Abstract
Issues regarding biomagnification of mercury (Hg) due to its persistence, bioaccumulation, and toxicity in freshwater lakes have gained much attention in the last two decades especially in remote regions of the world where anthropogenic inputs are considered as negligible. In this study, spatial distribution of total mercury (THg), interspecific accumulation patterns, trophic transfer, and associated health risks in fish of freshwater lakes (357–3107 masl) in Azad Kashmir, Pakistan, were investigated. THg concentrations in the regions were 0.20 ± 0.08 μg g−1 in glacial, 0.54 ± 0.21 μg g−1 in rural, and 1.35 ± 0.46 μg g−1 in urban region. Omnivorous, herbivorous, and carnivorous fish showed THg concentrations of 0.94, 0.85, and 0.49 μg g−1. Regional, lake, trophic level, and specie-specific differences of THg accumulation were found significant in the study. Among growth parameters, length and age varied significantly among species, trophic levels, and lakes, whereas weight showed significant variation among lakes as well. Condition factor (K) showed significant differences within species, lakes, and trophic levels. Biomagnification was observed in all lakes with the trophic magnification slopes (TMS) ranging from 0.03 to 0.20 with an average of 0.094 ± 0.07. Isotopic values of nitrogen (δ15N) and condition factor were found to dominate THg accumulation trends; however, no significant health risks were found in the study.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury is a widespread, semi-volatile, [1] ubiquitous, and persistent [2] environmental pollutant due to its highly mobile nature in the environmental matrices and food webs [3, 4]. It is released from variety of natural and anthropogenic sources including volcanic eruptions, re-emissions, fossil fuel and biomass burning, industrial processes and waste incineration, etc. [5, 6]. Greater than 90% of mercury in the atmosphere consists of elemental mercury, which is highly volatile with an atmospheric residence time of 0.5–2 years. It undergoes long-range atmospheric transport (LRAT) which allows it to travel long distances and making it a transboundary pollutant. This peculiar nature of Hg has gained it special attention, making it a potential threat to the aquatic ecosystems in regions having no point sources of mercury pollution [5]. Possessing the characteristics of persistence, bioaccumulation, biomagnification, and toxicity, it affects human and ecosystem health [5, 6] and causing deleterious effects on the immune system, kidneys, neurological system, and cardiovascular system of humans and wildlife [7]. Owing to the neurotoxic effects of methyl mercury, the International Program of Chemical Safety (IPCS) has listed it as one of the six most dangerous chemicals [8]. The Agency for Toxic Substances and Disease Registry has listed Hg on third place among the toxic substances in the Substance Priority List (SPL) [9]. In addition, it is regarded as “one of the top ten chemicals or groups of chemicals of major public health concern” [10].
Humans are exposed to toxic metals like mercury and selenium through eating fish contaminated with these metals. It is widely recognized that 90% of human health risk occurs by the consumption of metal-contaminated fish [11], making human health risk assessments via dietary exposure of mercury important. Fish accumulate and biomagnify Hg to dangerous levels for human consumption, and lakes, among other aquatic systems, are their essential habitats which act as sentinels and integrators of environmental variations in and beyond their watersheds and airshed [12, 13].
Fish is a suitable bioindicator for tracing metal pollution as they occupy a range of trophic levels and usually positioned at the top of the food web in aquatic ecosystems. They contain highest mercury concentrations as compared with baseline producers and other consumers having the ability to bioconcentrate mercury which is further biomagnified in the food chain [14]. Assimilation of Hg in fish takes place by ingestion of suspended matter, dietary uptake, and exchange via lipophilic membranes, i.e., gills, and through absorption on tissues and membranes [14]. Water-borne mercury is up taken by fish through skin and gills. However, dietary uptake is the most dominant pathway of Hg in fish, and 80–90% of MeHg and 32–92% of inorganic mercury comes from diet [15]. Trophic dynamics (community composition and feeding relationships) have been identified as important drivers of methylmercury (MeHg) bioaccumulation in lakes and reservoirs [16]. The relative contribution of benthic and pelagic pathways in energy transfer in lake food webs has implications in Hg distribution and should be quantified to understand and predict the patterns of Hg accumulation in top predator fish in lakes [17]. Disagreements exist between benthic [18, 19] and pelagic feeding pathways [20, 21] being the most efficient for trophic transfer of THg and MeHg to higher trophic level consumers. Studies have shown higher concentrations of THg observed in benthic predatory fish as compared with the pelagic ones [16]. However, higher levels of Hg have also been reported in pelagic fish. [22]. The physiological characteristics of fish (growth rate, length, age, weight) explain the discrepancies and variability of Hg concentrations and biomagnification [16, 23]. Growth factors such as size and age of fish, increase in trophic levels, and food web complexity have been positively related to Hg concentrations of organisms in various studies [24, 25].
Accumulation of Hg in fish depends upon the species of mercury available for uptake, species, and physiological characteristics of fish (size, age, weight, feeding habits, and mobility) [26]. Upon entering the biological systems, Hg (> 95% in muscles) tends to bind with selenium- and sulfur-containing compounds, and cysteine is the major thiol-containing peptide in fish muscles that make complexes with Hg [15]. After the dietary exposure, Hg accumulates in the gut and is then transferred to other tissues at a slower rate. It bioaccumulates and biomagnifies from the base line consumers to the highest of the predators, i.e., piscivorous fish in the aquatic ecosystems [15, 27]. The organic form of mercury is excreted slowly in aquatic organisms (2.8 times) as compared with inorganic mercury, which results in the elevated Hg concentrations in fish which are old and at the top of aquatic food webs. In addition, larger organisms living in colder environments also have lower elimination rate and thus accumulate more Hg with time. Similarly, predatory fish contains highest levels of mercury as the concentrations of mercury increases with trophic levels with Hg increasing more readily [16].
Levels of Hg in fish and its biomagnification along the food chain depend, among other factors, on the inherent ecosystem characteristics [28, 29]. Altitude is considered as an important factor when reporting fish Hg concentrations. Greater deposition and retention of mercury in high altitude lakes resulted in high fish Hg levels [29]; however, in the opposite case, the characteristics of lake watersheds play an important role in explaining the trends of fish Hg concentrations [30]. The variability of Hg levels in fish populations are related, in part, to the nature of ecosystems [31, 32], such as a forested landcover shows positive relationships with fish mercury concentrations at a landscape scale. [33, 34]. Temperature is another factor that can influence the methylation rates of mercury and its level in fish. As at high altitudes, temperatures are low all year around, and this could result in lower methylation rates than in temperate environments, thus contributing to low mercury and methylmercury concentrations observed in fish populations [30].
