Abstract
This study was carried out to evaluate the effects of selenium supplementation on glycemic control, lipid profiles, and biomarkers of inflammation and oxidative stress in patients undergoing for coronary artery bypass grafting (CABG) surgery. This randomized, double-blind, placebo-controlled trial was performed among 33 patients undergoing for CABG surgery, aged 40–85 years old. Subjects were randomly allocated into two groups to intake either 200 μg/day selenium supplements as selenium yeast (n = 17) or placebo (n = 16) for 4 weeks. Glycemic control, lipid profiles, and biomarkers of inflammation and oxidative stress were assessed at baseline and at the end of trial. After the 4-week intervention, selenium supplementation significantly decreased fasting plasma glucose (FPG) (β, 6.76 mg/dL; 95% CI, − 13.13, − 0.40; P = 0.03), insulin (β, − 1.14 μIU/mL; 95% CI, − 2.01, − 0.28; P = 0.01); homeostasis model of assessment-estimated insulin resistance (HOMA-IR) (β − 0.35; 95% CI, − 0.62, − 0.08; P = 0.01); and total-/HDL-cholesterol ratio (β − 0.31; 95% CI, − 0.51, − 0.09; P = 0.008); and significantly increased HDL-cholesterol levels (β, 2.72 mg/dL; 95% CI, 0.89, 4.55; P = 0.005) compared with the placebo. Moreover, selenium supplementation led to a significant reduction in high-sensitivity C-reactive protein (hs-CRP) (β, − 0.68 mg/L; 95% CI, − 1.18, − 0.17; P = 0.01) and malondialdehyde (MDA) (β, − 0.27 μmol/L; 95% CI, − 0.47, − 0.07; P = 0.009), and a significant elevation in total glutathione (GSH) levels (β, 77.33 μmol/L; 95% CI, 56.11, 98.55; P < 0.001) compared with the placebo. Selenium supplementation did not affect other metabolic profiles. Overall, our study demonstrated that selenium supplementation for 4 weeks to patients undergoing for CABG surgery had beneficial effects on FPG, insulin, HOMA-IR, total-/HDL-cholesterol ratio, HDL-cholesterol, hs-CRP, GSH, and MDA levels, but did not affect other metabolic profiles. Clinical trial registration number: http://www.irct.ir: IRCT2017090533941N22.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery bypass grafting (CABG) is a well-accepted therapeutic approach in people with symptomatic multivessel coronary artery disease (CAD) [1]. Based on the prior evidence, systemic inflammatory response syndrome, myocardial injury, and organ dysfunction are implicated as the most important side effects of cardiovascular surgery [2]. In addition, metabolic disturbances and chronic inflammatory state were caused by chronic disease in patients undergoing cardiac procedure contribute to the occurrence of postoperative clinical complications [3]. There is evidence indicating that a depletion of antioxidative trace elements such as selenium before cardiac operation is associated with a higher incidence of atrial fibrillation and mortality rate following surgery [4, 5]. Increased oxidative damage in cardiac surgery is accompanied by an additional reduction in the trace elements concentrations, such as selenium, copper, and zinc and subsequent reduction of selenoenzymes which may lead to postoperative multiorgan dysfunction [6].
The trace elements including selenium, copper, and zinc are essential for maintaining the oxidative balance [7]. Cardiac surgery using cardiopulmonary bypass provokes ischemia-reperfusion-mediated oxidative stress [8]. Antioxidant supplementation before cardiac surgery may prevent arterial fibrillation and reduce hospital stay [9]. Bahmani et al. [10] demonstrated that selenium supplementation for 12 weeks to patients with diabetic nephropathy had beneficial effects on insulin levels and insulin resistance, but did not affect fasting glucose, insulin sensitivity, and lipid profiles. In a meta-analysis conducted by Ju et al. [11], selenium supplementation to patients with coronary heart disease (CHD) significantly reduced C-reactive protein (CRP) levels, and significantly glutathione peroxidase activity. Moreover, administration of a new conditioning supplement containing selenium, glutamine, L-carnitine, vitamin C, and vitamin E for 7 days to nondiabetic patients scheduled for CABG led to better perioperative glycemic control and decreased post-CABG wound infection [12].
