Abstract
Signal crayfish (Pacifastacus leniusculus) is an invasive species displacing native European crayfish from their natural habitats. The elemental composition of the population from the southern Baltic coastal river and the potential health hazards are not known. The aim of the conducted research was to assess the quantitative content of Al, As, Ca, Cd, Cu, Fe, K, Mg, Na, Ni, Pb, Se, and Zn in meat, hepatopancreas, and exoskeleton in a population from Wieprza River (Poland) and compare the results with the recommendations of daily human consumption. Analysis also involved the composition of water and sediments. The concentrations of elements were analyzed using an Atomic Absorption Spectrometer. The bioconcentration factor (BCF) of elements in the signal crayfish was much higher from water than from sediments. Bioaccumulation of elements differed between the particular parts of the body of crayfish, e.g., Ca showed extreme predominance in the exoskeleton, while in meat exhibited a predominance of K, Na, Ca, and Mg. Among trace elements, crayfish meat was the richest in Zn, Cu, and Fe. The concentrations of non-essential Cd, Pb, and As were low compared to other determined elements. The highest concentrations of As, Cd, Cu, Fe, Ni, and Se were found in the hepatopancreas, while the highest levels of Al and Pb were found in the exoskeleton. Generally, it was found that the meat of P. leniusculus can be a perfect supplement to the human diet, and the consumption of 100 g of meat per day did not exceed the dietary reference values for essential elements and also for Al, As, Cd, Ni, and Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Similar to fish, edible crustaceans (crabs, shrimps, crayfish, or lobsters) are a valuable source of macroelements and microelements essential for humans, such as Ca, K, Mg, Na, Fe, Cu, Zn, Se, Al, and Ni. They may also contain toxic heavy metals (Cd, Pb) and hypothetically toxic elements (such as As). It should be remembered that after exceeding the levels necessary to maintain biochemical balance, heavy metals included in the group of essential elements (Zn, Cu, Al) may also pose a threat to health and life. Therefore, it is advisable to determine and monitor the elemental composition of crustaceans [1,2,3,4].
Of the five native European crayfish species (Souty-Grosset et al. 2006), only two, the noble crayfish (Astacus astacus) and Danube crayfish (Astacus leptodactylus), have inhabited the waters of Poland. As recently as the 1920s, Poland was the second largest exporter of crayfish for consumption in Europe, i.e., after Russia. However, as a result of the crayfish plague and its high sensitivity to pollution, these species are currently very rare in Poland and under strict protection [5].
Around 1860, the American spiny-cheek crayfish (Orconectes limosus) was introduced to European waters, initially in a fish farm in Barnówko on the Myśla River (N-W Poland). The species quickly reached other surface waters [6] and is currently the most common crayfish in European waters [5].
Signal crayfish (Pacifastacus leniusculus) were imported to Europe in 1970 because of their high commercial value. Currently, it is present at nearly 40 sites in Poland, with the largest population in the Wieprza river basin [5]. The species spreads via the Wieprza River towards the Baltic brackish waters. It is expected to soon reach the Polish coastal waters of the Baltic Sea and other coastal rivers [7]. Importantly, signal crayfish displaces the native noble crayfish due to its greater aggressiveness, height, and fertility and is also a carrier of the crayfish plague [8]. In addition, it is a typical trophic opportunist; it feeds on microzooplankton, perifitone, detritus, and macrophytes [9].
The high nutritional value of signal crayfish [10], and at the same time the need to reduce its population [11], suggests the possibility of intensive commercial catches. However, the tissues of crustaceans may accumulate high quantities of metals (both essential and non-essential) due to contamination of their benthic habitats [12, 13]. Bioaccumulation of metals in the body of signal crayfish, similar to other benthic crustaceans, probably occurs via absorption from the water, sediments, and ingestion of food [14]. Essential metals, such as Zn, Cu, Na, Mg, and Mn, which have many biological roles and are necessary for the proper functioning of the organism, can be toxic in elevated quantities [15, 16]. Non-essential metals as Pb and Hg do not play any role in metabolism and are toxic even at low concentrations [12].
Crustaceans are also good bioindicators of the quality of the environment [3, 17] and may be unsuitable for human consumption if metal standards are exceeded [18]. As P. leniusculus is considered a gastronomic homologue of A. astacus in Europe [7], the lack of data on the content of metals in the meat of these crustaceans in the southern Baltic coastal rivers is all the more surprising.
Given the above, the aim of the conducted research was to (i) assess the content of macroelements and microelements in meat collected from the abdomen and claws, the hepatopancreas and exoskeleton of female and male signal crayfish colonizing the lower part of the Wieprza River and (ii) compare the determined concentrations of elements with recommendations for their daily intake (dietary reference intakes for Ca, Cu, Fe, K, Mg, Na, Se, and Zn and acceptable daily intake for Al, As, Cd, Ni, and Pb).
Material and Methods
Study Area
The Wieprza is one of the largest rivers flowing into the central part of the southern Baltic (length 125 km, river basin area 2170 km2). The stream slope in its upper course is 3.09–6.53 PSU (practical salinity unit), and the bottom consists mainly of rocks and gravel. In the lower parts of the stream, the slope does not exceed about 0.5 PSU, and gravel and sand dominate the bottom. The Wieprza, compared to other rivers of the southern Baltic, is considered to have high-quality water.
Sampling Procedure
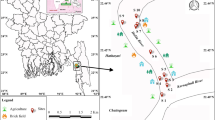
Signal crayfish (12 males, 8 females) were caught between August and September 2017 using fyke nets, at distances from 1.5 to 6.5 km of the mouth of the Wieprza River to the Baltic Sea (Fig. 1). Each crayfish was properly cleaned by rinsing with deionized water to remove debris, plankton, and other external adherents. Immediately after cleaning, the crayfish were stored in a container, preserved in crushed ice, and then transferred to the laboratory and frozen at − 20 °C until analyzed.
