Abstract
From the time of dietary intake to their utilization, the number of important interactions occurs among mineral elements, which can affect their bioavailability because of similarity in physicochemical properties and common absorptive pathways. However, the studies that have analyzed the interactions among copper, iron, and zinc have conflicting results and need further exploration. HT-29 cells grown to confluence in 6-well plates were incubated with increasing concentrations (0 to 200 μM) of Cu, Fe, and Zn for 3 and 6 h for uptake studies. Interaction studies involved measuring the uptake of metal in the presence of 0:1–4:1 ratio of the other metal for 3 h using atomic absorption spectrophotometer. The concentration of metal biomarkers and cytokines was also measured in the cell lysate following extracellular supplementation. The presence of 50 μM Zn significantly decreased (P < 0.05) cellular Cu uptake in HT-29 cells at 0.5:1 Cu:Zn ratio and also the cellular Fe uptake at the ratios 0.5:1, 2:1, and 4:1 Fe:Zn. The presence of 50 μM Fe significantly (P < 0.05) decreased cellular Cu uptake at the ratios 1:1, 2:1, and 4:1 Cu:Fe. The concentration of metallothionein responded significantly (P < 0.05) to changes in extracellular Zn concentration (supplementation and depletion). There was a decrease in concentration of IL-1β and TNF-α (P < 0.05) with an increasing extracellular concentration of Cu and Fe. The results of the study indicated that the presence of one mineral in the diet and multi mineral supplement may influence the bioavailability of the other mineral. Copper and iron may find application in promoting gut health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mineral levels in the body are kept in a state of steady harmony and at fine-tuned ratios which ensure optimal functioning of the organ system. The deficiency of minerals (Cu, Zn, and Fe) in the body leads to an increased absorption from the diet or vice versa, thereby correcting imbalance. In an attempt to self-regulate, the body makes internal adjustments due to which a surplus amount of one often creates deficiency of other minerals. From the time of dietary intake to utilization of mineral elements in the body, a large number of metabolic interactions take place affecting their nutritional status [1]. Awareness of these metal-metal interactions is important to develop effective strategies in order to improve micronutrient status [2]. Multi mineral supplements, which are complex formulations of several minerals, do not convey the whole truth as depicted on their labels, and an important consideration of mineral-mineral interaction is often left unchecked. Micronutrients with similar physicochemical characteristics and shared absorption pathways compete for transport proteins, and the bioavailability is affected [2]. The detailed information on the safe upper intake level of the nutrients is important to minimize the adverse effects on mineral absorption caused by interactions between minerals [3]. Information is scanty about the optimal molar ratios of the minerals for development of different formulations. Also, a unifying hypothesis is not yet established for the interactions that take place among Cu, Zn, and Fe. Therefore, the present study was carried out with an aim to establish the antagonistic or synergistic interactions among Cu, Zn, and Fe at different molar ratios.
The human colon adenocarcinoma cell line HT-29 was first established in 1964 from a primary tumor of a 44-year-old Caucasian female by Fogh and Trempe [4]. HT-29 is a suitable model to depict metal bioavailability at the level of the intestine since the expression of proteins characteristic to the human intestinal epithelium in vivo is found to be similar [5]. Identification of biomarkers that respond to metal status in the body is difficult under in vivo conditions. However, the bioavailability of metals can be studied using various intracellular biomarkers that respond to extracellular metal concentration. Ferritin—an intracellular globular protein complex consisting of 24 protein subunits—is a primary iron storage protein in both the eukaryotic and prokaryotic organisms. In one of the studies carried out by Glahn et al., it was reported that the synthesis of ferritin in Caco-2 cells is in proportion to the iron content of the culture medium. Therefore, ferritin formation in Caco-2 cells was considered a sensitive biomarker of Fe uptake and a determinant of iron bioavailability [6]. Low iron bioavailability leading to iron deficiency anemia in individuals is a major nutritional problem throughout the world. Food and mineral supplements rich in iron are used to circumvent this nutritional disorder, and hence, measurement of iron bioavailability in supplements becomes a necessity. The intracellular ferritin levels’ response to increasing iron concentration in the HT-29 cell line may be useful to understand the iron homeostatic mechanisms involved in uptake, transport, and absorption process. Metallothionein, a family of small–molecular weight proteins with a high content of cysteine residues, is present in many tissues, and the magnitude of its RNA and protein expression depends on the amount of zinc absorbed [7]. The relationship of intestinal metallothionein synthesis in HT-29 cells to supplemented and depleted zinc has not been explored. An intracellular mechanism operates to regulate Zn flux by the small intestine and account for the regulatory phase of the absorption process [8] wherein Zn enhances the accumulation of MT. Zn homoeostasis is maintained in the gut through the absorption and excretion mechanisms operating at the level of the intestine [9].