Selenoproteins have different biological functions, including regulating insulin signaling and glucose metabolism [13]. In addition, selenium may improve lipid profiles by reducing the expression of lipogenic enzyme genes [14]. Selenium also modulates the reactive oxygen species (ROS) and inhibits the nuclear factor-kappa B (NF-κB) pathway which result in decreased oxidative damage and suppressed the production of inflammatory markers [8]. This evidence highlights the significance of selenium supplementation in patients undergoing for CABG surgery. Therefore, the present study conducted to examine the effects of selenium supplementation on glucose homeostasis parameters, lipid profiles, and markers of inflammation and oxidative stress in patients undergoing for CABG surgery.
Subjects and Methods
Participants
In a randomized, double-blind, placebo-controlled trial, registered in the Iranian registry of clinical trials (http://www.irct.ir: IRCT2017090533941N22), this study was conducted at a cardiology clinic affiliated to Arak University of Medical Sciences (AUMS), Arak, Iran, between December 2017 and August 2018. Diagnosis of CAD was conducted based on the criteria of the American Heart Association [15]. Exclusion criteria were as follows: consuming selenium supplements within the last 3 months, having cardiac surgery within the past 3 months, or significant renal of hepatic failure and change in the dosage and kind of medications. This investigation was done according to the principals of the Declaration of Helsinki and the study protocol was approved by the ethics committee of AUMS. All patients were informed about the aims and protocol of the study. Written informed consent was obtained from all subjects prior to the intervention.
Study Design
At the onset of the study, to decrease potential confounding effects, all participants were stratified balanced block randomization. Stratified balanced block randomization with block size of 4 was used based on the following variables: age (< 50 vs. ≥ 50 years) and BMI (> 25 vs. ≥ 25 kg/m2). Then, participants in each block were randomly allocated into two treatment groups to intake either 200 μg selenium supplements as selenium yeast (n = 17) or placebo (n = 16) per day for 4 weeks. Patients were requested not to change their routine physical activity or usual diets throughout the study and not take any antioxidant medications or supplements during the 4-week intervention which might affect the results of the study. Selenium and its placebos were produced by Webber Naturals Company (Mississauga, Canada) and Barij Essence Pharmaceutical Company (Kashan, Iran), respectively. Both selenium supplements and placebos had similar packaging and patients and researchers were unaware of the content of the package until the end of study. Randomization assignment was performed using computer-generated random numbers. Randomization and allocation were concealed from the investigators and participants until the final analyses were completed. The randomized allocation sequence, enrolling participants, and allocating them to interventions were conducted by a trained staff member at the clinic. Compliance with the intake of supplements and placebos was determined by examining the tablet containers. In addition, participants received a daily reminder message on their cell phones to take their supplements regularly. All participants completed 3-day dietary records (two weeks’ days and one-weekend day) at week 1, 2, and 4 of the trial. The dietary records were based on estimated values in household measurements. To obtain nutrient intakes of participants according to 3-day food records, we used Nutritionist IV software (First Databank, San Bruno, CA) adopted for the Iranian food pattern. It must be kept in mind that in this study, we provided 7-day menu cycles to participants. The diets were planned using a “calorie count” system. To control for dietary intakes of participants throughout the study, the dietitian was calling the participants to resolve their probable problems. All participants spent about 45 min with a dietitian learning the basics of their diets.
Assessment of Anthropometric Measures
Weight (Seca, Hamburg, Germany) was assessed at baseline and after the 4-week intervention in cardiology clinic by a trained staff member. Height (Seca, Hamburg, Germany) was determined by a nonstretched tape measure to the nearest 0.1 cm. BMI was determined as weight in kg divided by height in meters squared.
Outcomes
Insulin levels and the homeostasis model of assessment-insulin resistance (HOMA-IR) were considered as the primary outcomes and lipid profiles, and biomarkers of inflammation and oxidative stress were considered as the secondary outcomes. Ten milliliters of fasting blood samples were collected at baseline and after the 4-week intervention at Arak reference laboratory, Arak, Iran. Fasting plasma glucose (FPG) was measured on the day of blood collection. Serum insulin levels were assessed by an ELISA kit (DiaMetra, Milano, Italy) with inter- and intra-assay coefficient variances (CVs) below 5%. HOMA-IR and the quantitative insulin sensitivity check index (QUICKI) were determined according to the standard formula [16]. Enzymatic kits (Pars Azmun, Tehran, Iran) were applied to evaluate FPG and lipid profiles with inter- and intra-assay CVs below 5%. Serum high sensitivity (hs)-CRP levels were determined by a commercial ELISA kit (LDN, Nordhorn, Germany) with inter- and intra-assay CVs below 7%. The plasma nitric oxide (NO) levels were determined using Griess method [17]. Plasma TAC levels by the method of ferric reducing antioxidant power developed by Benzie and Strain [18], total glutathione (GSH) using the method of Beutler et al. [19], and malondialdehyde (MDA) concentrations by the thiobarbituric acid reactive substances spectrophotometric test [20] were determined with inter- and intra-assay CVs below 5%.
