Abstract
The pressed powder sample is a common method for elemental analysis using X-ray fluorescence analysis whereas suitable light hydrocarbon materials should be added to the sample as a binder. The present study demonstrates the applicability of using different commercial binders for elemental analysis of urinary stone samples. In order to confirm the obtained results, a comparison with pure chemical grade binders was presented. Different commercial and pure binders were tested for quantitative elemental analysis of urinary stones, namely, cellulose, starch, wax, and urea. Energy dispersive X-ray fluorescence (EDXRF) was used for elemental analysis. Differential thermal analysis was used to estimate the loss on ignition (LOI) in the urinary stone samples. The signal to background ratios (I/IB) of the different detected elements in the commercial and pure binders were calculated, compared, and studied at eight different photon energies starting from 2.5 up to 37 keV. Standard-less quantitative analysis method based on the fundamental parameter approach was applied for elemental analysis of selected urinary stones. The commercial and low-cost binders could be an excellent alternative binder for urinary stone analysis using energy dispersive X-ray fluorescence. The commercial binders could provide an advantage as pure chemical grade binders or even better especially at photon energy higher than 10 keV. The best commercial binder candidate was found to be the wax. The quantitative analysis results using commercial and pure chemical grade binders give good agreement results, which indicate the applicability of commercial binders for quantitative elemental analysis of urinary stones in the form of pressed powder samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The accuracy and reliability of the X-ray fluorescence (XRF) techniques basically depend on the sample preparation of the material to be analyzed. A great portion of the errors of the quantitative analysis goes along with sample preparation. Sample preparation method should be rapid, cheap, and free from systematic error due to impurity contamination. As the analyzed sample mainly contains low Z elements such as the urinary stones, more precautions should be considered for sample preparation. In case of the direct elemental analysis of urinary stone samples, heterogeneity challenge of the cutting bulk urinary stone sample surface could be recognized. In addition, the polished surface will not be ideal due to the hard and soft components of these types of samples. Consequently, non-ideal measured intensities will be obtained if the heterogeneity of the sample is clear and the roughness of the surface of the sample is not ideal. As the urinary stone samples are heterogeneous, the samples should be converted into a homogenous fine powder with a particle size less than 50 μm to be analyzed in the XRF setups. The powder samples are either fused with a suitable flux or directly analyzed or pressed into pellets of constant density with or without binders [1]. Sample fusion method has many drawbacks such as the expected contamination from additives, the high cost of fusion procedures starting from using platinum crucibles and lithium tetraborate or related salts, up to the use of high temperature via fusion machine or muffle furnace ranging from 800 to 1000 °C. In addition, the complete recovery of the fused mixture seems to be impossible and part of them remains inside the platinum crucible. Furthermore, the annealing process in some cases may take several hours to be completed and the fused sample may partially be recrystallized during the cooling process, and consequently, the heterogeneities in microscale cannot be avoided. Using pressed powder pellet and loose powder, elemental analysis of Cr, As, Se, Cd, Hg, and Pb was quantified in soil samples using wavelength dispersive X-ray fluorescence (WDXRF) spectrometry [2]. The pressed powder samples were prepared without binders at a pressure of 300 kgf/cm2 whereas the loose powder samples were placed into a polyethylene cup with a polypropylene film of thickness 6 μm. A good agreement between pressed powder pellets and loose powder samples was obtained. In the case of pressing samples, the error of the light elements from Na (Z = 11) to Cl (Z = 17) decreases by using pressed pellets. Due to the existence of light elements in the urinary stone samples, the pressed pellets are more desirable. In case of pressing the samples without binder, extra pressures up to 50 T/in.2 may have to be employed if the self-bonding properties of the sample are poor. However, the high pressure pelletizing may cause a fracture of a pellet. Therefore, adding a suitable binder to the sample before pelletizing could be necessary even it may cause an inter-element interference and particle size effects. However, the inter-element interference and the particle size effects could be eliminated by grinding the sample together with the binder to an fine or ultrafine powder and pelletizing them at high pressure. The binding agent must have a good self-bonding property, low absorption, stable under vacuum, free from inter-element interferences, and free from signification elemental contamination. Normally, binders work as a glue that holds the sample together [3]. A great number of binding agents have been successfully used namely, starch, chromatographic cellulose, Lucite, boric acid, polyvinyl alcohol, urea, and wax. Although the use of binding agents gives the sample extra strength and eliminates the inter-element effects, the background radiation coming from the scattering of the sample increase and the dilution of the sample will decrease the sensitivity of certain elements. Therefore, the use of a small quantity of binding agents and mixed it very well with the sample could offer an alternative solution.