Water samples were collected in polyethylene bottles from 4 sites near places where the crayfish were collected. At each sampling site, the polyethylene sampling bottles were rinsed at least three times before sampling was done. The cleaned bottles were immersed about 0.5 m below the water surface. After filling, the bottles were transported to the laboratory in a cooler box.
Surface sediment samples (0–10 cm) were taken from the same 4 sites as the water using a Peterson grab sampler and then kept on ice before being transported to the laboratory. Furthermore, all the samples were kept away from metallic materials to avoid contamination.
Elemental Analysis
Four tissue samples (abdomen and claw meat, hepatopancreas and exoskeleton) were collected from each signal crayfish. Elemental composition was determined separately in each collected sample (i.e., 4 samples per each crayfish). Samples of 1.000 ± 0.01 g (wet weight) were digested in 10 mL concentrated HNO3 (ultra pure, Merck, Germany) in a high-pressure microwave digester (Speedwave Xpert, Bergoff, Eningen Germany). After digestion, the samples were diluted with Milli-Q (18.2 MΩ) water to 25 mL.
Water samples from the River Wieprza were digested with concentrated HNO3, at a volume ratio of 10:1. Bottom sediments, after drying to a constant weight at 90 °C, were sieved through a 2-mm sieve. The sediment fraction thus obtained was digested with concentrated HNO3, at a ratio of 2 g dry weight of sediment to 5 mL of HNO3. Digestion time is 30 min, and temperature is 200 °C. The resulting solution was diluted with deionized water to 25 mL.
The elements were determined using a Hitachi ZA3000 Series Polarized Zeeman Atomic Absorption Spectrometer (Hitachi High-Technologies Corporation, Tokyo, Japan). Ca, K, Na, and Mg were determined by flame atomic absorption spectroscopy (FAAS) in an air-acetylene flame. The concentrations of Al, As, Cd, Cu, Fe, Ni, Pb, and Zn were measured using a graphite furnace atomic absorption spectroscopy (GFAAS). Calibration curves were established using certified standard solutions (1000 mg/L) from Scharlau (Spain) for Mg, Ca, K, Na, and Fe and from Merck (Germany) for Al, As, Cd, Cu, Ni, Pb, and Zn.
Accuracy Tests
The analytical method was tested using the reference material Fish muscle ERM-BB422 (European Reference Materials, European Commission—Joint Research Center, Institute for Reference Materials and Measurements, Geel, Belgium). The recovery of elements was 95–105%; the accuracy for the reference material was 95% (for Mg, As), 96% (for Cd, Se, and Fe), 102% (for Ca), 101% (for K and Cu), and 105% (for Na, and Zn).
Body Condition Index
Each caught signal crayfish was measured (TL—total length from tip of rostrum to margin of telson, ± 1 mm) with a caliper, and wet weight (± 0.1 g) was determined using a portable scale (RADWAG WPE 300). According to Maguire and Klobučar [19], body condition indices were calculated by Fulton Condition Factor (FCF), according to the equation:
where W—weight of the signal crayfish (g); TL—total length of signal crayfish (cm). Body condition indices (FCF) of the males and females were used to assess potential inter-gender differences in condition.
Bioconcentration Factor
The accumulation of elements in crayfish tissue from the Wieprza River was measured using bioconcentration factor (BCF). According to Jitar et al. [20] and Vrhovnik et al. [21], BCF is defined as follows:
where Cb—concentration of elements in the meat, hepatopancreas or exoskeleton; C—concentration of elements in sediments or water.
Nutritional Quality and Potential Risks to Consumers
In order to assess the nutritional quality and potential risk of signal crayfish consumption, the intake of elements per 100 g of wet weight of meat from the abdomen and claws of the male and female crayfish was estimated, similar to Barrento et al. [1] who used the procedures of the Food and Agriculture Organization of the United Nations, the World Health Organization of the United Nations, and the US Food and Drug Administration. We calculated the average intake (AI) of each element per 100 g wet weight edible portion of meat and compared (i) K, Na, Ca, Mg, Zn, Cu, Fe, and Se levels with the dietary reference intake (DRI) of adult females and males aged between 9 and 70 years set per day by the Food and Nutrition Board, Institute of Medicine, US National Academy of Science [22] and (ii) Al, Ni, Pb, Cd, and As levels with acceptable daily intake (ADI), as described by Salahinejad and Aflaki [23].
Statistical Analysis
Differences in element concentrations between the sexes, tissues, water, and sediments were tested with analysis of variance (ANOVA) and Tukey post-hoc test. The differences were considered significant at P < 0.05. Correlations between the length and weight of signal crayfish were tested with a Pearson's correlation coefficient, at a confidence level α = 0.05. Statistical analysis was performed using Statistica 12.0 software [24].
Results
Water and Sediment
The waters in the signal crayfish fishing area were most abundant in Ca (36.6 ppm), then Na (4.4 ppm), Mg (2.72 ppm), and K (1.28 ppm). Concentrations of other elements ranged from 0.0002 ppm (Se and Cd) to 0.091 ppm (Fe) in the following descending series: Fe > Zn > Al > Ni > Cu > Pb > As > Se = Cd (Table 1).
The bottom sediments of the Wieprza river had significantly higher (P < 0.05) metal concentrations than water (except Ca, P = 0.3662). The sediments were most abundant in Fe (3033 ppm), Al (1210 ppm), Mg (836 ppm), and K (501 ppm). Concentrations of other elements ranged from 0.0057 ppm (Se) to 44.6 ppm (Ca) in the following descending series: Ca > Na > Na > Zn > Pb > Cu > Ni > As > Cd > Se (Table 1).