The intestinal mucosa consists of a layer of epithelial cells joined together by tight junctions. It is supported by a transcellular pathway for the absorption of nutrients and a complex immune system [10]. Along with their role in nutrient absorption, enterocytes play a major role in the initiation and regulation of the mucosal immune response in the intestine [11, 12]. A large number of regulatory molecules like the cytokines and growth factors are secreted by the intestinal epithelial cells. The mucosal lining of the gut generally forms a barrier that separates the luminal contents from the immune cells present beneath. Any abnormal activation of the underlying immune cells leads to an overproduction of the inflammatory cytokines (tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6) which damages the intestinal epithelial monolayers leading to a state of mucosal inflammation and therefore disturbing the overall gut health and integrity [13, 14]. We tried to study the responsiveness of HT-29 cells to increasing metal supplementation to see what role metals can play in maintaining the overall gut health and integrity by influencing the levels of pro-inflammatory cytokines. Studies in the past have shown that a proper balance between the pro-inflammatory and inflammatory cytokines is needed to prevent intestinal epithelial inflammation and promote gut health [15].
Therefore, the present study was carried out with the following aims and objectives: (a) to study the effect of interactions between metals, (b) to study biomarkers that respond to metal concentration in culture media which helps in maintaining cellular homeostasis, and (c) to identify cytokine changes in response to extracellular metal concentration.
Material and Methods
HT-29 Cell Lines
HT-29 cells were obtained from NCCS, Pune at passage numbers 25–40, and routinely grown in DMEM (Dulbecco’s modified Eagle’s medium) high glucose (D7777, Sigma-Aldrich) + 10% fetal bovine serum (FBS) (HiMedia Laboratories) in a 75-cm2 tissue culture flask to provide stock cultures of cells. The cells were incubated at 37 °C in humidified air (95%) and CO2 (5%). The medium was changed every second day and cells were passaged at approximately 70% confluence. For experimental purpose, approximately, 1 × 105 cells were grown in 6-well plates at 37 °C and 5% CO2 until confluency.
Cell Viability by MTT Assay
The viability of cells was measured using MTT assay which is a colorimetric assay that measures the reduction of yellow 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) by a mitochondrial enzyme succinate dehydrogenase [16].
Dose- and Time-Dependent Treatment of Metals in Cultured Cells
Once confluent, HT-29 cells were supplemented with different concentrations (0–200 μM concentration) of Zn (ZnSO4·7H2O), Cu (CuSO4·5H2O), and Fe (FeCl3·3H2O) for 3 and 6 h to study dose- and time-dependent uptake of metals by the cell. All the chemicals used were obtained from Sigma-Aldrich, St. Louis, MO, USA.
Interaction Studies
In a set of experiments, confluent HT-29 were treated with Zn at concentration of 50 μM along with varying concentration of Cu at 25, 50, 100, and 200 μM. Similarly, in another set, same concentrations of Cu were used in the medium, keeping Fe at a constant level of 50 μM. In third set of experiments with a constant 50 μM Zn, variation in Fe concentration (25, 50, 100, and 200 μM) was made and interactions were studied between the two metals for 3 h.
Preparation of Cellular Extracts for Metal Analysis and Estimation of Intracellular Metal Concentration
After the treatment of metals alone or in combination with other metals, cells were washed thrice with phosphate-buffered saline (PBS), treated with commercially available trypsin (0.25% (w/v) trypsin-0.53 mM ethylenediaminetetraacetic acid (EDTA)), and cell suspension collected in sterilized microcentrifuge tubes. The cell suspension was then centrifuged at 3000 rpm for 15 min. The cell pellet obtained was washed thrice with 1X PBS and dissolved in appropriate volume of 5% HNO3. The cell suspension was digested overnight at 95 °C to destroy the organic matter. The concentration of Cu, Zn, and Fe in the cell lysates obtained after digestion was determined using appropriate dilutions in 1% HNO3 by flame atomic absorption spectrophotometer (Thermo Electron Corporation, UK M6 Spectro with integrated software SOLAR AA). The intracellular concentration of Cu, Zn, and Fe was determined to measure the effect of extracellular added metal concentration (both dose- and time-dependent metal concentration) on the uptake of metal by the cells and also the effect of extracellular added concentration of one metal on the cellular concentration of the second metal (interaction studies).