In the present study, pure chemical grade and commercial binders were checked for quantitative elemental analysis of selected urinary stone samples collected from King Abdulaziz specialist hospital (KASH), Taif, Saudi Arabia. The applicability of using low-cost binders (commercial) as well as the pure chemical grade ones was investigated. The main binders used in the present work are urea, wax, starch, and cellulose [4, 5]. All of these binders compose of low Z elements which prevent caking of the surface of the sample, get stable pellet, and reduce matrix effect [6].
Materials and Methods
Sampling and Chemical Reagents
Based on 26 factors including type of stones, recurrence, diet, obesity, hypertension, smoking, diabetes, water intake … etc., the urinary stone samples were collected from King Abdulaziz specialists hospital (KASH) in Taif city, Saudi Arabia. Some stones were received in powder form due to the influence of the medical treatments with ultrasonic waves, and others were obtained in the form of intact stones after surgical operation. Urinary stones with a diameter equal or greater than 20 mm were selected for the present study. The selected urinary stone samples were carefully washed several times using double distilled water in order to reomve urine, blood, and residue of organic matter. Afterward, the samples were dried in an oven furnace at 70 °C for 12 h. Later, the urinary stone samples were crushed and grounded in an agate mortar to be homogenized powder. The selected urinary stone samples were mixed well with the different binders individually with ratios of sample: binder = 0.45:2 g. This ratio was considered afterward in the standard-less quantitative analysis software based on the fundamental paramter approach. A group of commercial and pure chemical grade binders was used in order to select the most appropriate and low-cost binder for pressed powder samples. Pure chemical grade binders have been used with the urinary stone samples, namely, urea (Biotechnology, Bio-Basic Inc., Canada), cellulose (Fluka, Sigma-Aldrich Chemi GmbH, Germany), wax (Fluka, Sigma-Aldrich Chemi GmbH, Germany), and starch from corn (Sigma-Aldrich, USA). In addition, one commercial product of cellulose, urea, and wax binders purchased from the local markets was also used. In the case of starch binder, four commerical binders were used, namely, Foster (S-C1), Gethai (S-C2), Queen (S-C3), and Unifood (S-C4). The mixed samples (urinary stone and binder) were placed in Al cup and pressed at pressure of 10 tons. The pressed samples were directly analyzed by EDXRF spectrometer.
Energy Dispersive X-ray Fluorescence Spectrometer
The quality of the quantitative elemental analysis results of the selected urinary stones with a different type of binders was checked by an energy dispersive X-ray fluorescence (Quant’X EDXRF, Thermo Fisher Scientific Inc., USA). Direct excitation of the pressed urinary stone samples was carried out using an air-cooled end window X-ray tube with Rh target. The applied X-ray voltage of the X-ray tube is up to 50 kV (1 kV increments) and the electrical current is up to 2 mA (0.02 mA increments). A beryllium window of thickness 76 μm was used in front of the exit window of the X-ray tube. A thermoelectric air-cooled silicon drift detector (SDD) with Be window of thickness ≤ 7.6 μm was used to evaluate the characteristic X-ray lines coming from the sample under study. The SDD has an area of 15 mm2 and 155 eV full width at half maximum (FWHM) resolution for Mn-Kα line at 1500 counts/s. The electrical pulses of the characteristic X-ray are amplified and transmitted to a readout system using a multi-channel spectrometer of 32-bit and 4096 channel. The incident angle (at which X-ray impacts the sample) is 55° and the X-ray detector Takeoff angle (at which X-ray leaves the sample to the detector) is 45°. Eight transmission filters at different photon energy ranges starting from 4 to 50 keV are used in order to collect the characteristics X-ray lines from the sample starting from low Z elements up to lanthanide group. These filters are Non, cellulose, Al, Thin Pd, Medium Pd, Thick Pd, Thin Cu, and Thick Cu. The first two filters were operated under vacuum whereas the others operated under atmospheric pressure. A collimator of a diameter 8.8 mm was used in order to confine the radiation coming from the X-ray tube and to avoid X-rays from the sample holder. Standard-less elemental quantitative analysis method based on the fundamental parameter (FP) approach was applied using UniQuant software. The use of the FP approach reduces the need for standards compared with the empirical methods. However, a pre-calibration was carried out using a set of pure elements (Al, Ti, Cr, Fe, Ni, Mo, Sn, W, and Pb) in order to correct the elemental drifts.