Generally, the concentrations of elements recorded in the waters were significantly lower (P < 0.05) than their concentrations in the signal crayfish tissues. Concentrations of Al, As, Fe, Ni, and Pb in sediments were significantly higher (P < 0.05) than in the signal crayfish tissues. Only Cd concentration in the tissues and Mg concentration in the exoskeleton did not differ significantly from their concentrations in sediments (P = 0.1159; P = 0.9877, respectively) (Supplementary material—Table S1).
Body of Signal Crayfish
Male signal crayfish were characterized by a statistically significantly longer length (TL) and body mass compared to the females (Table 2). We found a significant linear correlation between these parameters, both in females (r = 0.60) and males (r = 0.89). Body condition (FCF) was significantly different between the sexes (Table 2).
Elements in the Body of Signal Crayfish
We determined a very wide range of concentrations of elements in the tested parts of the body of the signal crayfish, from 0.0028 ppm (Pb in the meat from male claws) to 192,500 ppm (Ca in the female exoskeleton) (Table 3). Generally, the elements could be arranged in a descending order as shown in Table S2. On this basis, three trends could be observed.
The first trend was observed in the meat from the abdomen and claw without division into sex. In this group, the order of all elements was always in the same descending order (K > Na > Ca > Mg > Zn > Cu > Fe > Al > Ni > Se > As > Cd > Pb). In meat from male and female claws, the differences between elements were always significant. However, in the meat from male abdomens, Cu, Fe, and Al concentrations did not differ significantly (PCu/Fe = 0.3122; PCu/Al = 0.2705; PFe/Al = 0.8904), and in the meat of female abdomens, there was no statistically significant difference between Cu and Fe (P = 0.0611), between Fe and Al (P = 0.5124), and between As and Cd (P = 0.0536).
The second trend could be observed in hepatopancreas, both in males (M-Hp) and females (F-Hp), for which K and Na concentrations did not differ significantly (P = 0.8112 for M-Hp, P = 0.8466 for F-Hp). There was also a shift of Fe in the series, and its concentration did not differ significantly from the concentration of Mg (P = 0.2729 for M-Hp, P = 0.0754 for F-Hp). At the same time, there were no significant differences between the Zn and Cu (P = 0.1667), Ni and Se (P = 0.0697), and Se and Cd (P = 0.4481) in the hepatopancreas of the females.
The third trend was observed in the exoskeleton of the crayfish, in which Ca was in first place, and Se and Pb changed places in comparison to the order in the meat of crayfish. A common feature of the male (M-Ex) and female exoskeletons (F-Ex) was the lack of significant differences in concentrations between Cu and Zn (P = 0.8596 for M-Ex, P = 0.09930 for F-Ex) and As and Cd (P = 0.1074 for M-Ex, P = 0.5525 for F-Ex). In addition, there were no significant differences in concentrations between K and Na (P = 0.0527), Fe and Al (P = 0.6555), Ni and Pb (P = 0.9368), and As and Se (P = 0.0598) in the female exoskeleton.
Macroelements
The signal crayfish body was generally dominated by macroelements, with Ca, Mg, and Na most abundant in the exoskeleton Ca levels in the exoskeleton were about 100 times higher than K or Na and over 200 times higher than Mg (Table 3). The highest concentrations of K were recorded in the meat from the abdomen of both males and females (detailed order of the various parts of the body in terms of the content of macroelements is shown in Table S2).
When comparing the crayfish meat, we showed that the highest concentrations were recorded for K and the lowest for Mg (Table 3). The meat taken from male claws did not differ significantly in the macroelement levels from the meat from female claws (Table 3). In the meat of male abdomen, the concentrations of Mg and Na were significantly higher than in females (P = 0.023992 and P = 0.02159, respectively), while for Ca, the differences were opposite (P = 0.00777), and for K the concentration, differences were not significant (Table 3).
It was also found that in meat taken from male claws there was significantly less K than in meat from their abdomens (P = 0.000681). However, in meat taken from female claws, there was significantly more Na than in meat from their abdomens (P = 0.04356) (Table S3). The concentrations of the remaining macroelements did not differ significantly in the compared samples of meat collected from the claws and abdomen both in males and females (Table S3).
In the hepatopancreas (see Table S2), K and Na both dominated, and concentrations of these elements, as well as Ca and Mg, were usually significantly higher in the hepatopancreas of females than males (Table 3). Sodium concentration was usually significantly higher in the hepatopancreas of the crayfish than in meat, and K concentration was significantly lower in the male hepatopancreas than in meat (Table S3).
Trace Elements
The trace elements were characterized by a wide range of concentrations and high variability between the tested body parts of the crayfish (Table 3). The microelements could be divided into three groups. The first group included Al, Cu, Fe, and Zn; the second group for Ni and Se; and the third group for As, Cd, and Pb.
In the first group, we included trace elements with the highest concentrations, and the range of concentrations ranged from 2.19 ppm (Al in meat of male claws) to 221 ppm (Fe in the hepatopancreas of males) (Table 3). Generally, in the meat of the crayfish, the predominant element was Zn (over 50 ppm in the claws and about 20 ppm in the abdomen), and Fe was dominant in the hepatopancreas and the exoskeleton (about 200 ppm and 100 ppm, respectively). In the hepatopancreas, there was also a high concentration of Cu and Zn (about 20 ppm and 36 ppm, respectively). In contrast, the exoskeleton, along with Fe, was also rich in Al (around 80 ppm). In addition, concentrations of Al, Cu, Fe, and Zn in the remaining parts of the body ranged from about 2 ppm to about 10 ppm (Table 3).
In the group consisting of Ni and Se, higher concentrations were recorded for Ni (from 0.184 ppm in females exoskeleton to 0.995 ppm in male hepatopancreas) and lower for Se (from 0.012 ppm in male exoskeleton to 0.258 ppm in the hepatopancreas of females) (Table 3). Comparing samples taken from males with samples collected from females, it was shown that in the case of Se only, the female exoskeleton was significantly more abundant in this element than the male exoskeleton. In the case of Ni, significantly higher concentrations were noted in the hepatopancreas and exoskeleton of males than females, while the meat of female claws had significantly more Ni than meat of male claws (Table 3).