Alpine regions show rapid changes in climatic, biological, and environmental characteristics with altitude, e.g., high surface roughness along the altitudinal gradient led to increased atmospheric deposition, increased precipitation, and lower soil emissions due to low temperatures. [35]. Due to the weak capabilities of self-purification and self-recovery, these high-altitude water systems such as rivers, and lakes are considered as pristine regions but are more susceptible toward environmental changes. Such remote water systems are considered as early warning sites for environmental change [36]. Studies have shown that environments in these regions are critically sensitive to the atmospheric Hg deposition through LRAT, condensation, and enrichment of transported pollutants [37, 38]. More importantly, forest regions with unprotected surface waters have high concentrations of Hg in the biota, thus being sensitive to Hg deposition [36]. Following LRAT and passing through a series of chemical reactions in the atmosphere, it gets deposited in the remote and high mountainous areas of the world [5]. The majority of atmospheric mercury (~ 40%) from the local and regional anthropogenic sources deposits to the aquatic waterbodies. In addition, surface runoffs, precipitation, glacial melt, and flooding also carry mercury in the aquatic systems [39]. Once mercury gets into the aquatic system, it converts into methyl mercury by the sulfate- and iron-reducing bacteria (SRBs and FeRBs) most predominantly at the sediment water interface and becomes available for uptake and biomagnification in the food chains particularly in fish [40, 41]. The decreased capacity of fragile ecosystems to dilute contaminants like Hg led to a concern regarding risk posed by the contaminant to humans [42].
Asia has a major contribution in anthropogenic Hg emissions where the largest share of Hg emissions comes from fossil fuel combustion and biomass burning [43,44,45]. In recent decades, efforts have been made to reveal consistent co-variation of background Hg levels in lakes and changes in fish Hg in response to regional and global depositions of Hg in remote and mountainous regions [46]. However, comparative studies of Hg accumulation and biomagnification along a range of altitudinal gradients incorporating regional differences are needed to be conducted. The lesser Himalayan region in Pakistan consists of a range of high-altitude glaciated freshwater lakes to the remote rural lakes with forested watershed. Down the altitudinal gradient is the highly impacted urban reservoir [47]. The study area gives access to the three kinds of lakes needed for a comparative study, making it possible to evaluate the dynamics of mercury in regions with different source attribution. This study was designed to evaluate the spatial distribution of Hg in fish; evaluate interspecific accumulation patterns related to feeding habits and growth parameters; analyze trophic transfer; and finally assess the risk to human health imposed by the consumption of these fish in the selected glacial, rural, and urban freshwater lakes of Azad Kashmir, Pakistan. The lakes were categorized according to the geography, land use, demographics, and the sources of mercury pollution (Table 1). The least populated area with glacial watershed having no point sources of mercury pollution defines the glacial lake, whereas sparsely populated areas with forested watershed having inputs from biomass burning were characterized as the rural ones, and the densely populated areas with forested and non-forested urban watershed having industrial and domestic pollution sources comprised the urban lakes.
Materials and Methods
Study Area
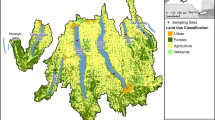
Freshwater lakes selected for the study lie in the lesser Himalayan region in the states of Azad Jammu and Kashmir at longitude 73–75° and latitude 33–36°. The GIS constructed map of study area is depicted in Fig. 1. Five lakes were selected for sampling along the attitudinal range of 357–3107 masl, including one reservoir (Mangla Dam) based on their watersheds, i.e., glacial/forested. The study area was divided into three types of lakes, i.e., glacial (Shounter (3107 masl)), rural (Banjosa (1792 masl) and Chikar, 1325 masl)), and urban (Mangla (357 masl) and Subri (742 masl)) depending upon the water inflows and surrounding areas. Lakes directly fed with the glaciers are termed as glacial lakes; those fed by snowmelt or precipitation with surrounding rural areas (forests/dispersed population) are here referred to as the rural lakes, and the lakes fed by either river or precipitation present in an urban area are termed as urban lakes. Glacial lake including Lake Shounter is present in the watershed of river Jhelum, whereas Chikar and Banjosa were categorized as rural lakes. Lake Chikar lies in the watershed of river Jhelum also. Lake Banjosa is fed by River Kunhar and outflows into the watershed of Mangla ultimately flowing in river Jhelum. The man-made Mangla dam fed by both perennial (Jhelum and Poonch) and non-perennial rivers (Kanshi and Khad) and outflows in river Jhelum, covering a total area of 26,500 ha [47], was also selected to investigate the impact of anthropogenic sources along with Subri, which is fed by River Neelum and empties finally into river Jhelum, and both were categorized as urban lakes (Table 1). Higher-altitude glacial fed lakes are expected to be mainly under the influence of LRAT of pollutants along with the rural sources of biomass burning with no industrial source. The rural region with lakes consisted of forested or mountainous watersheds with pollutant inputs directly from the atmosphere and nearby sources of biomass burning and tourism. The urban region consisted of Mangla dam and Subri was under the influence of pollutant inputs from anthropogenic sources on a greater scale as compared with the other regions.
Sample Collection, Processing, and Analysis
Gill nets with varying mesh sizes (10–100 mm) were used to capture fish from lakes, and samples were stored in plastic zipper bags, brought to the laboratory in an icebox, and stored in a freezer until further processing. The specimen was identified by fish taxonomist with the help of local names, photographs, and morphological characteristics obtained from Fishbase.org. The collected fish specimen belonged to the following seven species: Labeo rohita, Oreochromis aureus, Hypophthalmichthys molitrix, Sperata seenghala, Tor putitora, Channa marulius, and Monopterus cuchia. The study was approved by the Bioethics Committee of Quaid-i-Azam University, Islamabad, Pakistan. All the adopted procedures were in compliance with ethical guidelines. Additional details about the sampling sites and fish characteristics are given in Tables 1 and 2, respectively.
Fish Sample Processing and Tissue Preparation
In the laboratory, fish were thawed, and their lengths and weights were measured. Fish scales were collected for determining age at ×10 resolution from a LED microscope. The age was determined based on scales, and the measurements were confirmed by a fisheries expert [48]. Afterwards, dissection was performed using stainless steel dissection tools, rinsed with 50% solution of 65% nitric acid and distilled water between each dissection. Muscles were obtained between the pectoral fin and vent of fish, packed in zippers, and stored at 4 °C until analysis.
Analysis of Mercury in Fish Muscle Tissues
-
a.
Sample preparation and analysis
To prepare the samples for analysis, acid digestion was performed. Pyrex-made glassware was used in the process of digestion. The glassware was soaked in 10% nitric acid (Merck, Germany) overnight followed by rinsing with distilled water and left for air-drying. Fish muscles were digested following the protocol described by Lomonte et al. [49]. Briefly, 0.5 g of muscle was weighed to the nearest millimeter, soaked in 10 ml of 65% HNO3, and left for overnight digestion at room temperature. Afterwards, samples were digested on hotplate at 95–100 °C for 2 h. One millimeter of 35% H2O2 was added before turning off the hot plate until the effervescence stopped. Digestate were filtered with Whatman filter paper no. 41, diluted, and analyzed with atomic absorption spectroscopy.
-
b.
Quality assurance and quality control (QA/QC)
The procedures for QA and QC were followed accordingly during the analysis to ensure the viability of results obtained. Blanks, duplicates, and certified reference material (CRM) were used to ensure quality control of the method. Throughout each sampling session, duplicates and blanks were taken regularly in each batch of 10 samples. The instrument was calibrated each time by obtaining a calibration curve from the standards of 10, 30, 50, 75, and 100 ug l−1. The relative standard deviation for duplicate sample analysis was less than 10% for THg. CRM DORM-2 with analytical recovery of 84.4% (mean ± SD 3.917 ± 0.121 mg kg−1) was used. The detection limit was 0.006 mg kg−1. All the glassware used in the sample preparation until analysis were made of Pyrex and was washed properly with nitric acid and distilled water. Chemicals used in the experimental process were of analytical grade and purchased from Merck (Germany).