Thermal Analysis
In the case of urinary stone samples, the major constituents are water and organic compounds which are not possible to detect by EDXRF spectrometer. Therefore, the estimation of loss on ignition (LOI) seems to be essential for the standard-less elemental quantitative analysis method. In order to estimate the loss on ignition for the urinary stone samples, differential thermal analysis was used. Thermal gravimetric analysis (TGA), derivative thermogravimetry (DTG), and differential scanning calorimetry (DSC) (Shimadzu Simultaneous DTG-60H thermal analyzer, Japan) were carried out in order to investigate the behavior of the inorganic constitutes in the urinary stone samples as a function of temperature and to calculate the loss on ignition accurately. Only a small amount of urinary stone samples (5–10 mg) is required [7,8,9]. The atmosphere of the vicinity of the sample was standardized whereas the thermal measurements were carried out under a nitrogen atmosphere with a flow rate of 30 mL min−1 in order to avoid extra oxidation at high temperatures. Two crucibles made of aluminum/platinum was used one for the urinary stone sample and the other used an empty case for calibration. The temperature range is from the room temperature to 1000 °C with a heating rate of 10 °C/min.
Results and Discussions
Prior to the elemental quantitative analysis of the pressed urinary stone samples, the detected impurities in both pure chemical grade and commercial binders were characterized by EDXRF spectrometer in order to subtract it during the quantitative analysis process. The different binders (pure chemical grade and commercial) were pressed in Al cup and measured as a sample. Afterward, the binders were mixed well with the selected urinary stone samples, and the quantitative elemental analysis of the selected urinary stone samples has been investigated. For this purpose, eight different filters covering the whole photon energy range starting from the low Z elements up to the lanthanide groups were used. Table 1 shows more details about the eight filters and the related photon energy ranges used in the present work.
Impurities in Binders
Low Z Element Filters (Non, Cellulose, and Aluminum)
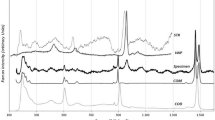
The applied voltages for filter 1 (no filter), filter 2 (cellulose, (C6H10O5)n), and filter 3 (aluminum, Al) were 4, 8, and 12 kV, respectively. The characteristic K-lines for the elements from Na (Z = 11) up to Mn (Z = 25) could be excited and measured. Although L-lines for the elements from Zn (Z = 30) to Sm (Z = 62) could be measured, there is no L-lines from those elements were recognized in all binders. Both filters 1 and 2 operate under vacuum in order to avoid Ar-K lines, but filter 3 (low Zc) operates under atmospheric pressure. Figure 1 depicts an example of the XRF spectra of the different type of binders using cellulose filter at applied voltage up to 8 kV. Remarkable high Na contents were found only in the commercial binder of urea. Constant intensities of Al-Kα at 1.487 keV line for all pure chemical grade and commercial binders indicate to the same source which is the Al cup in which the binders were pressed inside it. As the H, C, and O elements are the main components of all binders, the low absorption of the different binders could facilitate the penetration of the X-ray beam to the Al cup itself. High intensities of Kα-lines of Si (1.74 keV), P (2.013 keV), S (2.308 keV), Cl (2.622 keV), K (3.312 keV), and Ca (3.690 keV) could be recognized with all types of starch binders. Therefore, starch binders are not suitable at low photon energy up to Sc. However, P-lines disappear in other types of binders. In cellulose, wax, and urea binders, traces of Si, S, Cl, K, and Ca cannot be avoided and should be considered during the quantitative analysis. However, in the case of the pure binders of cellulose and wax, the intensities of these elements decreased remarkably and reach to the minimum values as shown in Fig. 1. In case of filter 3 (low Zc, from 4.5 to 6.5 keV), there are no detected impurities in all binders either pure chemical grade or commercial with the exception of very trace detection of Ti in all binders especially with cellulose binders. The small counts of Ti could be treated during background correction. It could be summarized that the most adopted binders at low photon energy up to 6 keV seem to be pure chemical grade and/or the commercial binders of cellulose and wax.