Statistical Methods
We used the formula suggested for randomized clinical trials’ sample size calculation. Type one (α) and type two errors (β) were defined as 0.05 and 0.20 (power = 80%), respectively. According to the previous trial [10], we used 1.4 as the SD and 1.12 as the change in mean (d) of HOMA-IR as a primary outcome.
The Kolmogorov-Smirnov test was done to determine the normal distribution of variables. To detect differences in anthropometric measures and dietary intakes between the two groups, the independent-samples t test was used. Multiple linear regression models were used to evaluate treatment effects on study outcomes after adjusting for confounding parameters, including baseline values of each biochemical variable, age and BMI. The effect sizes were presented as the mean differences with 95% confidence intervals. P values < 0.05 were considered significant. The Statistical Package for Social Science version 18 (SPSS Inc., Chicago, Illinois, USA) were used for statistical analyses of this trial.
Results
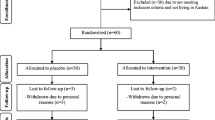
Firstly, we invited 48 participants; however, 8 subjects were excluded due to not meeting inclusion criteria (Fig. 1). Among participants in the selenium group, 3 persons (withdrawn due to personal reasons (n = 3)) and in the placebo group, 4 participants (withdrawn due to personal reasons (n = 4)) did not complete the trial. Finally, 33 participants (selenium (n = 17) and placebo (n = 16)) completed the trial. The rate of compliance in our study ranged between 90 and 100% in both groups. No side effects were reported after selenium supplementation in patients undergoing for CABG surgery throughout the study.
Mean age, weight, and BMI at baseline after the 4-week intervention of study participants were not statistically different between selenium and placebo groups (Table 1). In addition, consumption of antidiabetic and antilipidemic drugs, consumption of angiontensin-converting enzymes inhibitors (captopril and enalapril), aldosterone receptor blockers drugs, and blocker drugs (β- blocker: metoprolol and calcium channel blocker: amlodipin) at baseline after the 4-week intervention of study participants were not statistically different between the two groups.
Based on the 3-day dietary records obtained throughout the intervention, no significant difference was observed between the two groups in terms of micro- and macronutrients (data not shown).
After the 4-week intervention, selenium supplementation significantly decreased FPG (β, 6.76 mg/dL; 95% CI, − 13.13, − 0.40; P = 0.03); insulin (β, − 1.14 μIU/mL; 95% CI, − 2.01, − 0.28; P = 0.01); HOMA-IR (β, − 0.35; 95% CI, − 0.62, − 0.08; P = 0.01); and total-/HDL-cholesterol ratio (β, − 0.31; 95% CI, − 0.51, − 0.09; P = 0.008); and significantly increased HDL-cholesterol levels (β, 2.72 mg/dL; 95% CI, 0.89, 4.55; P = 0.005) compared with the placebo (Table 2). Moreover, selenium supplementation led to a significant reduction in hs-CRP (β, − 0.68 mg/L; 95% CI, − 1.18, − 0.17; P = 0.01) and MDA (β, − 0.27 μmol/L; 95% CI, − 0.47, −0.07; P = 0.009), and a significant elevation in GSH levels (β, 77.33 μmol/L; 95% CI, 56.11, 98.55; P < 0.001) compared with the placebo. Selenium supplementation did not affect other metabolic profiles.
Discussion
In the present study, we found that selenium supplementation for 4 weeks to patients undergoing for CABG surgery had beneficial effects on FPG, insulin, HOMA-IR, total-/HDL-cholesterol ratio, HDL-cholesterol, hs-CRP, GSH and MDA levels, but did not affect other metabolic profiles.