Stable Isotopic Analysis of Nitrogen
The subset of fish muscle samples was selected for stable isotope analysis to identify the natural abundance of N15. The samples were analyzed at the Pakistan Institute of Nuclear Science and Technology (PINSTECH). The samples were oven dried at 60 °C for 48–72 h, grounded to fine powder, and analyzed. For the determination of N15, steam distillation process was used. Ammonium, nitrate, and nitrite were measured in the alkali labile organic nitrogen compounds (fish muscles). In the process, sulfamic acid was used for the destruction of nitrite followed by the reduction of nitrate and nitrate by Devarda’s alloy, and at the end, magnesium oxide was used in the distillation of ammonium. Forms of nitrogen under analysis are converted to and determined as ammonium which is readily oxidized to nitrogen gas for mass spectrometer assay of 15N [50]. The stable isotopic values are expressed in δ notation as parts per mill deviation from a standard reference.
where R is 15N/14N.
Calculation of Fulton’s Condition Factor
The fish condition was calculated by following the calculations given by [51]
where W = total body weight of fish (g) and L = total length of fish (cm) [52].
Health Risk Estimation
Estimation of human health risk derived from fish consumption was evaluated using EDI and THQ, respectively.
-
(a)
Estimated daily intake
The estimated daily intake [26] of mercury by consumption of fish was determined by the following equation:
where EF is the exposure frequency (365 days year−1), ED is the exposure duration (70 years) [53], FIR is the food/fish ingestion rate (g day−1), and 2 kg annum−1 (5.4 g day−1 person−1) [54]. C is the metal concentration in fish (mg kg−1 or μg g−1), RfD is the daily oral reference dose (μg g−1 day−1), BW is the average body weight (60 kg for the Pakistani population) [53, 55], and AT is the average exposure time for the non-carcinogens (365 days year−1 × number of exposure years (70 years). EDI were compared with the PTDI values as suggested by the FAO/WHO Expert Committee on Food Additives (JECFA); 0.23 μg kg−1 day−1 for THg, and with RfD for MeHg established by US EPA, 0.1 μg kg−1 day−1 [56]
-
b
Target hazard quotient
Target hazard quotient (THQ); the ratio between exposure to the reference dose (RfD) is often used to assess the effects of non-carcinogenic risks. THQ was calculated by adopting the equation described by [57]:
THQ provides an indication of health hazards associated when the exposure of a specific contaminant occurs. Previous studies indicated that a contaminant’s ingestion dose is similar to the dose which is adsorbed in the body and that cooking procedures do not affect contaminant concentration.
Statistical Computation and Analysis
The statistical analysis of the data was carried out by using MS Excel 2016, SPSS (IBM 20), Minitab 18, and MVSP. Kolmogorov-Smirnov test was applied to check the normality of data, and tests for parametric stats were performed afterwards. Mean, standard deviation, and minimum-maximum were opted as measures of descriptive stats. One-way ANOVA at p = 0.05 was used to identify differences between fish morphometrics and metal concentration, and post hoc Tukey’s test was performed to analyze the differences in variables based on different grouping variables (region, lakes, trophic levels, specie). Multiple statistical analyses (Pearson correlation, regression, and principal component analysis (PCA)) were performed to evaluate relationships between fish morphometrics and THg concentrations. For PCA, Euclidean biplots were generated using Pearson’s similarity matrix on centered, standardized, and log ratio transformed data.
Results and Discussion
Spatial Distribution of THg
THg concentrations in the studied fish ranged from 0.02 to 1.89 μg g−1 with an average of 0.73 ± 0.54; 44% of fish were well below the standards for mercury in fish permitted by JECFA and EPA (0.5 μg g−1), whereas 56% of the fish exceeded permissible levels of mercury. THg concentrations in the three regions were glacial, 0.20 ± 0.08 < rural, 0.54 ± 0.21 < urban, 1.35 ± 0.46 μg g−1, respectively (Table 3; Fig. 2). Concentrations of THg varied significantly among the three regions (glacial, rural, and urban regions) and among sites (lakes) at p = 0.00 respectively (SI Table S3). Shounter (glacial lake) showed lowest THg concentrations in fish, having an average of 0.20 ± 0.08 ranging from 0.04 to 0.30 μg g−1). In the rural region, fish THg concentrations ranged from 0.10 to 0.98, having an average of 0.54 ± 0.21 μg g−1; in Chikar, 0.72 ± 0.13 (0.52–0.98) μg g−1; and Banjosa, 0.40 ± 0.15 (0.10–0.60) μg g−1, and followed by the urban region having mean fish THg concentration of 1.04 ± 0.57 μg g−1 ranging from 0.02 to 1.89 μg g−1; Mangla, 1.35 ± 0.46 (0.21–1.89) μg g−1; and Subri, 0.66 ± 0.47 (0.02–1.55) μg g−1.
Differences in climatological factors and source attribution resulted in the decreasing trend of THg concentrations from glacial to urban lakes [58]. The composition of watersheds, i.e., glacial (Shounter) or forested (Banjosa), also explains variations in THg [16]. Mercury concentrations in glacial region were comparable with similar high-altitude remote alpine systems with glacial watersheds in the Tibetan Plateau (0.21 μg g−1), Canada (La Ronge, 0.09 μg g−1; Wollaston, 0.08 μg g−1; and Reindeer, 0.12 μg g−1) [3, 17], Lake Crop (0.21 μg g−1), and La Sagne 0.18 μg g−1 from the French Alps [30] and were lower as compared with glacial lakes in Norway (0.66 μg g−1), Argentina (0.405 μg g−1), and Lake Meretta in Canada (0.53 μg g−1), respectively [16, 19, 59].
The values in rural lakes were comparable with the studies on similar rural systems (mountain originated) in Lego Menor in Canada (0.53 μg g−1) and Lake Norsjø of southern Norway (0.55 μg g−1), less as compared with Tinnsjø in Norway (0.96 μg g−1) [59], and more than in Africa (George, 0.03 μg g−1) [60] and the French Alps (Poursollet, 0.10 μg g−1) [30] due to differences in contamination sources, geographic location, and watersheds. Banjosa is surrounded by lush green forest cover, a source of organic matter (absent in the case of Shounter) on the surrounding mountains of the valley, and receives heavy snowfall during the winter season, changing the chemistry of Hg cycling [61]; Chikar was affected by earthquake, and the construction activities near the lake can result in mercury inputs in the lake [62].
The urban lake fish THg concentrations were comparable with other similar systems, impacted by anthropogenic activities, such as Three Gorges Reservoir, China (1.09 μg g−1) [12], Lake Norheim in Norway (1.68 μg g−1) [59], Tapajos River in Brazil (0.03–1.51 μg g−1) [63]. Due to the geographic location where the lakes are situated, forested watershed and population density around Subri explains the mercury inputs in Mangla and can be attributed to anthropogenic Hg inputs from the input tributaries and its location in urban setting, [47].
Interspecific Accumulation Patterns
The trends of increasing THg concentrations in the lakes were Shounter: Hyp mol < Lab roh < Spe see < Ore aur; Banjosa: Tor put < Spe see < Lab roh = Ore aur; Chikar: Tor put < Ore aur < Hyp mol < Spe see; Subri: Mon cuc < Cha mar < Hyp mol < Spe see < Lab roh; and Mangla: Cha mar < Hyp mol < Ore aur < Lab roh (Fig. 3; Table 3). Considering individual species, the increasing order of THg concentration was as follows; Mon cuc < Spe see < Cha mar < Tor put < Hyp mol < Ore aur < Lab roh with mean concentrations of 0.04 ± 0.02 < 0.31 ± 0.15 < 0.48 ± 0.32 < 0.82 ± 0.11 < 0.85 ± 0.52 < 0.86 ± 0.54 < 1.02 ± 0.6 μg g−1, respectively.