In the third group, included As, Cd, and Pd, and their concentrations oscillated in the ranges 0.033–0.079 ppm for As, 0.010–0.214 ppm for Cd, and 0.0028–0.1816 ppm for Pb (Table 3). In general, however, it can be pointed out that in this trace element group, As was dominant in meat, Cd in the hepatopancreas, and Pb in the exoskeleton (Table S2). For As we did not show any significant differences between analogous parts of the body of males and females. However, in the case of Cd and Pb, significantly higher concentrations of these elements were noted in the exoskeleton of females than males (for Cd) and in the abdominal meat of females compared to males (for Pb) (Table 3).
Based on the analysis presented in Table S4, comparing different parts of the body, the following relationships can be indicated:
- The hepatopancreas was the most abundant in the majority of the determined trace elements, and the concentrations of individual elements were usually significantly higher than their concentrations in the remaining parts of the crayfish. The exception was As and Ni determined in the female body. Arsenic showed no significant differences between the tested parts of females. In the case of Ni, its concentration in the hepatopancreas of the females was significantly higher only in relation to the concentration recorded in their exoskeleton.
-
Aluminum and Pb had the highest levels in the exoskeleton, significantly higher than those in other parts of the body (significant differences in these elements were not demonstrated by comparing the hepatopancreas and meat samples).
-
Individual body parts of the crayfish significantly differed in Zn levels.
-
Trace elements concentrations (except Zn) determined in the abdominal meat did not significantly differ from the concentrations indicated in claw meat.
-
Concentrations of As, Cd, Cu, and Ni (Ni only for male samples) determined in the exoskeleton did not significantly differ from the concentrations of these elements in the meat of crayfish.
Bioconcentration Factor
Table 4 shows the calculated BCF for the individual body parts of the signal crayfish. The body/water ratio of BCF values for all elements exceeded 1, the threshold level. At the same time, the body parts differed in the degree of accumulation. Generally, accumulation of most elements was lowest in the meat. This applied especially to As, Ca, Cd, Fe, and Pb (BCF at 49, 21, 84, 53, and 4, respectively). By comparison, BCF values in hepatopancreas were 110 (As), 940 (Cd), and 2154 (Fe) and in the exoskeleton 5178 (Ca) and 130 (Pb).
BCF body/sediment ratios were distinctly lower and did not exceed 1 for Al, As, Fe, Mg, Ni, and Pb. At the same time, it should be emphasized that for Cd the BCF meat/sediment ratio was 0.764, the hepatopancreas/sediment ratio was 8.5, and the exoskeleton/sediment ratio was 2.1
Comparison with Daily Intake Recommendations
As shown in Table 5, the consumption of a portion of 100 g of signal crayfish meat from the Wieprza River meets 3 to 10% of the dietary reference intake (DRI) for K, Ca, and Fe and a slightly higher DRI for Mg, Na, and Se (from approx. 10 to 30%). In the case of Zn DRI, it was lower for meat from abdomen of males and females (about 23%) and much higher for meat with their claws (over 60%). Only in the case of Cu was DRI exceeded, with the range from 88 to 164% of DRI.
Acceptable daily intake (ADI) was the lowest for Al and Pb (maximum 1.0% and 0.5% respectively). In the case of other elements, ADI was higher and on average was 1.7% (Cd), 2.6% (As), and 3.0% for Ni (Table 5).
Discussion
Water and Sediments
The Wieprza River, similarly to other coastal rivers (e.g., [25]), is characterized by good water quality. According to [26], its biological and physicochemical parameters place it second in purity. Its bottom sediments have been designated 1st class purity, and only occasionally do they have a harmful effect on living organisms. This study confirms that the elemental composition of the water and bottom sediments of the Wieprza River were typical for the rivers of this region, with low concentrations of Cu, Cd, Pb, Ni, and Zn, reflecting the low level of anthropopressure, similar to the study by Pokorny et al. [27].
Body of the Signal Crayfish
Invasive crayfish species are considered to be some of the most extensively distributed aquatic invasive species worldwide. In Europe, the signal crayfish (Pacifastacus leniusculus; Dana) is one of the most widespread and successful non-native crayfish species [28]. In the Baltic Sea basin, it is the largest water crustacean. It attains a total length of 180 mm [29], although some individuals may reach a length of over 200 mm. In Polish surface waters, caught individuals are usually from 110 to 140 mm long, and only about 5% of individuals are longer [30]. In this study, the average total length of caught crayfish was about 120 mm, with statistically significant differences between males and females in total length, weight, and condition index. Higher weight and condition index in males have also been observed in various crustacean populations [31], resulting from morphometric differences associated with sexual dimorphism, manifesting, inter alia, in larger size and weight of the male claws [32]. The larger size of males is related to the fact that the size of body is one of the important determinants of agonistic success and social status in crayfish.
Macroelements in the Crayfish Tissue
In our research, we found a very wide range of macroelement and microelement concentrations in the studied signal crayfish. Such differentiation has also been demonstrated in the body of other species of crayfish [12, 17, 33, 34] as well as other crustaceans [13, 35,36,37,38].
