein level in Korean adults. Front Public Health 11:1062741
Balkrishna A, Solleti SK, Singh H, Singh R, Bhattacharya K, Varshney A (2022) Herbo-metallic ethnomedicine ‘Malla Sindoor’ ameliorates lung inflammation in murine model of allergic asthma by modulating cytokines status and oxidative stress. J Ethnopharmacol 292:115120
Devcic J, Dussol M, Collin-Faure V, Pérard J, Fenel D, Schoehn G, Carrière M, Rabilloud T, Dalzon B (2022) Immediate and sustained effects of cobalt and zinc-containing pigments on macrophages. Front Immunol 13:865239
Harmon ME, Lewis J, Miller C, Hoover J, Ali AS, Shuey C, Cajero M, Lucas S, Zychowski K, Pacheco B et al (2017) Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities. J Eposure Sci Environ Epidemiol 27(4):365–371
Zhang L, Fang B, Wang H, Zeng H, Wang N, Wang M, Wang X, Hao Y, Wang Q, Yang W (2023) The role of systemic inflammation and oxidative stress in the association of particulate air pollution metal content and early cardiovascular damage: a panel study in healthy college students. Environ Pollut 323:121345
Takatani T, Eguchi A, Yamamoto M, Sakurai K, Takatani R, Taniguchi Y, Nakayama SF, Mori C, Kamijima M (2022) Individual and mixed metal maternal blood concentrations in relation to birth size: an analysis of the Japan Environment and Children’s Study (JECS). Environ Int 165:107318
Cheng BJ, Sheng J, Wang HL, Wang Y, Cao HJ, Li XD, Zhou TT, Meng XL, Nie HH, Wang SF et al (2023) Selenium attenuates the association of co-exposure to arsenic, cadmium, and lead with cognitive function among Chinese community-dwelling older adults. Environ Sci Pollut Res Int 30(13):36377–36391
Banerjee S, Dhar S, Sudarshan M, Chakraborty A, Bhattacharjee S, Bhattacharjee P (2023) Investigating the synergistic role of heavy metals in arsenic-induced skin lesions in West Bengal, India. J Trace Elem Med Biol 75:127103
Everson TM, Niedzwiecki MM, Toth D, Tellez-Plaza M, Liu H, Barr DB, Gribble MO (2021) Metal biomarker mixtures and blood pressure in the United States: cross-sectional findings from the 1999–2006 National Health and Nutrition Examination Survey (NHANES). Environ Health 20(1):15
Li W, Li X, Su J, Chen H, Zhao P, Qian H, Gao X, Ye Q, Zhang G, Li X (2023) Associations of blood metals with liver function: analysis of NHANES from 2011 to 2018. Chemosphere 317:137854
Colsoul ML, Goderniaux N, Onorati S, Dupuis S, Jamart J, Vanpee D, Berlin I, Galanti L (2023) Changes in biomarkers of endothelial function, oxidative stress, inflammation and lipids after smoking cessation: a cohort study. Eur J Clin Invest 53(8):e13996
Wiebe N, Ye F, Crumley ET, Bello A, Stenvinkel P, Tonelli M (2021) Temporal associations among body mass index, fasting insulin, and systemic inflammation: a systematic review and meta-analysis. JAMA Netw Open 4(3):e211263
Cho YH, Lee Y, Choi JI, Lee SR, Lee SY (2022) Biomarkers in metabolic syndrome. Adv Clin Chem 111:101–156
Merianos AL, Stone T, Jandarov RA, Mahabee-Gittens EM, Choi K (2022) Sources of tobacco smoke exposure and their associations with serum cotinine levels among U.S. children and adolescents. Nicotine Tobacco Res 25(5):1004–1013
Brunetti R, Hicklin H, Rackley J, Ahmad MI, Li Y, Soliman EZ (2019) Serum cotinine and silent myocardial infarction in individuals free from cardiovascular disease. Am J Cardiol 124(5):666–670
Sun J, Li Y, Zhao M, Yu X, Zhang C, Magnussen CG, Xi B (2023) Association of the American Heart Association’s new Life’s Essential 8” with all-cause and cardiovascular disease-specific mortality: prospective cohort study. BMC Med 21(1):116
Carrico C, Gennings C, Wheeler DC, Factor-Litvak P (2015) Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 20(1):100–120
Ajsuvakova OP, Tinkov AA, Aschner M, Rocha JBT, Michalke B, Skalnaya MG, Skalny AV, Butnariu M, Dadar M, Sarac I et al (2020) Sulfhydryl groups as targets of mercury toxicity. Coord Chem Rev 417:213343
Ouyang Y, Peng Y, Li J, Holmgren A, Lu J (2018) Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems. Metallomics 10(2):218–228
Hansen JM, Zhang H, Jones DP (2006) Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radical Biol Med 40(1):138–145
Valko M, Morris H, Cronin MT (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Bist P, Choudhary S (2022) Impact of heavy metal toxicity on the gut microbiota and its relationship with metabolites and future probiotics strategy: a review. Biol Trace Elem Res 200(12):5328–5350
Liu X, Zhang J, Si J, Li P, Gao H, Li W, Chen Y (2023) What happens to gut microorganisms and potential repair mechanisms when meet heavy metal(loid)s. Environ Pollut 317:120780