In order to confirm the suitability of cellulose and wax binders, Fig. 2 depicts the signal to background ratios (I/IB) of the different elements detected by the low Z element filters (low Za, Zb, and Zc) versus a different type of binders. As the present type of binders is used with the urinary stone samples, high detected signals of elements in the different binders are not desirable. Therefore, the highest (I/IB) values represent undesirable binders whereas as the (I/IB) ratios decrease as the binders could be more effective and applicable. As illustrated from Fig. 2, the highest (I/IB) was found for urea and starch (pure chemical and commercial) for the elements Si, K, and Ca. The highest (I/IB) of Si-Kα was found at the pure chemical grade urea binders and reaches to ~ 19.
In the case of K-Kα, the values of I/IB ratios for urea and starch binders reach up to 11.6 and 7.5, respectively. Similarly, the highest I/IB ratios for Ca were found in some commercial starch (~ 7.5). The high values of I/IB ratios for Si, K, and Ca with starch and urea binders seem to be problematic and a certain background correction will be essential in such cases. Similarly, starch commercial binders are not suitable for Cl-Kα whereas of I/IB ratios reach to 4.8 for starch commercial. In the case of Ti which is measured by the third filter (aluminum), all binders are applicable and the I/IB ratios are less than 2. Therefore, starch and urea binders could be considered as non-appropriate binders at low photon energy. Cellulose and wax binders (either pure chemical or commercial) seem to be suitable.
Medium Z Element Filters (Thin, Medium, and Thick Pd)
The medium filters are fabricated from palladium (thin, medium, and thick) and they work usually under atmospheric pressure. The applied voltages of these three filters are 16, 20, and 28 kV, respectively. Using these filters, the K-lines of elements from Cr (Z = 24) to Mo (Z = 42) can be covered which is related to the photon energy range from 5.0 to 20.0 keV. In addition, the L-lines of the elements from Nd (Z = 60) to U (Z = 92) can also be covered at the same photon energy range. Figure 3 illustrates an example of the EDXRF spectra of pure chemical grade and commercial types of binders at applied voltages of 20 kV using medium Pd filter (medium Zb). It is clear that the K-lines of Fe (Kα = 6.400 keV, Kβ = 7.059 keV) and Cu (Kα = 8.05 keV, Kβ = 8.90 keV) appear in most of the binders. Cellulose binders (pure and commercial) have less intensity of Fe impurities. In the case of starch, the intensity of Fe impurities is relatively higher than that found in the pure chemical grade starch binder. No Mn lines appear in all types of cellulose, starch, and pure urea binders. Furthermore, high counts of Mn were found only with the commercial binders of wax and urea. The remarkable low counts of Zn intensities have appeared in the most of the binder especially commercial and pure chemical grade binders of urea and starch. However, high intensities of Cu were recognized in all binders especially all types of cellulose and wax binders. In consistency to the usual expectation, the intensities of Mn, Fe, Cu, and Zn impurities in commercial wax are higher than those found in the pure chemical grade wax. On the other side, the intensities of Fe, Cu, and Zn impurities are relatively similar in commercial and pure chemical grade binders of cellulose, urea, and starch. At photon energy range from 10 to 20 keV, the thick Pd filter is used whereas the most type of binders is completely free from any type of impurities especially starch and wax binders as well as pure urea and commercial cellulose (Fig. 4). However, the characteristic K-lines of Br appear in commercial binders of urea and cellulose. In addition, the Sr-Kα line appears also with the commercial urea even its intensity is not significant. The coherent and incoherent scattering of Rh appears in all spectra which is originated from the Rh target of the X-ray tube. By calculating the signal to background ratios for Fe and Mn with filter 4 (Thin Pd), the I/IB values were less than 8 for all types of binders (Fig. 5). Cellulose and starch binders seem to have lowest values I/IB ratios for Mn and Fe. The commercial binders of starch and wax have the highest values I/IB ratios for Fe. On the other hand, the commercial urea and wax binders have also higher I/IB ratios for Mn. In the case of Cu and Zn impurities, commercial wax binder has the highest (I/IB) and the values reached to 8 for Cu-Kα. The other signal to background ratios (I/IB) for other binders are relatively similar with the exception of commercial cellulose whereas (I/IB) values reached to 4. At photon energy of 11.909 keV, the signal to background ratios (I/IB) of Br-Kα are less than 3 with the exception of the value Br-Kα in case of commercial urea which reaches to 20. Therefore, cellulose (pure and commercial) binders are the most suitable for the elements from Cr to Ni. If the samples under investigation have no iron, starch could be most applicable. Also, commercial urea is not suitable as a binder at this photon energy range.