Generally, Ca, Mg, Na, and K are the dominant macroelements in crustacean meat. They perform a number of important functions in living organisms, e.g., K is primarily an intracellular cation bound to protein and alongside with Na exerts tremendous influence on the protein molecule it is bound to [36]. The range of concentrations of these elements in crustaceans is very wide. For example, samples containing both meat and exoskeleton of Uca tangeri from Cross River (Nigeria) had 7180 ppm Ca, 7670 ppm Mg, 6300 ppm Na, and 6060 ppm K [36]. In contrast, the meat of Sudananautes africanus africanus from River Osun (Nigeria) contained 330 ppm Ca, 283 ppm Mg, 260 ppm Na, and 300 ppm K [35]. The meat of the signal crayfish, tested in our study, had similar concentrations of Ca (447–990 ppm) and Mg (219–293 ppm) and higher concentrations of Na (1445–1875 ppm) and K (2550–3127 ppm). The sequence of macroelements in signal crayfish meat was as follows K > Na > Ca > Mg, different than in their exoskeleton (Ca > Na > K > Mg), where Ca is the main component of the exoskeleton, in addition to chitin and proteins [39]. For example, in crustaceans surveyed by Boßelmann et al. [40], Ca constituted 17.2–27.8% of the mass of the exoskeleton (in our studies, it was approx. 19%). Similarly, high concentrations of Ca (13.4–15.3%) in the signal crayfish exoskeleton from Cache Creek and Putah Creek watersheds (CA, USA) were reported by Hothem et al. [41].
Trace Elements in the Crayfish Tissue
Trace elements, especially Fe, Cu, Zn, Se, and also (albeit to a small extent) Ni and Al, are essential for the functioning of organisms. In the signal crayfish meat, these elements formed the following series: Zn > Cu > Fe > Al > Ni > Se. The same order in meat from signal crayfish abdomens was also noted by Hothem et al. [41], and the reported concentrations were similar to those presented in our research and had the following ranges: 71–108 ppm (Zn), 20.7–51.9 ppm (Cu), 14.2–20.6 ppm (Fe), 5.41–8.18 ppm (Al), 0.98–2.14 ppm (Ni), and 0.75–0.95 ppm (Se). In contrast, Protasowicki et al. [33] in signal crayfish meat from Pobłędzkie Lake (Poland) showed the following order of Al > Zn > Cu > Fe > Ni, and concentrations were 34 ppm (Al), 9.9 ppm (Zn), 2.02 ppm (Cu), 1.29 ppm (Fe), and 0.016 ppm (Ni).
The order of elements also differed between the individual parts of the body of crustaceans. In the hepatopancreas, we showed a shift of Fe in relation to its position in the order of element concentrations observed in signal crayfish meat. The concentration of Fe in the hepatopancreas in relation to the meat concentration was about 70 times greater in males and about 30 in females; at the same time, it was close to the Mg concentration. In contrast, in the exoskeleton, the order was as follows: Fe ≥ Al > Cu > Zn > Ni > Pb (in relation to the meat there was a change in the location of Fe and Al, and Pb took the place of Se; see Table S2).
It is important to monitor toxic elements in crustaceans due to their ability to accumulate toxic elements [3, 12, 17]. In our study, we determined As, Cd, and Pb, and their concentrations were the lowest among the tested elements (Table 3). Their concentrations were also lower than those reported by Hothem et al. [41], who found the following ranges in the signal crayfish meat: 0.24–0.68 ppm (As), 0.02–0.04 ppm (Cd), and 0.08 ppm (Pb). Also, Protasowicki et al. [33], when compared to our results, found an order of magnitude higher level of Pb (0.023 ppm) and a much lower concentration of Cd (0.001 ppm).
Bioaccumulation of Elements
The elements can be accumulated in various ways in particular body parts of crustaceans [3, 33, 37, 38, 41]. For example, in our study, the bioaccumulation of As, Cd and Fe was as follows: hepatopancreas > exoskeleton > meat; for Na, Pb and Al—exoskeleton > hepatopancreas > meat and for K—meat > hepatopancreas > exoskeleton (detailed bioaccumulation is presented in Tables 2 and 3). Our results were consistent with those presented by other authors (e.g., [12, 33, 37, 38, 41]), who also usually observed higher concentrations of elements in the exoskeleton or hepatopancreas, and lower in meat.
As in the case of other crustaceans, signal crayfish had varying concentrations of elements and different ability to bioaccumulate them in different parts of the body as a result of multidirectional factors including the condition of the environment, chemical composition of food, metabolism, sexual dimorphism, and reproductive season [4, 12, 34, 36].
It is widely known that the content of elements in organisms depends on their concentration in the environment and, in general, is consistent with their content in the lithosphere and hydrosphere. Such a relation was also evident in our research, and the general trend of the determined elements in the body of crayfish was convergent with their levels in water, as well as in bottom sediments, although in the bottom sediments, Fe, Al, and Mg were most abundant (Table S2).
Higher concentrations of Fe and Al (as well as other elements) in bottom sediments than in water could be an indirect cause of a significant increase in the concentration of these elements in the exoskeleton of crayfish in relation to their meat levels. Chitin that builds the exoskeleton in crustaceans has a high sorption capacity with regard to metals [42, 43]. Similar relationships have been shown by Tunca et al. [34], indicating that the high concentration of Al in bottom sediments (their common component) may additionally explain the high content of Al in the exoskeleton of Astacus leptodactylus
Trace essential elements (Zn, Fe, Cu) and also Ni and Al are present in the body of crayfish, performing important functions in physiological processes. Although they occur in lower concentrations than Ca, Mg, K, or Na, they can be accumulated in concentrations that exceed the demand. Particularly, high concentrations of metals are noted in the hepatopancreas, the organ responsible for detoxification, which makes it susceptible to the bioaccumulation of metals and As [3, 12, 34, 41]. In our study, in addition to Fe, Cu and Ni, also As, Cd and Se had the highest levels in the hepatopancreas. According to Coretti et al. [17], the differences in the bioaccumulation of elements by detoxification tissues and other tissues may be expressed by the ratio of levels found in the hepatopancreas to those in the abdominal muscle (Hep/AbM). In the studied signal crayfish, the Hep/AbM ratios could be placed in the following descending order: Fe (41.0) > Cd (11.1) > Pb (3.5) > Cu (2.7) > As (2.3) > Se (2.2) > Ni (2.1) > Na (1.3) > Zn (1.0) > Ca (0.92) > Mg (0.86) > Al (0.80) > K (0.78). Similar regularities were reported by Goretti et al. [17] for crayfish from an area uncontaminated with metals, where Hep/AbM could be arranged in the following descending order: Pb (4.3) > Cd (3.6) > Zn (1.6) > Cu (0.8). The authors then argued that the Hep/AbM ratio is more suited to areas with a high metal contamination.