Rizzetto L, Fava F, Tuohy KM, Selmi C (2018) Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun 92:12–34
Liu Y, Zhang Z, Han D, Zhao Y, Yan X, Cui S (2022) Association between environmental chemicals co-exposure and peripheral blood immune-inflammatory indicators. Front Public Health 10:980987
Chen Z, Lu Q, Wang J, Cao X, Wang K, Wang Y, Wu Y, Yang Z (2022) The function of omega-3 polyunsaturated fatty acids in response to cadmium exposure. Front Immunol 13:1023999
Yan LJ, Allen DC (2021) Cadmium-induced kidney injury: oxidative damage as a unifying mechanism. Biomolecules 11(11):1575
Bjørklund G, Peana M, Dadar M, Chirumbolo S, Aaseth J, Martins N (2020) Mercury-induced autoimmunity: drifting from micro to macro concerns on autoimmune disorders. Clinical Immunol 213:108352
Bjørklund G, Dadar M, Mutter J, Aaseth J (2017) The toxicology of mercury: current research and emerging trends. Environ Res 159:545–554
Chuang YC, Wu SY, Huang YC, Peng CK, Tang SE, Huang KL (2022) Cell volume restriction by mercury chloride reduces M1-like inflammatory response of bone marrow-derived macrophages. Front Pharmacol 13:1074986
Ahn H, Kim J, Kang SG, Yoon SI, Ko HJ, Kim PH, Hong EJ, An BS, Lee E, Lee GS (2018) Mercury and arsenic attenuate canonical and non-canonical NLRP3 inflammasome activation. Sci Rep 8(1):13659
Rabolli V, Wallemme L, Lo Re S, Uwambayinema F, Palmai-Pallag M, Thomassen L, Tyteca D, Octave JN, Marbaix E, Lison D et al (2014) Critical role of aquaporins in interleukin 1β (IL-1β)-induced inflammation. J Biol Chem 289(20):13937–13947
Crowe W, Allsopp PJ, Watson GE, Magee PJ, Strain JJ, Armstrong DJ, Ball E, McSorley EM (2017) Mercury as an environmental stimulus in the development of autoimmunity - a systematic review. Autoimmun Rev 16(1):72–80
Huang JH, Tao L, Wu Y, He W, Wang JX, Chen X, Fu L (2023) Cobalt exposure and dyslipidemia in elderly population: the mediating role of systemic inflammation and lipid peroxidation. Environ Sci Pollut Res Int 30(17):50402–50411
Scammell MK, Sennett C, Laws RL, Rubin RL, Brooks DR, Amador JJ, López-Pilarte D, Ramirez-Rubio O, Friedman DJ, McClean MD et al (2020) Urinary metals concentrations and biomarkers of autoimmunity among Navajo and Nicaraguan men. Int J Environ Res Public health 17(15):5263
Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ (2020) Tobacco product use among adults - United States, 2019. MMWR Morb Mortal Wkly Rep 69(46):1736–1742
Kozak K, Antosiewicz DM (2023) Tobacco as an efficient metal accumulator. Biometals 36(2):351–370
Li X, Yu X, Luo K, Liu H, Fan X, Yin X, Zhao Q, Liu X, Yang Y (2023) Exposure to metals and the disruption of sex hormones in 6–19 years old children: an exploration of mixture effects. Ecotoxicol Environ Saf 250:114477
Li Y, Liu M, Cui Y, Zhu Z, Chen J, Zeng F, Gao M, Li Y, Huang F, Chen H (2022) Increased risk of testosterone deficiency is associated with the systemic immune-inflammation index: a population-based cohort study. Front Endocrinol 13:974773
Spurgeon DJ, Jones OA, Dorne JL, Svendsen C, Swain S, Stürzenbaum SR (2010) Systems toxicology approaches for understanding the joint effects of environmental chemical mixtures. Sci Total Environ 408(18):3725–3734
Lin S, Yang F, Hu M, Chen J, Chen G, Hu A, Li X, Fu D, Xing C, Xiong Z et al (2023) Selenium alleviates cadmium-induced mitophagy through FUNDC1-mediated mitochondrial quality control pathway in the lungs of sheep. Environ Pollut 319:120954
Gelman A (2007) Struggles with survey weighting and regression modeling. Stat Sci 22(2):153–164
Funding
This study was supported by grants from the National Natural Science Foundation of China (82073655, 82373672) and the funds for academic and technical leaders in Anhui province (2017D140) and the Clinical Medicine Discipline construction project of Anhui Medical University(2021lcxk043).
Author information
Authors and Affiliations
Contributions
Xiaoya Sun: Conceptualization and design, Data curation, Formal analysis, Writing – original draft. Yujie Deng: Study conception and design, Data curation, Investigation. Lanlan Fang: Supervision and validation, Visualization. Man Ni: Investigation, Methodology, Resources. Tao Zhang and Xinqi Wang: Investigation, Data curation, Formal analysis. Yuting Chen: Software and Supervision. Guoqi Cai: Validation and Visualization. Faming Pan: Funding acquisition, Writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Deng, Y., Fang, L. et al. Association of Exposure to Heavy Metal Mixtures with Systemic Immune-Inflammation Index Among US Adults in NHANES 2011–2016. Biol Trace Elem Res 202, 3005–3017 (2024). https://doi.org/10.1007/s12011-023-03901-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03901-y