Effect on Glycemic Control
CAD is accompanied by metabolic derangement, including poor glycemic control [21], atherogenic dyslipidemia [22], inflammatory state, and disrupted antioxidant defense system [23]. The current study showed that selenium supplementation for 4 weeks to patients undergoing for CABG surgery significantly decreased FPG, insulin levels and HOMA-IR, but did not affect QUICKI score. In a study conducted by Wang et al. [24], dietary selenium intake was negatively correlated with insulin resistance. In addition, a 12-week selenium supplementation to subjects with congestive heart failure (CHF) significantly reduced insulin concentrations and HOMA-IR, and significantly increased QUICKI score [25]. Although in patients with metabolic syndrome, selenium supplementation was not effective in reducing HOMA-IR [26]. Preoperative glycemic control can predict insulin sensitivity during cardiac surgery which is associated with major complications after surgery [27]. Several pathways involving selenium are known to influence insulin homeostasis. Selenium may induce insulin function by the activation of kinase involved in the insulin signaling cascade [28]. In addition, selenium increases peroxisome proliferator activated receptor gamma mRNA expression which is the key mediator of insulin sensitivity and influence the glucose and lipid uptake in peripheral tissue [29].
Effect on Lipid Profiles
We found that taking selenium supplements for 4 weeks by patients undergoing for CABG surgery led to a significant increase in HDL-cholesterol levels and a significant reduction in total-/HDL-cholesterol ratio, but did not affect other lipid profiles. Similarly, our previous study indicated that selenium supplementation at a dosage of 200 μg/day for 12 weeks to patients with CHF significantly improved HDL-cholesterol levels [25]. In addition, long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10, and selenium) in patients with multiple cardiovascular risk factors increased HDL-cholesterol levels, but did not influence other lipid profiles [30]. Rayman et al. [31] also reported that 300 μg/day selenium supplementation for 6 months to healthy volunteers with low selenium levels significantly increased HDL-cholesterol levels. However, a 3-month selenium supplementation to hemodialysis patients with selenium deficiency had no beneficial effect on lipid profiles [32]. HDL-cholesterol exhibit protective effects on endothelial cells. In addition, HDL-cholesterol is inversely associated with the risk of myocardial infarction and death [33]. Selenium may increase HDL-cholesterol levels through positive effect on the expression of apolipoprotein A-1 expression which provides the structure to the HDL particle and mediates the esterification of cholesterol during reverse cholesterol transport [29].
Effect on the Biomarkers of Inflammation and Oxidative Stress
We found that selenium administration for 4 weeks to patients undergoing for CABG surgery significantly increased plasma GSH, and significantly decreased serum hs-CRP and plasma MDA levels. Consistent with our findings, selenium supplementation to patients with hemodialysis for 3 months significantly reduced MDA concentrations [34]. Moreover, selenium supplementation alone or with topiramate for 45 days to subjects with epilepsy increased erythrocyte GSH levels [35]. In addition, in a meta-analysis conducted by Ju et al. [11], selenium supplementation to people with CHD significantly reduced CRP levels. However, a 12-week selenium supplementation to patients with diabetic nephropathy significantly increased GSH, but did not affect MDA and hs-CRP levels [36]. Inflammation and oxidative stress are related to the pathogenesis of atherosclerosis [37], and increase the risk of postoperative atrial fibrillation and graft failure [38, 39]. In patients with cardiac disease, GSH deficiency is associated with functional status and the structural cardiac abnormalities [40]. In addition, elevated CRP levels are associated with higher levels of complement protein which predict the development of atrial fibrillation [41]. Selenoproteins exert a direct inhibitory effect on the binding of NF-κB transcription factor to DNA through a reversible rupture of disulfide bridge fixation leading to decreased inflammation [42]. Selenoenzymes also take part in the cellular defense against oxidative stress through reducing free radicals and the regeneration of other antioxidants [28].
The current study had few limitations. The sample size was small. Future studies with longer duration of the intervention, and bigger sample size are needed to confirm the validity of our findings. In addition, we could not evaluate plasma selenium levels before and after the intervention.
Conclusions
Overall, our study supported that selenium supplementation for 4 weeks to patients undergoing for CABG surgery had beneficial effects on FPG, insulin, HOMA-IR, total-/HDL-cholesterol ratio, HDL-cholesterol, hs-CRP, GSH, and MDA levels, but did not affect other metabolic profiles.