Lead concentration was higher in the hepatopancreas in relation to the meat concentration, but the highest level was found in the exoskeleton, which can be attributed to its partial adsorption from bottom sediments (which contained about 20 times more Pb than the exoskeleton). One has also to remember the process of molting, during which the concentration of metals in the newly formed exoskeleton can increase, inter alia by their absorption from water, and then the metals can be eliminated during the molt [14, 43].
Bioconcentration Factor
The ability of an aquatic organism to absorb chemicals from the environment can be assessed by the bioconcentration factor (BCF). As reported by Tao et al. [44], a BCF value > 1 means that the organism has the potential to accumulate a chemical substance, but it is not considered significant unless it exceeds 100 or more. Based on Costanza et al. [45], it can be assumed that at BCF < 1000, the chemical substance is not significantly bioaccumulative; at a BCF in the range 1000–5000, the chemical substance is bioaccumulative; and at BCF > 5000, the chemical substance is highly bioaccumulative. Based on these assumptions, the crayfish analyzed in our study, similar to the research by Varol and Sünbül [46], had the potential to accumulate all the elements tested from the water (BCF > 1) and only Ca, Cu, Cd, K, Na, Se, and Zn from sediments. The accumulation of Al, Cu, K, Na, and Zn (for meat/water); Al, As, Cd, Cu, Fe, K, Na, Sn, and Zn (for hepatopancreas/water); and Al, Ca, Cd, Cu, Fe, Mg, Na, Pb, Se, and Zn (for exoskeleton/water) may be considered significant (BCF > 100). At the same time, it should also be pointed out that the accumulation of individual elements varied between body parts. For example, the BCF for Ca exceeded 5000 for the exoskeleton and only 21 for the meat, while the BCF for Cu exceeded 12,000 for the hepatopancreas and 3511 for the exoskeleton. The demonstrated diversity of BCF can probably be associated with the biochemical function of the elements in the life processes of the organism, and with the physiological function performed by the individual organs. Hence the high BCF values of elements necessary for proper functioning of the organism, such as Ca, Cu, K, Fe, Na, Se, or Zn, as well as toxic elements such as Cd or Pb in the hepatopancreas (responsible for detoxification) or in the exoskeleton, which can directly adsorb metals from water but also can be used by crustaceans in the process of removing toxic elements during exuviation [13, 16, 37, 44, 46].
The obtained BCF values were consistent with the data obtained, e.g., by Varol and Sünbül [46] or Vrhovnik et al. [21]. The investigated crayfish had a much higher bioaccumulation (including As, Cd, and Pb) from the water than from sediments (although in the case of Cd, the BCF values were also elevated for the body/sediment ratio). Generally, the signal crayfish accumulated elements from the water and, to follow Canpolat et al. [47], it can be concluded that the level of their concentration in water did not indicate a direct risk toxic to cancer.
Comparison with Daily Intake Recommendations
The Food and Agriculture Organization of the United Nations (FAO) recommends eating foods with varied composition, while at the same time preserving the biodiversity of resources. Numerous works emphasize that crustaceans are an important source of elements, but they vary in terms of meeting the daily demand of humans for particular elements [1, 2]. We have also demonstrated such differences in our research, with the majority of essential elements not exceeding the recommended daily intake. Only Cu was above DRI, although it was below the Permissible Maximum Tolerable Daily Intake (PMTDI 35 mg/day, after Barrento et al., [1]) for humans set by the WHO UN.
The DRI values estimated in our research were generally similar to those presented by Barrento et al. [1], for the muscle of brown crab Cancer pagurus. Particularly high difference was demonstrated for Se, whose DRI in our research was in the range of 15–30% and in Barrento et al. [1] it was 178–197%.
The consumption of signal crayfish meat does not pose a health hazard to people due to the content of Al, Ni, Pb, Cd, and As—elements for which the acceptable daily intake was determined. In the presented study, they did not exceed 4% ADI. Concentrations of Cd and Pb in meat (as in the remaining body parts of crayfish tested) did not exceed the values established by international regulations for their concentration in food products—for both elements maximum 0.50 ppm [48, 49].
Conclusions
The body size of the signal crayfish was typical for the most commonly caught crayfish of this species in the southern Baltic coastal area, with the males significantly larger than the females.
The exoskeleton and hepatopancreas were significantly different from meat in terms of the content of the elements determined. The exoskeleton was extremely rich in Ca and exhibited the highest bioaccumulation of Al and Pb (probably in the adsorption of metals from bottom sediments). In contrast, the bioaccumulation of As, Cd, Cu, Fe, Ni, and Se was the highest in the hepatopancreas. Meat had the highest Zn concentration, following the levels of macroelements—K, Na, Ca, and Mg. The bioconcentration factor (BCF) from water was much higher than from sediments.
Due to the low content of heavy metals in the water of the Wieprza River, it can be concluded that their availability from this source should not negatively affect the quality of meat of the signal crayfish living in this river. Nevertheless, one must bear in mind the ability of crayfish to accumulate metals, even when they live in habitats not strongly contaminated with heavy metals.
The content of essential elements in 100 g of meat did not exceed the dietary reference values, and the concentration of non-essential or toxic elements did not exceed the acceptable daily intake.
In conclusion, it should be emphasized that the meat of P. leniusculus from the Wieprza River is a great source of macroelements and microelements and can be a valuable source in the human diet.