Feeding Modes
The glacial region showed highest THg (μg g−1) concentrations in carnivores (0.22 ± 0.02) > omnivores (0.21 ± 0.09) > herbivores (0.12 ± 0.08). In the rural and urban lakes, the concentration followed the following trend: rural: herbivore (0.74 ± 0.02) > carnivore (0.57 ± 0.29) > omnivore (0.49 ± 0.18); urban: omnivore (0.45 ± 0.25) > herbivore (1.13 ± 0.41) > carnivore (0.38 ± 0.34), respectively. Significantly higher concentrations between species at p = 0.004 and lakes at p = 0.00 (SI Table S3) were recorded in Lab roh, 0.30 μg g−1 (Shounter); Spe see, 0.60 μg g−1 (Banjosa) and 0.98 μg g−1 (Chikar); Lab roh, 1.55 μg g−1 (Subri); and Hyp mol, 1.89 μg g−1 (Mangla) (Fig. 3; Table 3).
Taking into consideration the feeding modes of fish, higher concentrations in Lab roh and Ore aur can be attributed to their benthopelagic feeding and opportunistic feeding habits (omnivorous trophic guild), where both fish feed mostly on phytoplanktons, zooplanktons, insects, algae, and detritus [16]. Concentrations of THg in Spe see (demersal; column feeder ) were found comparable with the findings of Annual et al. [64], where higher concentrations in demersal or benthic living as compared with the pelagic fish species were found. Lower concentrations of Hg in Cha mar can be attributed to higher growth rate of this fish that leads to more elimination of mercury. Also, Cha mar occupies a lower trophic position as depicted by δ15N values (6.9 and 5.8) indicating that it occupies a lower position in the food web as compared with the omnivorous species. Hyp mol is a filter-feeding herbivore and is a benthopelagic fish, feeding from within the water column, thus showing lower THg concentrations. The negative correlation (r = − 0.69, p = 0.02) between THg and δ15N explains the concentration trends for the species, wherein lower nitrogen content feed contributes to greater to THg concentrations; thus, it is inferred that the higher proportion of inorganic mercury contributed to higher concentrations at the given time as the species is a surface filter feeder. A similar trend was reported in a study on mercury biogeochemical cycling in the Wuchang bream, China, where highest THg was found in herbivorous fish [65]. It has also been reported that the food web is brief in high mountain lakes, and in these lakes, fish feeds mainly on insects, and cannibalism is also observed. Furthermore, lakes with a rocky watershed contain less dissolved organic carbon (DOC) which elevates Hg methylation rates [30]. However, the biotic transfer efficiency of bottom feeding fish can also be a reason of such lower levels of THg and can be attributed to the opportunistic feeding preferences (feeding on a variety of plant and animal matters) and bottom feeding habitat [17].
High concentrations of Hg in carnivorous fish at a higher trophic level as compared with the herbivorous and omnivorous fish at lower trophic levels were reported, and also higher concentrations were found in demersal as compared with the pelagic fish species [64]. A complex interaction of physiological characteristics of fish and mercury cycling drives the accumulation of mercury in an ecosystem. THg concentrations in Baihua Reservoir, China, were found to be highest in zooplankton-feeding big head carp than in the carnivorous fish [65]. Higher THg concentrations in omnivorous fish were found downstream to the Cabixi hydropower plant as well [66].
Growth Parameters
In the urban lakes, fish THg concentrations were inversely associated with increasing length (r = 0.52, p = 0.00). However, rural and glacial region showed negligible positive effects on THg accumulation (Table 4). Length was found to be negatively associated with THg in carnivorous and herbivorous fish. Omnivorous species were found to show a minimal positive association of length on THg accumulation. So THg concentration in fish was explained by the dominating regional and site-specific influences. A similar positive correlation is reported for B. intermedius in Lake Awasa [67]. A significant negative correlation with the length of fish was found in all lakes at p < 0.05. In addition, lower concentrations in carnivorous fish of urban lakes (Fig. 2) are explained by their weight, length, and condition factor (Table 3; SI Fig S1; SI Table S3), and they were older and heavier as compared with the herbivorous and omnivorous species that can result in lower assimilation of mercury, and also in older fish, the increase in fish mass may also lead to the bio-dilution of metals in muscles leading to lower concentrations despite being at higher trophic levels [5].
The weight of fish slightly positively affected fish THg concentrations in the rural lakes (r = 0.14, p = 0.09) (Table 4). Age was found to have a significant positive relation with THg concentrations (r = 0.4 and p = 0.00) (Table 4) in the urban region. Lab roh, Ore aur, and Tor put showed significant positive associations of log THg with weight (r = 0.12, p = 0.00) and log THg and age (r = 0.53, p = 0.00) for the carnivorous species respectively (Table 4), wherein age showed positive association with weight (r = 0.54, p = 0.02) and mercury (r = 0.61, p = 0.01), which describes an increase in THg as the fish ages (r = 0.57, p = 0.01) (Table 4). Age, however, showed a positive correlation with the condition factor in the two urban lakes (r = 0.61 in Subri and r = 0.57 in Mangla at p < 0.05). In addition, significant specie-specific variations in length, weight, condition factor (p = 0.00), and THg accumulation (p = 0.004) were found. Also, significant differences between the omnivorous, herbivorous, and carnivorous fish were found in the length, weight, condition factor (p = 0.00), and THg accumulation (p = 0.005) (SI Table S3).
The decreasing trend of fish THg with length can be explained by differences in fish movements and metabolism. It has been reported that smaller and younger fish can move to areas with higher contaminant concentrations as they show more movement due to their large home ranges, especially in lakes [66]. The ontogenetic dietary shifts of fish should also be taken into consideration, wherein the consuming diet with variable contaminant concentrations than the usual diet is observed [68]. Non-linear trends of mercury accumulation with fish length have been reported. A negative association of mercury vs fish length (mostly salmonids) has been reported in Lake Nahuel Huapi, Argentina, wherein the mercury concentrations decreased with size until 300 mm (30 cm) and then increased. [16]. A negative correlation (r = 0.64) has been observed for Dourada in the Tapaios River, which is related to fish diet, i.e., the specimen feed upon uncontaminated or low contaminated diet. The variance observed in the relationships between metal concentration and fish size, as measured among different fish species, is related to the differences in ecological needs, swimming behaviors, and metabolic activity [69].
The age of fish is a valuable indicator to explain the differences in contaminant concentrations in the muscle tissues. In this study, age is positively correlated with THg accumulation in urban fish, which can be due to slow elimination rates (half-life), leading to higher Hg concentrations as the fish ages [70]. The complexity of ecosystem behavior to the environmental variables lead to adaptation of fish according to the prevailing conditions, i.e., changing environmental variables that play a certain role in ichthyofaunal mercury contamination [58].