Cell Culture Experiments to Determine Cellular Concentration of Metallothionein, Ferritin, Ceruloplasmin
HT-29 cells grown in 6-well plates once confluent were treated with 10 μM, 25 μM, 50 μM, and 100 μM Zn and 1.2 mM diethylenetriaminepentaacetic acid (DTPA) concentration for a period of 6 h to see the effect of supplementation and depletion (DTPA) of Zn (extracellular) on the metallothionein biomarker (studied using Human MT1E (metallothionein 1E) ELISA Kit, Elabscience) in addition to the control. The measurement of ferritin (Fe biomarker) involved supplementing confluent HT-29 cells in 6-well plates to increasing iron concentration (25 μM, 50 μM, 100 μM, and 200 μM Fe) for 3 h in addition to the control (Bio-Detect ferritin (ELISA) kit). For ceruloplasmin (Cu biomarker) measurement, HT-29 cells were supplemented with 25 μM and 50 μM Cu for 3 h in addition to control cells to see the effect of supplemental copper on the intracellular concentration of Cu (Euro Diagnostic kit based on turbidimetry).
Preparation of Cell Lysate for Estimation of Ferritin, Metallothionein, and Ceruloplasmin
Cells grown to confluence were treated with extracellularly supplemented and depleted metal, washed thrice with 1X PBS, and trypsinized, and cell suspension collected in autoclaved Eppendorf. The cell suspension was then centrifuged at 3000 rpm for 15 min. The cell pellet obtained was washed thrice and suspended in PBS. Cell lysates were prepared by sonicating for 2 × 10 s at 20 kHz (Ultra Sonic Liquid Processor, SONICS & MATERIAL, USA). The cell lysate obtained was stored at − 80 °C until analysis.
Metallothionein, Ferritin, and Ceruloplasmin Protein Expression
Metallothionein concentration in the cell lysates was determined using Sandwich ELISA method (Elabscience). Intracellular ferritin concentration was estimated by quantifying ferritin protein using Sandwich ELISA at 450 nm (Bio-Detect ferritin kit). The concentration of ferritin in cell culture lysates was determined from a suitable calibration curve and finally expressed as nanograms of ferritin per mg protein. The protein concentration in cell culture lysates was determined using Bradford reagent. Ceruloplasmin was determined by turbidimetry method (Euro Diagnostic Systems).
Cellular Concentration of Pro-inflammatory Cytokines
HT-29 cells were treated to increasing extracellular concentration of Cu, Zn, and Fe for 3 h in 6-well plates. The preparation of cell lysates for cytokines is the same as discussed above for the biomarker studies (metallothionein, ferritin, and ceruloplasmin). The concentration of cytokines (IL-6, IL-1β, TNF-α, and IFN-γ) was then determined in the cell lysates after appropriate dilutions using ELISA method (Elabscience).
Statistical Analysis
Data analysis was performed using GraphPad Prism 5 software. Data is presented as mean ± SD. Unpaired t tests were used to study interactions between the two metals. Analysis of differences between the different treated cell line groups to study metal-dependent biomarkers and inflammatory cytokines was done using one-way analysis of variance (ANOVA) with Bonferroni multiple comparison post hoc tests. Statistical significance was accepted at P < 0.05 for all tests.
Result
The percentage viability of cells subjected to 0–200 μM Cu, Zn, and Fe concentrations calculated using MTT assay was found to be 85–90%.
- 1.
Metal uptake and interaction: Dose- and time-dependent metal uptake by the cells is demonstrated in Fig. 1a–c. There was a dose-dependent uptake of Zn, Cu, and Fe by HT-29 cells at both 3 h and 6 h. Interaction of metals is shown in Fig. 2a–c. Presence of Zn at 50 μM concentration inhibited uptake of Cu and Fe at all ratios tested except at equimolar concentration (Fig. 2a, c). Similarly, Fe at 50 μM inhibited uptake of Cu if latter was present in higher concentrations, i.e., 2 and 4 times (Fig. 2b).