References
Barrento S, Marques A, Teixeira B, Analceto P, Carvalho ML, Vaz-Pires P, Nunes ML (2009) Macro and trace elements in two populations of brown crab Cancer pagurus: ecological and human health implications. J Food Compos Anal 22:65–71. https://doi.org/10.1016/j.jfca.2008.07.010
Barrento S, Marques A, Teixeira B, Carvalho ML, Vaz-Pires P, Nunes ML (2009) Influence of season and sex on the contents of minerals and trace elements in brown crab (Cancer pagurus, Linnaeus, 1758). J Agric Food Chem 57:3253–3260. https://doi.org/10.1016/j.jfca.2015.03.002
Rainbow PS (2007) Trace metal bioaccumulation: models, metabolic availability and toxicity. Environ Int 33:576–582. https://doi.org/10.1016/j.envint.2006.05.007
Islam B, Mia B, Razzaque A, Sarker M, Rahman R, Jalil A, Rahim A, Roy DK (2016) Investigation on mineral composition of freshwater crab (Paratelphusa lamellifrons) of Padma River near Rajshahi City, Bangladesh. Int J Fish Aquatic Studies 4(6):236–240
Skorupski J, Szenejsko M, Śmietana P, Panicz R, Keszka S, Czerniejewski P, Soroka M, Orłowska L, Albrycht M, Zatoń-Dobrowolska M, Moska M, Kirczuk L, Rymaszewska A (2017) Invasive alien species - identification of threats to protect biodiversity. Wyd. Green Federation “GAJA”, Polish Society of Conservation Genetics LUTREOLA.
Schulz R, Śmietana P (2001) Occurrence of native and introduced crayfish in Northeastern Germany and Northwestern Poland. B Fr Peche Pisci 361:629–641. https://doi.org/10.1051/kmae:2001009
Dobrzycka-Krahel A, Skóra ME, Raczyński M, Szaniawska A (2017) The signal crayfish Pacifastacus leniusculus - distribution and invasion in the Southern Baltic Coastal River. Pol J Ecol 65(3):445–452
Filipová L, Petrusek A, Matasová K, Delaunay C, Grandjean F (2013) Prevalence of the crayfish plague pathogen Aphanomyces astaci in populations of the signal crayfish Pacifastacus leniusculus in France: evaluating the threat to native crayfish. PLoS One 8(7):e70157. https://doi.org/10.1371/journal.pone.0070157
Momot WT, Gowing H, Jones PD (1978) The dynamics of crayfish and their role in aquatic ecosystems. Am Midl Nat 99(1):10–35
Harlioğlu MM, Holdich DM (2001) Meat yields in the introduced freshwater crayfish, Pacifastacus leniusculus (Dana) and Astacus leptodactylus Eschscholtz, from British waters. Aquac Res 32:411–417. https://doi.org/10.1046/j.1365-2109.2001.00577.x
Regulation EU, 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species
Kouba A, Buřič M, Kozák P (2010) Bioaccumulation and effects of heavy metals in crayfish: a review. Water Air Soil Pollut 211:5–16. https://doi.org/10.1007/s11270-009-0273-8
Rainbow PS, Luoma SN (2010) Trace metals in aquatic invertebrates. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota: interpreting tissue concentrations. Taylor and Francis Books, Boca Raton, pp 231–252
Reichmuth JM, Weis P, Weis JS (2010) Bioaccumulation and depuration of metals in blue crabs (Callinectes sapidus Rathbun) from a contaminated and clean estuary. Environ Pollut 158:361–368
Nieboer E, Richardson DHS (1980) The replacement of the nondescript “heavy metals” by a biologically and chemically significant classification of metal ions. Environ Pollut 1B:3–26
Rainbow PS (2002) Trace metal concentrations in aquatic invertebrates: why and so what? Environ Pollut 120:497–507
Goretti E, Pallottini M, Ricciarini MI, Selvaggi R, Cappelletti D (2016) Heavy metals bioaccumulation in selected tissues of red swamp crayfish: an easy tool for monitoring environmental contamination levels. Sci Total Environ 559:339–346. https://doi.org/10.1016/j.scitotenv.2016.03.169
Francesconi KA (2010) Arsenic species in seafood: origin and human health implications. Pure Appl Chem 82(2):373–381
Maguire I, Klobučar G (2011) Size structure, maturity size, growth and condition index of stone crayfish (Austropotamobius torrentium) in North-West Croatia. Knowl Manag Aquat Ecosyst 401:12. https://doi.org/10.1051/kmae/2011026
Jitar O, Teodosiu C, Oros A, Plavan G, Nicoara M (2015) Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechol 32:369–378
Vrhovnik P, Arrebola JP, Serafimovski T, Dolenec T, Smuc NR, Dolenec M, Mutch E (2013) Potentially toxic contamination of sediments, water and two animal species un Lake Kalimanci, FYR Macedonia: relevance to human health. Environ Pollut 180:92–100
Ross C, Taylor CL, Yaktine AL, del Valle HB (2011) Dietary reference intakes for calcium and vitamin D. National Academies Press, Washington. https://doi.org/10.17226/13050
Salahinejad M, Aflaki F (2010) Toxic and essential elements content of black tea leaves and their tea infusion consumed in Iran. Biol Trace Elem Res 134:109–117. https://doi.org/10.1007/s121011-00908449-z
StatSoft Inc. (2016) Statistica (data analysis software system), version 12.1. www.statsoft.com
Senze M, Kowalska-Góralska M, Pokorny P, Dobicki W, Polechoński R (2015) Accumulation of heavy metals in bottom sediments in Baltic Sea catchment rivers affected by the operations of petroleum and natural gas mines in Western Pomerania, Poland. Pol J Environ Stud 24(5):2167–2175