Fulton’s condition factor (FCF) is an index used to illustrate growth rate and changes in nutritional condition of fish, and it also indicates changes in fish condition in response to environmental contaminants or stress [51, 71]. Regression analysis revealed the significant positive effect of mercury accumulation on fish condition in the urban fish (r = 0.50, p = 0.00) (SI Fig S1; SI Table S1). Similar positive relationships were found between the condition factor and mercury concentrations for catfish (p < 0.05) and yellowhead catfish (p < 0.05) in the Yangtze River, China [69]. This implies that the fish were in better condition, showing growth in the urban region, being more tolerant toward contaminant concentrations, as compared with the sophisticated systems in remote glacial regions [72]. The moderate condition factor in younger and heavier fish shows that these fish can effectively assimilate mercury from their diet and are inefficient at its elimination resulting in increased mercury load due to their slow growth rate [73]. However, the fact that fish moves along different concentration gradients in their lifetime can also be taken into consideration here. Older fish do not show lower metabolic activities and dilution of tissue metal concentrations if the concentrations are higher in the surrounding water than the capacity of these factors; thus, metals show continued accumulation, showing positive relationships with fish condition [69].
Trophic Transfer of Mercury
The trophic transfer of mercury was examined by using nitrogen stable isotopic data, which overall indicated an increase in THg with increase in nitrogen content (r = 0.27; p = 0.02). Nitrogen stable isotopic data indicated an increase in THg with increase in nitrogen content (r = 0.27; p = 0.02). In Shounter, omnivorous fish (Lab roh and Ore aur) showed highest δN15 (11.56 ± 1.43 and 11.43 ± 0.95‰) closely followed by carnivorous Spe see and herbivorous Hyp mol (11.02 ± 0.94 and 10.99 ± 1.38‰). This can be explained by the isotopic carryover from the previous years and presence of recalcitrant material in time series that was not assimilated and due to the small physiological differences in isotope fractionation [67, 74]. The values show that the two omnivorous and one carnivorous species all lie at somewhat the same trophic level in Shounter. No relationship of δN15 was observed with either of the length, weight, and age; it can be inferred that the nitrogen content of the fish is primarily determined by the type of diet the fish consumes; both content and pathways (pelagic/benthic) should be considered. The omnivorous fish from Banjosa showed lower δN15 as compared with that of Shounter (10.24 ± 2.72 for Lab roh and 10.70 ± 1.04 for Ore aur). These were followed by Spe see (9.81 ± 3.41‰) and Tor put (6.01 ± 0.98‰). Lower δN15 values of Banjosa can be related to the fact that nitrogen contents in glacial fed lakes (dissolved inorganic nitrogen, DIN) are higher as compared with those in snowmelt fed lakes [75]. The enrichment of δN15 from Tor put to Spe see was found to be 3.8‰, indicating that the two species lie at two different trophic levels in this lake. However, the discrimination of δN15 from Spe see to Lab roh was 0.43, indicating the possibility of no intermediate trophic levels between these species. The average isotopic content of Chikar was found to be 10.64 ± 1.63‰, with order of increasing δN15 in fish as Tor put < Hyp mol < Spe see < Ore aur. Hyp mol and Ore aur showed similar mercury concentrations, yet the δN15 values indicates that the two species are at different trophic levels with the omnivorous species at a higher trophic level and the herbivore at a lower trophic level. Although Spe see showed the highest concentration of mercury in Chikar, it occupies a lower trophic position to Ore aur at an enrichment of 0.55‰. These differences in the trophic positions between omnivores and carnivore indicate differences in the diet consumed. The order of increasing δN15 in Subri fish was Mon cuc < Cha mar < Hyp mol < Spe see < Lab roh. Concentrations of mercury increased also in the same manner. A significant positive correlation coefficient of 0.821 at p = 0.000 indicated an increase in THg (ug g−1) and is followed by increasing δN15 values, i.e., higher trophic–level organisms accumulate more mercury. In Mangla, the order of increasing δN15 in fish was Cha mar < Hyp mol < Ore aur < Lab roh, and a similar order was followed by THg concentrations. A positive correlation coefficient of 0.586 at p = 0.011 indicated increase in THg with increasing nitrogen content of the biota.
Regression analysis showed a positive regression slope with significant regression slopes in glacial, rural, and urban regions (Table 4; Fig. 4; SI Fig. S2). In this study, the phenomenon of biomagnification was well observed in all the lakes with the highest intensity in Subri. Shounter showing a TMS of 0.21 (p = 0.00); similar to glacial lakes in Norway (0.20) [22], Banjosa and Chikar showed TMS of 0.06 and 0.03 with p = 0.01 and 0.03, similar to the biomagnification factor for log MeHg vs δ15N in Lake Nahuel Huapi, Patagonia, Argentina [16]. This is attributed to the isotopic carryover from the previous years and the presence of recalcitrant material in time series that was not assimilated and due to the small physiological differences in isotope fractionation. Lower δ15N values of lake Banjosa can be related to the fact that nitrogen contents in glacial fed lakes (dissolved inorganic nitrogen, DIN) is higher as compared with those in snowmelt fed lakes [75]. Mangla showed TMS of 0.04 with p = 0.03, whereas Subri showed an insignificant positive TMS (0.14), respectively, and a similar mean TMS of 0.14 ± 0.06 is reported in the aquatic ecosystem of New Brunswick, Canada [73]. Our results were found similar to the TMS (0.06–0.20, p < 0.05) between log THg vs δ15N, reported in the aquatic ecosystems of Canada [73], and were close to the global TMS range of 0.1–0.3 as reported by Lavoie et al. [20]. The TMS for our study was similar in range of 0.07–0.32 as reported in the Canadian food web of St. Lawrence. The variability in the TMS suggests that the trophodynamics of mercury is different at different sites [74]. Positive regression biomagnification slopes are also reported for Canadian lakes [19]. In this study, biomagnification of mercury in all selected lakes was observed at significant levels.
Principal component analysis (PCA) was executed to examine the relative dominance of the selected variables defining trophic transfer in terms of nitrogen isotopic ratios. Axis 1 of the PCA explained 51.06% of the data; axis 2 explained 25.02%; and axis 3 explained 14.10% showing a cumulative percentage of 90.15%, respectively. Axis 1 of the PCA biplots was strongly dominated by length, weight, age, and THg. δ15N was dominant on PC 2, and K, on PC 3, with variable loadings of 0.74 and 0.72, respectively (Fig. 5; Table 5). Species grouped based on trophic levels showed that the carnivorous species in our study consisted of greater length and weight but exhibited lower condition factor, i.e., had lower nutrition, so they were found to have the least THg concentration which is gained by diet [51]. The herbivorous fish showed least δ15N and were smaller and younger as compared with carnivorous fish and showed more THg as they showed strong association with condition factor as compared with the carnivorous fish. The omnivorous fish were found to have smaller lengths and ages and a higher condition factor, i.e., better nutrition resulting in higher dietary inputs of mercury [5]. So it can be inferred that the smaller and younger fish contained higher THg concentrations, due to the variety of food they consume containing variable THg concentrations. Among species, the ones also having generalist diet (Ore aur and Lab roh) were smaller and younger, showed better condition factor, and exhibited higher THg, whereas Hyp mol; the herbivores were younger but heavier with higher condition factor as compared with the carnivorous fish showing higher THg concentration than Spe see and Cha mar which were old and heavy and had least condition factor. Among lakes, fish of Mangla and Chikar (urban lakes) were mostly smaller and younger exhibiting more condition factor, thus showing more THg as compared with the fish of rural lakes, which showed lower THg concentrations.