High Z Element Filters (Thin and Thick Cu)
At high Z element, thin and thick Cu filters were used under atmospheric pressure and applied voltages of 40 and 50 kV, respectively. At this photon energy range, only the characteristic K lines of the elements from Sr (Z = 38) to Ba (Z = 56) could be detected. No L-lines can be measured with these two filters. Figure 6 represents an example of the X-ray fluorescence spectra of the different binders at applied voltages up to 50 kV using a thick Cu filter. As seen from Fig. 6, only the characteristic K-lines of Zr (Kα = 15.746 keV and Kβ = 17.687 keV) appear for all type of binders either pure or commercial and it originates from the EDXRF instrument and there is no need to calculate the signal to background ratio in this case. The Zr and Rh K-lines could be treated as a permanent background for all type of binders. It is clear that there are no impurities that appeared at photon energy range from 14 to 37 keV which means the applicability of all binders either pure or commercial at this range. As a conclusion, pure wax, cellulose, and urea are the best candidate at all photon energy ranges. At the two filters “Med Za” and “Med Zb”, the commercial wax was not completely suitable, but it has some trace elements. At photon energy above 14 keV, all the different types of commercial and pure chemical grade binders are completely suitable.
Thermal Analysis of Urinary Stones
The urinary stone samples belong to the group of bio-mineral materials. Its constituents are generally organic and inorganic substances in crystalline and non-crystalline structures. In order to estimate the loss on ignition of the present urinary stones, the ratios of inorganic and organic contents of the selected urinary stone samples (#18 and #23) have been determined by their simultaneous thermogravimetric-differential thermal analyses (TGA-DTA). The selected urinary stone samples have almost the same thermal behavior that contains five distinct weight loss stages. Their TGA-DTA thermograms are shown in Fig. 7. According to the X-ray diffraction pattern of sample #18, it contains calcium oxalate monohydrate and uric acid in the ratio of 1:3 as the major components. When heated, it decomposes in five well-defined steps. At temperatures ranging from 100 to 200 °C, a first weight loss of 5.10% with an endothermic peak was observed, corresponding to the loss of water of crystallization [10]. At temperatures ranging 400 to 550 °C, second and third weight loss steps of 63.58% with two exothermic and endothermic peaks were observed, corresponding to the decomposition of 2.5 mol of uric acids. At temperatures ranging 550–630 °C, a fourth weight loss of 13.43% with an endothermic peak was observed, corresponding to the decomposition of 0.5 Mol of uric acid as well as the decomposition of anhydrous calcium oxalate, with the loss of carbon monoxide. At temperatures ranging 630–800 °C, the fifth and final weight loss step was determined to be 7.78%, corresponding to the decomposition of calcium carbonate to calcium oxide with the loss of carbon dioxide [11, 12]. The composition of sample #23 has been found to be 1 mol of calcium oxalate dihydrate and 1.5 mol of ammonium urate. As seen in Fig. 7, the pyrolysis of sample #23 exhibits two broad endothermic peaks at around 150 and 250 °C accompanied by a total mass loss of 13.94%, assignable for the removal of two water molecules of crystallization as well as 1.5 mol of ammonia. At temperatures ranging 420–610 °C, third and fourth weight loss steps of 51.92% with two exothermic and endothermic peaks were observed, corresponding to the decomposition of 1.0 mol of uric acid as well as the decomposition of anhydrous calcium oxalate. At temperatures ranging 620–800 °C, the final weight loss step was determined to be 12.44%, corresponding to the remained uric acid, in addition to the decomposition of calcium carbonate to a mixture of calcium oxide and carbon dioxide. Based on the defined five distinct weight loss steps of urinary stone samples #18 and #23, their total loss of ignitions was found to be 89.9% and 77.6%, respectively. These quantities were considered during the calculations of the elemental quantitative analysis.