The state of the environment in the West Pomeranian Voivodeship. Report 2018. Voivodeship inspectorate of environmental protection, Szczecin, pp. 192. (in Polish)
Pokorny P, Senze M, Dobicki W, Kowalsak-Góra M, Polechoński R (2013) Geochemical assessment of Western Pomerania watercourse. Przem Chem 92(9):1768–1771
Hudina S, Zganec K, Hock K (2015) Differences in aggressive behaviour along the expanding range of an invasive crayfish: an important component of invasion dynamics. Biol Invasions 17:3101–3112
Souty-Grosset C, Holdich DM, Noel P (2006) Atlas of crayfish of Europe. Museum National d’Histoire Naturalle, Paris
Śmietana P, Krzywosz T (2006) Determination of the rate of growth of Pacifastacus leniusculus in Lake Pobłędzie using polymodal length frequency distribution analysis. B Fr Peche Pisci 380–381:1229–1244
Rebrina F, Skejo J, Lucić A, Hudina S (2015) Trait variability of the signal crayfish (Pacifastacus leniusculus) in a recently invaded region reflects potential benefits and trade-offs during dispersal. Aquat Invasions 10(1):41–50
Capurro M, Galli L, Mori M, Salvidio S, Arillo A (2015) Reproductive cycle of Pacifastacus leniusculus (Dana) (Crustacea: Decapoda) from the Brugneto Lake (Liguria, northwest Italy). Ital J Zool 82(3):366–377. https://doi.org/10.1080/11250003.2015.1022235
Protasowicki M, Własow T, Rajkowska M, Polna M, Bernard A (2013) Metal concentrations in selected organs of crayfish – Orconectes limosus and Pacifastacus leniusculus from Mazurian Lakes. J Elem 18(4):683–694. https://doi.org/10.5601/jelem.2013.18.4.537
Tunca E, Ucuncu E, Ozkan AD, Ulger ZE, Cansizoglu AE, Tekinay T (2013) Differences in accumulation and distribution profile of heavy metals and metalloid between male and female crayfish (Astacus leptodactylus). B Environ Contam Tox 90:70–577. https://doi.org/10.1007/s00128-013-0960-4
Adeyeye EI, Olanlokun JO, Falodun TO (2010) Proximate and mineral composition of whole body, flesh and exoskeleton of male and female common West African fresh water crab Sudananautes africanus africanus. Pol J Food Nut Sci 60(3):213–216
Jimmy UP, Arazu VN (2012) The Proximate and mineral composition of two edible crabs Callinectes amnicola and Uca tangeri (Crustecea: Decapoda) of the Cross river, Nigeria. Pak J Nutr 11(1):78–82
Nędzarek A, Czerniejewski P, Drost A, Harasimiuk F, Machula S, Tórz A, Masalski P (2017) The distribution of elements in the body of invasive Chinese mitten crabs (Eriocheir sinensis H. Milne-Edwards, 1853) from Lake Dąbie, Poland. J Food Compos Anal 60:1–9
Nędzarek A, Czerniejewski P, Tórz A (2019) Macro- and trace elements in Chinese mitten crabs (Eriocheir sinensis) from Szczecin Lagoon, Poland – implications for human health. Aquaculture 506:229–237. https://doi.org/10.1016/j.aquaculture.2019.03.042
Pires C, Marques A, Carvalho ML, Batista I (2017) Chemical characterization of Cancer pagurus, Maja Squinado, Necora puber and Carcinus maenas shells. Poul Fish Wildl Sci 5(1):1–6. https://doi.org/10.4172/2375-446X.1000181
Boßelmann F, Romano P, Fabritius H, Raabe D, Epple M (2007) The composition of the exoskeleton of two crustacean: the American lobster Homarus americanus and the edible crab Cancer pagurus. Thermochim Acta 463:65–68
Hothem RL, Bergen DR, Bauer ML, Crayon JJ, Meckstroth AM (2007) Mercury and trace elements in crayfish from Northern California. B Environ Contam Tox 79:628–632. https://doi.org/10.1007/s00128-007-9304-6
Rana MS, Halim MA, Safiullah S, Mamun Mollan M, Azam MS, Goni MA, Kamal Hossain M, Rana MM (2009) Removal of heavy metal from contaminated water by biopolymer crab shell chitosan. J Appl Sci 9(15):2762–2769
Bergey LL, Weis JS (2007) Molting as a mechanism of depuration of metals in the fiddler crab, Uca pugnax. Mar Environ Res 64:556–562
Tao Y, Yuan Z, Xiaona H, Wein M (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotox Environ Safe 81:55–64
Costanza J, Lynch DG, Boethling RS, Arnot JA (2012) Use of the bioaccumulation factor to screen chemicals for bioaccumulation potential. Environ Toxicol Chem 31(10):2261–2268
Varol M, Sünbül MR (2018) Biomonitoring of trace metals in the Keban Dam Reservoir (Turkey) using mussels (Unio elongatulus eucirrus) and crayfish (Astacus leptodactylus). Biol Trace Elem Res 185(1):216–224
Canpolat O, Eroglu M, Dusukcan M (2016) Transfer factor of some heavy metals in muscle of Cyprinus carpio. Fresenius Environ Bull 25(11):4988–4994
Commission Regulation (EU) No 420/2011 of 29 April 2011 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. https://www.fsai.ie/uploadedFiles/Reg420_2011.pdf. Accessed 9 March 2018
Food and Agriculture Organization (FAO) Heavy metal regulations – Faolex. Legal Notice no. 66/2003. http://faolex.fao.org/docs/pdf/eri42405.pdf. Accessed 6 March 2018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 878 kb)
Rights and permissions
About this article
Cite this article
Nędzarek, A., Czerniejewski, P. & Tórz, A. Macroelements and Trace Elements in Invasive Signal Crayfish (Pacifastacus leniusculus) from the Wieprza River (Southern Baltic): Human Health Implications. Biol Trace Elem Res 197, 304–315 (2020). https://doi.org/10.1007/s12011-019-01978-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01978-y