Potential Human Health Risk Estimation
The distribution of mercury in fish based upon species and lakes is provided in Table 3. Non-carcinogenic risks of mercury exposure to human health were estimated by calculating EDI and THQ. EDI values were compared to the toxic daily intake (TDI) (RfD and TDIs established by EFSA, USEPA, and JECFA) [56]. The TDI for mercury has been established by European and American health advisories. The European Food Safety Authority (EFSA) has established a PTWI of 1.3 μg kg−1 for MeHg (0.19 μg kg−1 day−1), lower than the PTWI established by JECFA (1.6 μg kg−1 week−1 and 0.23 μg kg−1 day−1) [76, 77]. The US EPA has established a reference dose for chronic oral exposure to MeHg to be 0.1 μg kg−1 day−1, respectively [56].
These TDIs and RfDs are used to assess risks to humans without posing a significant health implication to the human body [26, 57]. Table 6 summarizes the estimated daily intakes and target hazard quotients, respectively. Results indicate that there exist no significant and appreciable health risk to humans from mercury via fish consumption in our study area. This implies that the study area contains no point sources of mercury contamination at a larger scale, and the anthropogenic influence is still under control. No EDI value crossed the established TDIs. Although the detected mercury levels were below the RfD and TDIs, the biomagnification of mercury in fish and accumulation of mercury in different tissues of human body can lead to adverse effects. In addition, the synergistic effects of different contaminants can also be taken into consideration in a situation where exposure of more than one metal takes place [78].
Target hazard quotients (THQ) were calculated for the estimation of non-carcinogenic effects of mercury. A THQ < 1 indicates no risk, implying that the daily exposure of metal or contaminant is lower as compared with the adopted oral reference dose and is not likely to cause any adverse health effect to exposed population during lifetime [79, 80]. In our study, the THQ values were less than 1, implying no significant health risk to the human population who consumes these fish. The results can provide a significant basis to concoct the public health policies and safety protocols for mercury in fish most importantly in those areas which do not possess any point sources of mercury pollution.
Conclusions
The study presents the spatial distribution of THg, interspecific accumulation patterns with respect to feeding habits and growth parameters, trophic transfer, and the associated health risks in selected freshwater lakes of Azad Kashmir, Pakistan. The spatial distribution was found to be in line with global trends, i.e., glacial < rural < urban, with significant variation (p = 0.00). Specie-specific accumulation, trophodynamics, and growth parameters were found to affect the metal concentrations in fish. The site-specific (p = 0.00), specie-specific (p = 0.004), and trophic level (p = 0.005) differences were found to be significant in the study. All the lakes under consideration showed biomagnification of THg with trophic magnification slopes (TMS) varying between 0.03 and 0.20, revealing that the trophic transfer of mercury is site specific with different sites having different capacities and efficiencies to biomagnify mercury along the food web. However, estimated health risk showed no significant health risks; THQ values were less than one, implying no significant health risk to the population. Pakistan is a signatory to the Minamata Convention on mercury, making it necessary to conduct studies related to the distribution and dynamics of mercury in the remote and pristine regions to help achieve global benchmarks. This study can provide a significant baseline to carry out source-attributed studies regarding contamination of aquatic bodies at higher altitudes in remote and pristine environments and also as a basis to concoct the public health policies and safety protocols for mercury in fish, most importantly in areas with no point sources of mercury pollution. Also, it will serve as an initial baseline for comparison with future assessment and biomagnification studies of mercury to global THg database.
Abbreviations
- ASTDR:
-
Agency for Toxic Substances and Disease Registry
- Hg:
-
Mercury
- LRAT:
-
Long-range atmospheric transport
- SPL:
-
Substance Priority List
- THg:
-
Total mercury
- TMF:
-
Trophic magnification factor
- TMS:
-
Trophic magnification slope
References
Belger L, Forsberg BR (2006) Factors controlling Hg levels in two predatory fish species in the Negro river basin, Brazilian Amazon. Sci Total Environ 367(1):451–459
Jinadasa B, Fowler SW (2019) Critical review of mercury contamination in Sri Lankan fish and aquatic products. Mar Pollut Bull 149:110526
Zhang Q, Pan K, Kang S, Zhu A, Wang W-X (2014) Mercury in wild fish from high-altitude aquatic ecosystems in the Tibetan Plateau. Environ Sci Technol 48(9):5220–5228
Nimick DA, Caldwell RR, Skaar DR, Selch TM (2013) Fate of geothermal mercury from Yellowstone National Park in the Madison and Missouri Rivers, USA. Sci Total Environ 443:40–54
Shao J, Shi J, Duo B, Liu C, Gao Y, Fu J, Yang R, Jiang G (2016) Mercury in alpine fish from four rivers in the Tibetan Plateau. J Environ Sci 39:22–28
Ha E, Basu N, Bose-O’Reilly S, Dórea JG, McSorley E, Sakamoto M, Chan HM (2017) Current progress on understanding the impact of mercury on human health. Environ Res 152:419–433
Evers DC, Burgess NM, Champoux L, Hoskins B, Major A, Goodale WM, Taylor RJ, Poppenga R, Daigle T (2005) Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 14(1-2):193–221
Monikh FA, Karami O, Hosseini M, Karami N, Bastami AA, Ghasemi AF (2013) The effect of primary producers of experimental aquatic food chains on mercury and PCB153 biomagnification. Ecotoxicol Environ Saf 94:112–115
ASTDR (2017) Substance Priority List. http://www.atsdr.cdc.gov/SPL/. Accessed 14 Nov 2020
WHO (2017) Mercury and health. https://www.who.int/news-room/fact-sheets/detail/mercury-and-health. Accessed 22-03-2020
Monroy M, Maceda-Veiga A, de Sostoa A (2014) Metal concentration in water, sediment and four fish species from Lake Titicaca reveals a large-scale environmental concern. Sci Total Environ 487:233–244
Baker MR, Schindler DE, Holtgrieve GW, St. Louis VL (2009) Bioaccumulation and transport of contaminants: migrating sockeye salmon as vectors of mercury. Environ Sci Technol 43(23):8840–8846
Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH (2007) Methylmercury exposure and health effects in humans: a worldwide concern. AMBIO 36(1):3–11
Monferrán MV, Garnero P, de los Angeles Bistoni M, Anbar AA, Gordon GW, Wunderlin DA (2016) From water to edible fish. Transfer of metals and metalloids in the San Roque Reservoir (Córdoba, Argentina). Implications associated with fish consumption. Ecol Indic 63:48–60