References
Salzberg SP, Adams DH, Filsoufi F (2005) Coronary artery surgery: conventional coronary artery bypass grafting versus off-pump coronary artery bypass grafting. Curr Opin Cardiol 20:509–516
Murphy GJ, Angelini GD (2004) Side effects of cardiopulmonary bypass: what is the reality? J Card Surg 19:481–488
Charlesworth DC, Likosky DS, Marrin CA et al (2003) Development and validation of a prediction model for strokes after coronary artery bypass grafting. Ann Thorac Surg 76:436–443
McDonald C, Fraser J, Shekar K, Clarke A, Coombes J, Barnett A, Pearse B, Fung L (2016) Low preoperative selenium is associated with post-operative atrial fibrillation in patients having intermediate-risk coronary artery surgery. Eur J Clin Nutr 70:1138–1143
Koszta G, Kacska Z, Szatmari K, Szerafin T, Fulesdi B (2012) Lower whole blood selenium level is associated with higher operative risk and mortality following cardiac surgery. J Anesth 26:812–821
Stoppe C, Schalte G, Rossaint R et al (2011) The intraoperative decrease of selenium is associated with the postoperative development of multiorgan dysfunction in cardiac surgical patients. Crit Care Med 39:1879–1885
Klotz LO, Kroncke KD, Buchczyk DP, Sies H (2003) Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr 133:1448s–1451s
Benstoem C, Goetzenich A, Kraemer S, Borosch S, Manzanares W, Hardy G, Stoppe C (2015) Selenium and its supplementation in cardiovascular disease--what do we know? Nutrients 7:3094–3118
Ali-Hassan-Sayegh S, Mirhosseini SJ, Rezaeisadrabadi M, Dehghan HR, Sedaghat-Hamedani F, Kayvanpour E, Popov AF, Liakopoulos OJ (2014) Antioxidant supplementations for prevention of atrial fibrillation after cardiac surgery: an updated comprehensive systematic review and meta-analysis of 23 randomized controlled trials. Interact Cardiovasc Thorac Surg 18:646–654
Bahmani F, Kia M, Soleimani A, Asemi Z, Esmaillzadeh A (2016) Effect of selenium supplementation on glycemic control and lipid profiles in patients with diabetic nephropathy. Biol Trace Elem Res 172:282–289
Ju W, Li X, Li Z, Wu GR, Fu XF, Yang XM, Zhang XQ, Gao XB (2017) The effect of selenium supplementation on coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol 44:8–16
Akbarzadeh M, Eftekhari MH, Shafa M, Alipour S, Hassanzadeh J (2016) Effects of a new metabolic conditioning supplement on perioperative metabolic stress and clinical outcomes: a randomized, placebo-controlled trial. Iran Red Crescent Med J 18:e26207. https://doi.org/10.5812/ircmj.26207
Jablonska E, Reszka E, Gromadzinska J et al (2016, 2016) The effect of selenium supplementation on glucose homeostasis and the expression of genes related to glucose metabolism. Nutrients 8(12)
Dhingra S, Bansal MP (2006) Modulation of hypercholesterolemia-induced alterations in apolipoprotein B and HMG-CoA reductase expression by selenium supplementation. Chem Biol Interact 161:49–56
Luepker RV, Apple FS, Christenson RH et al (2003) Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 108:2543–2549
Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT (2013) Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care 36:845–853
Tatsch E, Bochi GV, Pereira Rda S et al (2011) A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem 44:348–350
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Beutler E, Gelbart T (1985) Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med 105:581–584
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9:515–540
Bornfeldt KE, Tabas I (2011) Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 14:575–585
Lahoz C, Mostaza JM, Tranche S, Martin-Jadraque R, Mantilla MT, López-Rodriguez I, Monteiro B, Sanchez-Zamorano MA, Taboada M (2012) Atherogenic dyslipidemia in patients with established coronary artery disease. Nutr Metab Cardiovasc Dis 22:103–108
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Wang Y, Lin M, Gao X, Pedram P, du J, Vikram C, Gulliver W, Zhang H, Sun G (2017) High dietary selenium intake is associated with less insulin resistance in the Newfoundland population. PLoS One 12:e0174149. https://doi.org/10.1371/journal.pone.0174149
Raygan F, Behnejad M, Ostadmohammadi V, Bahmani F, Mansournia MA, Karamali F, Asemi Z (2018) Selenium supplementation lowers insulin resistance and markers of cardio-metabolic risk in patients with congestive heart failure: a randomised, double-blind, placebo-controlled trial. Br J Nutr 120:33–40