Quantitative Elemental Analysis of Pressed Urinary Stones
The quantitative elemental analysis of two selected urinary stone samples has been carried out using the standard-less analysis method based on the fundamental parameter approach. The method was established earlier [13,14,15] and used for many different applications such as hematite, sediments, and plant samples [16,17,18]. Accurate descriptions of the present EDXRF geometry, the absorption coefficients of the specimen compositions, transition probabilities, fluorescence yields, the matrix, and the irradiated area of the sample have been considered due to their important role for the quality of the quantitative analysis results. In addition, direct and indirect excitations were considered during the iteration procedure of the quantitative analysis calculation. Before measuring the urinary stone samples by the EDXRF spectrometer, few milligram of the samples was treated by the thermal analysis in order to estimate the loss on ignition accurately. Once the percentage of the loss on ignition was determined for each sample, the raw urinary stone samples mixed with selected binder were measured by the EDXRF spectrometer. During the iteration procedures of the fundamental parameter method, the loss on ignition should be inserted to the software in order to get accurate quantitative analysis results. Prior to the final calculation of the elemental analysis of the urinary stone samples, the spectra of pure and/or commercial binders should be subtracted from the related spectra of the prepared urinary stone samples in order to eliminate the influence of the existed impurities in the binders. The elemental quantitative analysis results including the standard deviation expressed as 3 sigma of the selected urinary samples (#18 and #23) are presented in Tables 2 and 3. Pure chemical grade and commercial binders of cellulose, wax, starch, and urea were used in order to explore the applicability of using all of them in the case of pressed powder urinary stone samples. The commercial starch C3 was selected to be used for the present elemental quantitative analysis. The number of detected elements could be varied from sample to another based on the natural and the type of urinary stone. The main element in present two urinary stone samples is calcium which indicates the existence of calcium oxalate in these two samples. The obtained quantitative analysis results revealed that there is a generally good agreement between the quantitative analysis results either with different pure or commercial binders. At low photon energy, the low Z elements such as S, K, P, and Cl were quantified even these elements exist in some type of binders as impurities. Based on the standard deviation values mentioned in Tables 2 and 3, the relative standard deviation for each element was calculated. It was found that the relative standard deviation with pure chemical grade and commercial starch binders reached to 22% and 26% (sample 18) and 19% and 15% (sample 23) for P, respectively. In the case of K determination using pure chemical grade and commercial starch binders, the relative standard deviation reached to 33% and 23% (sample 18) and 12% and 17% (sample 23), respectively. However, the most of the relative standard deviations of the quantitative analysis results for Cl and S decrease to less than 10% for the samples 18 and 23 with starch binders (pure chemical grade and commercial). The most of other type of binders (pure/commercial) gives good agreement quantitative analysis results for S, K, P, and Cl with a relative standard deviation of less than 10%. In case of high Z elements, the quantitative elemental analyses for the elements Ca, Fe, Ni, Ti, Cu, Zn, Se, Sr, and Br using different binders are in good agreements either the pure or the commercial binders for both selected samples. It was confirmed that there are no impurities in the different binders (pure/commercial) at high photon energy. Therefore, all type of binders either pure chemical grade or commercial can be used effectively with the pressed samples, especially at high photon energy.
ND not detected
Conclusions
At low Z elements, the pure chemical grade of wax was found to be the most suitable binder for excitation up to Sc (Z = 21) whereas impurities like Si, P, S, Cl, K, and Ca were found in another type of binders especially in starch binders. Therefore, the pure chemical grade and commercial starch binders are not suitable for elemental quantitative analysis at low photon energy. Although of using pure chemical grade binders, some impurities could be found and these impurities should be taken into account during the elemental quantitative analysis of the urinary stone samples. Based on the elemental quantitative analysis of the selected urinary stone samples, the feasibility of using commercial chemical binders was demonstrated especially for the elements of atomic number > 21. In this case, the detected trace elements should be treated using background correction before performing quantitative analysis. The main advantages of using commercial chemical binders are the availability and the low cost. In the present study, the most suitable binder commercial binder was commercial wax and it works very well at eight different photon energy ranges. Without any doubt, the different types of binders either pure chemical grade or commercial ones could be used for pressed samples for XRF spectrometer at photon energy higher than 15 keV.