Bradley MA, Barst BD, Basu N (2017) A review of mercury bioavailability in humans and fish. Int J Environ Res Public Health 14(2):169
Arcagni M, Juncos R, Rizzo A, Pavlin M, Fajon V, Arribére MA, Horvat M, Guevara SR (2018) Species-and habitat-specific bioaccumulation of total mercury and methylmercury in the food web of a deep oligotrophic lake. Sci Total Environ 612:1311–1319
Kidd KA, Muir DC, Evans MS, Wang X, Whittle M, Swanson HK, Johnston T, Guildford S (2012) Biomagnification of mercury through lake trout (Salvelinus namaycush) food webs of lakes with different physical, chemical and biological characteristics. Sci Total Environ 438:135–143
Eagles-Smith CA, Ackerman JT, Willacker JJ, Tate MT, Lutz MA, Fleck JA, Stewart AR, Wiener JG, Evers DC, Lepak JM (2016) Spatial and temporal patterns of mercury concentrations in freshwater fish across the Western United States and Canada. Sci Total Environ 568:1171–1184
Lescord GL, Kidd KA, Kirk JL, O’Driscoll NJ, Wang X, Muir DC (2015) Factors affecting biotic mercury concentrations and biomagnification through lake food webs in the Canadian high Arctic. Sci Total Environ 509:195–205
Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM (2013) Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ Sci Technol 47(23):13385–13394
Ethier A, Scheuhammer A, Bond D (2008) Correlates of mercury in fish from lakes near Clyde Forks, Ontario, Canada. Environ Pollut 154(1):89–97
Økelsrud A, Lydersen E, Fjeld E (2016) Biomagnification of mercury and selenium in two lakes in southern Norway. Sci Total Environ 566:596–607
Lavigne M, Lucotte M, Paquet S (2010) Relationship between mercury concentration and growth rates for walleyes, northern pike, and lake trout from Quebec lakes. N Am J Fish Manag 30(5):1221–1237
Schafer HA, Hershelman GP, Young DR, Mearns AJ (1981) Contaminants in ocean food webs. Coast Water Res Pro Bienn Rep Years 1982:17–28
Verdouw JJ, Macleod CK, Nowak BF, Lyle JM (2011) Implications of age, size and region on mercury contamination in estuarine fish species. Water Air Soil Pollut 214(1-4):297–306
Castilhos Z, Rodrigues-Filho S, Cesar R, Rodrigues AP, Villas-Bôas R, de Jesus I, Lima M, Faial K, Miranda A, Brabo E (2015) Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ Sci Pollut Res 22(15):11255–11264
Liu G, Cai Y, O'Driscoll N (2011) Environmental chemistry and toxicology of mercury. John Wiley & Sons, Inc., Hoboken
Rognerud S, Grimalt J, Rosseland B, Fernandez P, Hofer R, Lackner R, Lauritzen B, Lien L, Massabuau J, Ribes A (2002) Mercury and organochlorine contamination in brown trout (Salmo trutta) and arctic charr (Salvelinus alpinus) from high mountain lakes in Europe and the Svalbard archipelago. Water Air Soil Pollut 2(2):209–232
Blais JM, Charpentié S, Pick F, Kimpe LE, Amand AS, Regnault-Roger C (2006) Mercury, polybrominated diphenyl ether, organochlorine pesticide, and polychlorinated biphenyl concentrations in fish from lakes along an elevation transect in the French Pyrénées. Ecotoxicol Environ Saf 63(1):91–99
Marusczak N, Larose C, Dommergue A, Paquet S, Beaulne J-S, Maury-Brachet R, Lucotte M, Nedjai R, Ferrari CP (2011) Mercury and methylmercury concentrations in high altitude lakes and fish (Arctic charr) from the French Alps related to watershed characteristics. Sci Total Environ 409(10):1909–1915
Drevnick PE, Roberts AP, Otter RR, Hammerschmidt CR, Klaper R, Oris JT (2008) Mercury toxicity in livers of northern pike (Esox lucius) from Isle Royale, USA. Comp Biochem Physiol C Toxicol Pharmacol 147(3):331–338
Lindeberg C, Bindler R, Bigler C, Rosén P, Renberg I (2007) Mercury pollution trends in subarctic lakes in the northern Swedish mountains. AMBIO 36(5):401–405
Drenner RW, Chumchal MM, Jones CM, Lehmann CM, Gay DA, Donato DI (2013) Effects of mercury deposition and coniferous forests on the mercury contamination of fish in the South Central United States. Environ Sci Technol 47(3):1274–1279
Shanley JB, Bishop K (2012) Mercury cycling in terrestrial watersheds. Mercury in the Environment: Pattern and Process
Zhang H, R-s Y, X-b F, Sommar J, Anderson CW, Sapkota A, X-w F, Larssen T (2013) Atmospheric mercury inputs in montane soils increase with elevation: evidence from mercury isotope signatures. Sci Rep 3:3322
Phillips VJ, St. Louis VL, Cooke CA, Vinebrooke RD, Hobbs WO (2011) Increased mercury loadings to western Canadian alpine lakes over the past 150 years. Environ Sci Technol 45(6):2042–2047
Szopka K, Karczewska A, Kabała C (2011) Mercury accumulation in the surface layers of mountain soils: a case study from the Karkonosze Mountains, Poland. Chemosphere 83(11):1507–1512
Fu X, Feng X, Dong Z, Yin R, Wang J, Yang Z, Zhang H (2010) Atmospheric gaseous elemental mercury (GEM) concentrations and mercury depositions at a high-altitude mountain peak in south China. Atmos Chem Phys 10(5):2425–2437
Pirrone N, Cinnirella S, Feng X, Finkelman R, Friedli H, Leaner J, Mason R, Mukherjee A, Stracher G, Streets D (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10(13):5951–5964
Chen CY, Dionne M, Mayes BM, Ward DM, Sturup S, Jackson BP (2009) Mercury bioavailability and bioaccumulation in estuarine food webs in the Gulf of Maine. Environ Sci Technol 43(6):1804–1810
Razavi NR, Arts MT, Qu M, Jin B, Ren W, Wang Y, Campbell LM (2014) Effect of eutrophication on mercury, selenium, and essential fatty acids in Bighead Carp (Hypophthalmichthys nobilis) from reservoirs of eastern China. Sci Total Environ 499:36–46
Azevedo-Silva CE, Almeida R, Carvalho DP, Ometto JP, de Camargo PB, Dorneles PR, Azeredo A, Bastos WR, Malm O, Torres JP (2016) Mercury biomagnification and the trophic structure of the ichthyofauna from a remote lake in the Brazilian Amazon. Environ Res 151:286–296
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47(10):4967–4983
Blackwell BD, Driscoll CT (2015) Deposition of mercury in forests along a montane elevation gradient. Environ Sci Technol 49(9):5363–5370
Li P, Feng X, Qiu G, Shang L, Li Z (2009) Mercury pollution in Asia: a review of the contaminated sites. J Hazard Mater 168(2-3):591–601
Fliedner A, Rüdel H, Knopf B, Weinfurtner K, Paulus M, Ricking M, Koschorreck J (2014) Spatial and temporal trends of metals and arsenic in German freshwater compartments. Environ Sci Pollut Res 21(8):5521–5536
Ali U, Sweetman AJ, Riaz R, Li J, Zhang G, Jones KC, Malik RN (2018) Sedimentary black carbon and organochlorines in Lesser Himalayan Region of Pakistan: relationship along the altitude. Sci Total Environ 621:1568–1580
Horká P, Ibbotson A, Jones J, Cove R, Scott L (2010) Validation of scale-age determination in European grayling Thymallus thymallus using tag-recapture analysis. J Fish Biol 77(1):153–161
Lomonte C, Gregory D, Baker AJ, Kolev SD (2008) Comparative study of hotplate wet digestion methods for the determination of mercury in biosolids. Chemosphere 72(10):1420–1424