Tabrizi R, Akbari M, Moosazadeh M, Lankarani KB, Heydari ST, Kolahdooz F, Mohammadi AA, Shabani A, Badehnoosh B, Jamilian M, Assarian A, Asemi Z (2017) The effects of selenium supplementation on glucose metabolism and lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 49:826–830
Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T (2010) The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 95:4338–4344
Steinbrenner H (2013) Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic Biol Med 65:1538–1547
Donma MM, Donma O (2016) Promising link between selenium and peroxisome proliferator activated receptor gamma in the treatment protocols of obesity as well as depression. Med Hypotheses 89:79–83
Shargorodsky M, Debby O, Matas Z, Zimlichman R (2010) Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab (Lond) 7:55. https://doi.org/10.1186/1743-7075-7-55
Rayman MP, Stranges S, Griffin BA, Pastor-Barriuso R, Guallar E (2011) Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Ann Intern Med 154:656–665
Omrani H, Golmohamadi S, Pasdar Y, Jasemi K, Almasi A (2016) Effect of selenium supplementation on lipid profile in hemodialysis patients. J Renal Inj Prev 5:179–182
Luscher TF, von Eckardstein A, Simic B (2012) Therapeutic targets to raise HDL in patients at risk or with coronary artery disease. Curr Vasc Pharmacol 10:720–724
Salehi M, Sohrabi Z, Ekramzadeh M, Fallahzadeh MK, Ayatollahi M, Geramizadeh B, Hassanzadeh J, Sagheb MM (2013) Selenium supplementation improves the nutritional status of hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Nephrol Dial Transplant 28:716–723
Yurekli VA, Naziroglu M (2013) Selenium and topiramate attenuates blood oxidative toxicity in patients with epilepsy: a clinical pilot study. Biol Trace Elem Res 152:180–186
Bahmani F, Kia M, Soleimani A, Mohammadi AA, Asemi Z (2016) The effects of selenium supplementation on biomarkers of inflammation and oxidative stress in patients with diabetic nephropathy: a randomised, double-blind, placebo-controlled trial. Br J Nutr 116:1222–1228
Siti HN, Kamisah Y, Kamsiah J (2015) The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc Pharmacol 71:40–56
Zakkar M, Ascione R, James AF, Angelini GD, Suleiman MS (2015) Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol Ther 154:13–20
Gaudino M, Antoniades C, Benedetto U, Deb S, di Franco A, di Giammarco G, Fremes S, Glineur D, Grau J, He GW, Marinelli D, Ohmes LB, Patrono C, Puskas J, Tranbaugh R, Girardi LN, Taggart DP, Ruel M, Bakaeen FG (2017) Mechanisms, consequences, and prevention of coronary graft failure. Circulation 136:1749–1764
Damy T, Kirsch M, Khouzami L, Caramelle P, le Corvoisier P, Roudot-Thoraval F, Dubois-Randé JL, Hittinger L, Pavoine C, Pecker F (2009) Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PLoS One 4:e4871. https://doi.org/10.1371/journal.pone.0004871
Dernellis J, Panaretou M (2006) Effects of C-reactive protein and the third and fourth components of complement (C3 and C4) on incidence of atrial fibrillation. Am J Cardiol 97:245–248
Forceville X (2006) Seleno-enzymes and seleno-compounds: the two faces of selenium. Crit Care 10:180
Funding
The current study was founded by a grant from the Vice-chancellor for Research, AUMS, and Iran.
Author information
Authors and Affiliations
Contributions
ZA contributed in conception, design, statistical analysis and drafting of the manuscript. AK and EA contributed in data collection and manuscript drafting. Z.A. supervised the study.
Corresponding author
Ethics declarations
This investigation was done according to the principals of the Declaration of Helsinki and the study protocol was approved by the ethics committee of AUMS. All patients were informed about the aims and protocol of the study. Written informed consent was obtained from all subjects prior to the intervention.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamali, A., Amirani, E. & Asemi, Z. Effects of Selenium Supplementation on Metabolic Status in Patients Undergoing for Coronary Artery Bypass Grafting (CABG) Surgery: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res 191, 331–337 (2019). https://doi.org/10.1007/s12011-019-1636-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-1636-7