References
Li XL, An SQ, Liu YX, Yu ZS, Zhang Q (2018) Investigation of a high-pressure pressed powder pellet technique for the analysis of coal by wavelength dispersive X-ray fluorescence spectroscopy. Applied Radiation and Isotopes 132: 170–177. https://doi.org/10.1016/j.apradiso.2017.11.003
Shibata Y, Suyama J, Kitano M, Nakamura T (2009) X-ray fluorescence analysis of Cr, as, se, cd, hg, and Pb in soil using pressed powder pellet and loose powder methods. X-Ray Spectrom 38(5):410–416. https://doi.org/10.1002/xrs.1195
Kuz’mina TG, Troneva MA, Kononkova NN, Romashova TV (2017) Error of sample preparation in pressing emitters for X-ray fluorescence analysis. Journal of Analytical Chemistry 72(3):272–278
International Atomic Energy Agency (1997) Sampling, storage and sample preparation procedures for X ray fluorescence analysis of environmental materials. International Atomic Energy Agency, IAEA, Vienna
Jenkins R (1999) X-ray fluorescence spectrometry, vol 152. Chemical Analysis-A series of monographs on analytical chemistry and its application, Second edn. Johan Wiley & Sons, Inc., New York
Beckhoff B, Kanngießer B, Langhoff N, Wedell R, Wolff H (2007) Handbook of practical X-ray fluorescence analysis. Springer Science & Business Media, Berlin
Haines PJ, Reading M, Wilburn FW (1998) Chapter 5 - Differential Thermal Analysis and Differential Scanning Calorimetry. In: Brown ME (ed) Handbook of Thermal Analysis and Calorimetry, vol 1. Elsevier Science B.V., pp 279–361. doi:https://doi.org/10.1016/S1573-4374(98)80008-3
Hatami M, Ganji DD, Sheikholeslami M (2017) Chapter 1 - introduction to differential transformation method. In: Differential transformation method for mechanical engineering problems. Academic Press, pp 1–54. doi:https://doi.org/10.1016/B978-0-12-805190-0.00001-2
Wagner M (2018) Chapter 21 - Overview of standard methods for thermal analysis. In: Thermal analysis in practice. Carl Hanser Verlag GmbH & Co. KG, pp 336–346. https://doi.org/10.3139/9781569906446.021
Berényi M, Liptay G (1971) The use of thermal analysis in medical science with special reference to nephroliths. J Therm Anal 3(4):437–443. https://doi.org/10.1007/bf02188652
Mitchell BD, Birnie AC (1970) Biological Materials. In: Mackenzie RC (ed) Differential thermal analysis, vol 1. Academic Press, London, pp 673–704
Keattch CJ, Dollimore D (1975) An introduction to thermogravimetry, 2nd edn. Heyden, Enlarged
Sherman J (1954) The correlation between fluorescent X-ray intensity and chemical composition. ASTM Spec Tech Publ I57:27–33
Sherman J (1955) The theoretical derivation of fluorescent X-ray intensities from mixtures. Spectrochim Acta 7:283–306
Shiraiwa T, Fujino N (1966) Theoretical calculation of fluorescent X-ray intensities in fluorescent X-ray spectrochemical analysis. Jpn J Appl Phys 5:886–899
Shaltout AA, Gomma MM, Ali-Bik MW (2012) Utilization of standardless analysis algorithms using WDXRF and XRD for Egyptian iron ore identification. X-Ray Spectrom 41(6):355–362
Shaltout AA, Moharram MA, Mostafa NY (2012) Wavelength dispersive X-ray fluorescence analysis using fundamental parameter approach of Catha edulis and other related plant samples. Spectrochim Acta B At Spectrosc 67:74–78
Shaltout AA, Welz B, Ibrahim MA (2011) Influence of the grain size on the quality of standardless WDXRF analysis of river Nile sediments. Microchem J 99(2):356–363
Acknowledgments
The authors gratefully acknowledge the volunteers who participated in this research.
Funding
This study has no any financial support.
Author information
Authors and Affiliations
Contributions
Abdallah A. Shaltout, Maram M. Dabi, Mohamed M. Ibrahim, Ahmed S. Al-Ghamdi, and Essam El-Naggar certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaltout, A.A., Dabi, M.M., Ibrahim, M.M. et al. Applicability of Low-Cost Binders for the Quantitative Elemental Analysis of Urinary Stones Using EDXRF Based on Fundamental Parameter Approach. Biol Trace Elem Res 195, 417–426 (2020). https://doi.org/10.1007/s12011-019-01884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01884-3