Bremner JM, Keeney DR (1965) Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal Chim Acta 32:485–495. https://doi.org/10.1016/S0003-2670(00)88973-4
Jin S, Yan X, Zhang H, Fan W (2015) Weight–length relationships and Fulton’s condition factors of skipjack tuna (Katsuwonus pelamis) in the western and central Pacific Ocean. PeerJ 3:e758
Łuczyńska J, Paszczyk B, Łuczyński MJ (2018) Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol Environ Saf 153:60–67
Ali H, Khan E (2018) Assessment of potentially toxic heavy metals and health risk in water, sediments, and different fish species of River Kabul, Pakistan. Hum Eco Risk Assess Int J 24(8):2101–2118
FAO F (2012) Agriculture Organization of the United Nations. 2012. FAO statistical yearbook
Mahmood A, Malik RN, Li J, Zhang G (2014) Human health risk assessment and dietary intake of organochlorine pesticides through air, soil and food crops (wheat and rice) along two tributaries of river Chenab, Pakistan. Food Chem Toxicol 71:17–25
USEPA (2014) Methylmercury (MeHg) (CASRN 22967-92-6). Referenced dose for chronic oral exposure (RfD) integrated risk information system. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=73. Accessed 1/04 2020
USEPA (2014) HHRAP: Chapter 7 Characterizing Risk and Hazard | US EPA ARCHIVE DOCUMENT https://archive.epa.gov/epawaste/hazard/tsd/td/web/pdf/05hhrap7.pdf. Accessed 1/04 2020
Nevado JB, Martín-Doimeadios RR, Bernardo FG, Moreno MJ, Herculano AM, Do Nascimento J, Crespo-López ME (2010) Mercury in the Tapajós River basin, Brazilian Amazon: a review. Environ Int 36(6):593–608
Økelsrud A, Lydersen E, Moreno C, Fjeld E (2017) Mercury and selenium in free-ranging brown trout (Salmo trutta) in the River Skienselva watercourse, Southern Norway. Sci Total Environ 586:188–196
Poste AE, Muir DC, Guildford SJ, Hecky RE (2015) Bioaccumulation and biomagnification of mercury in African lakes: the importance of trophic status. Sci Total Environ 506:126–136
Siddiqui M, Moinuddin A, Nasrullah K, Khan I (2010) A quantitative description of moist temperate conifer forests of Himalayan region of Pakistan and Azad kashmir. Int J Biol Biotechnol 7(3):175–185
Basharat M, Rohn J, Khan MR (2014) Effect of drawdown of Karli Lake, A Case Study of Karli landslide hazard in District Hattian, Northeast Himalayas of Pakistan. Life Sci J 11(9):610–616
Lino A, Kasper D, Guida Y, Thomaz J, Malm O (2018) Mercury and selenium in fishes from the Tapajós River in the Brazilian Amazon: an evaluation of human exposure. J Trace Elem Med Biol 48:196–201
Anual ZF, Maher W, Krikowa F, Hakim L, Ahmad NI, Foster S (2018) Mercury and risk assessment from consumption of crustaceans, cephalopods and fish from West Peninsular Malaysia. Microchem J 140:214–221
Feng X, Meng B, Yan H, Fu X, Yao H, Shang L (2018) Biogeochemical cycle of mercury in reservoir systems in Wujiang River Basin. Springer, Southwest China
Cebalho EC, Díez S, dos Santos FM, Muniz CC, Lázaro W, Malm O, Ignácio AR (2017) Effects of small hydropower plants on mercury concentrations in fish. Environ Sci Pollut Res 24(28):22709–22716
Yohannes YB, Ikenaka Y, Nakayama SM, Saengtienchai A, Watanabe K, Ishizuka M (2013) Organochlorine pesticides and heavy metals in fish from Lake Awassa, Ethiopia: insights from stable isotope analysis. Chemosphere 91(6):857–863
Arcagni M, Rizzo A, Juncos R, Pavlin M, Campbell LM, Arribére MA, Horvat M, Guevara SR (2017) Mercury and selenium in the food web of Lake Nahuel Huapi, Patagonia, Argentina. Chemosphere 166:163–173
Yi Y, Zhang S (2012) The relationships between fish heavy metal concentrations and fish size in the upper and middle reach of Yangtze River. Procedia Environ Sci 13:1699–1707
Sackett DK, Cope WG, Rice JA, Aday DD (2013) The influence of fish length on tissue mercury dynamics: implications for natural resource management and human health risk. Int J Environ Res Public Health 10(2):638–659
Parente T, Hauser-Davis R (2013) The use of fish biomarkers in the evaluation of water pollution. In: de Almeida EA, & de Oliveira Ribeiro, C. A. (ed) Pollution and fish health in tropical ecosystems. CRC Press, Boca Raton, pp 164–181
Ahmed AS, Sultana S, Habib A, Ullah H, Musa N, Hossain MB, Rahman MM, Sarker MSI (2019) Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PLoS One 14(10):e0219336
Jardine TD, Kidd KA, O’Driscoll N (2013) Food web analysis reveals effects of pH on mercury bioaccumulation at multiple trophic levels in streams. Aquat Toxicol 132:46–52
Lavoie RA, Hebert CE, Rail J-F, Braune BM, Yumvihoze E, Hill LG, Lean DR (2010) Trophic structure and mercury distribution in a Gulf of St. Lawrence (Canada) food web using stable isotope analysis. Sci Total Environ 408(22):5529–5539
Saros JE, Rose KC, Clow DW, Stephens VC, Nurse AB, Arnett HA, Stone JR, Williamson CE, Wolfe AP (2010) Melting alpine glaciers enrich high-elevation lakes with reactive nitrogen. Environ Sci Technol 44(13):4891–4896
Food, Nations AOotU (2012) The state of world fisheries and aquaculture. FAO Rome
SCOOP E (2004) Report from Task 3.2. 11: assessment of the dietary exposure to arsenic, cadmium, lead and mercury of the population of the EU Member States. European Commission, Directorate-General Health and Consumer Protection SCOOP report
Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M (2011) Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378(9793):815–825
Bogdanović T, Ujević I, Sedak M, Listeš E, Šimat V, Petričević S, Poljak V (2014) As, Cd, Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chem 146:197–203
Zhuang P, Z-a L, McBride MB, Zou B, Wang G (2013) Health risk assessment for consumption of fish originating from ponds near Dabaoshan mine, South China. Environ Sci Pollut Res 20(8):5844–5854
Acknowledgments
The authors are grateful to Mr. Tariq Javed, principal scientist, Isotope Analysis Division, Pakistan Institute of Nuclear Science and Technology (PINSTECH), for aiding in the stable isotopic analysis of nitrogen. In addition, we deeply thank the Department of Environmental Sciences, Fatima Jinnah Women University, Rawalpindi, for the analysis of THg at their facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• A comparative study on distribution and biomagnification of THg in freshwater lakes
• Site and species-specific differences in THg accumulation were observed.
• Feeding modes and growth parameters explained variability in concentrations.
• Trophic transfer of THg was observed with regression slopes > 0.
• No significant health risk was found.
Supplementary Information
ESM 1
(DOCX 77.5 kb)
Rights and permissions
About this article
Cite this article
Hina, N., Riaz, R., Ali, U. et al. A Quantitative Assessment and Biomagnification of Mercury and Its Associated Health Risks from Fish Consumption in Freshwater Lakes of Azad Kashmir, Pakistan. Biol Trace Elem Res 199, 3510–3526 (2021). https://doi.org/10.1007/s12011-020-02479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02479-z