- 2.
Expression of metal biomarkers: The intracellular concentration of ceruloplasmin, ferritin, metallothionein in response to extracellular supplementation and depletion is depicted in Fig. 3. The concentration of metallothionein in HT-29 cells responded to extracellular Zn concentration with significant increase being observed at 100 μM Zn in comparison with the control (P < 0.001). The concentration of metallothionein decreased significantly on addition of DTPA—an extracellular Zn chelator (1.2 mM) in comparison with 50 μM Zn (P < 0.01) and 100 μM Zn (P < 0.001) (Fig. 3a). The concentration of ceruloplasmin in HT-29 cells increased in response to extracellular added Cu in HT-29 cells. When HT-29 cells were supplemented with increasing Cu concentration (25 μM Cu and 50 μM Cu), the concentration of ceruloplasmin in HT-29 cells increased proportionately although the change was insignificant (Fig. 3b). The concentration of ferritin in HT-29 cells also increased proportionately (although insignificantly) in response to increasing extracellular added Fe concentration (Fig. 3c).
- 3.
Expression of cytokines in response to extracellular metal concentrations: The concentrations of cytokines, i.e., TNF-α, IFN-γ, IL-1β, and IL-6, were studied in HT-29 cells. It was observed that the cellular concentration of TNF-α decreased significantly (P < 0.05) with increased Fe supplementation in the medium (Fig. 4a). The concentration of IL-1β also decreased with increasing Fe concentration with significant (P < 0.05) changes being observed at 100 μM Fe with respect to 25 μM Fe (Fig. 4b). With increasing Fe concentration, significant (P < 0.05) changes were observed at 50 μM with respect to both the control and 25 μM Fe in case of IL-6 and with respect to only 25 μM Fe for IFN-γ (Fig. 4c) (Fig. 4d). The concentration of both TNF-α and IL-1β in HT-29 cells decreased with increasing Cu concentration with significant (P < 0.05) decrease being observed at 200 μM Cu with respect to 25 μM Cu and 50 μM Cu in case of TNF-α (Fig. 5a) and at 200 μM Cu with respect to the control, 25 μM Cu and 50 μM Cu in case of IL-1β (Fig. 5b). No significant change was observed in IL-6 and IFN-γ concentration with increasing Cu concentration (Fig. 5c, d).
Fig 1 Fig 2 Fig 3 The concentration of metal-dependent biomarkers in response to extracellular added metal concentration. a Metallothionein. b Ceruloplasmin. c Ferritin. *, significant w.r.t control (P < 0.001); #, significant w.r.t 10 μM Zn (P < 0.001); @, significant w.r.t 20 μM Zn (P < 0.001); $, significant w.r.t 50 μM Zn (P < 0.05 for 100 μM Zn and P < 0.01 for DTPA); ^, significant w.r.t 100 μM Zn (P value < 0.001)
Fig 4
Discussion
Cu, Zn, and Fe are essential elements involved in various important metabolic pathways. Although the three metals play an important role in the body, taking an excess of one nutrient can affect the absorption of other metals. Various studies in the past have been carried out to study the effect of combined supplementation of two metals, but no conclusive evidence has been reached regarding the exact mechanism and direction of interaction among Cu, Zn, and Fe [17], and the doses and the ratios of these metals necessary to make these interactions are still debatable. A unifying hypothesis is not yet established for the effects or imbalances among these elements. We have tried using different ratios of the metals keeping the concentration of one of the metals constant for a limited period of 3 h only which was found sufficient enough for uptake studies using the HT-29 cell line. Most of the studies in the past have been carried out using radioactive isotopes of a particular metal and limited to uptake studies of single metal.
The dietary level of Cu, Zn, and Fe is known to influence the absorption of each other over a given time frame at the intestinal level [18]. In the present study, we have tried to study the interaction among Cu, Zn, and Fe when the cells were supplemented with a metal either alone or in combination with the second metal for a period of 3 h.
Dose-Dependent Cu Uptake and Its Interaction with Zn and Fe
When HT-29 cells were supplemented with Cu alone for 3 and 6 h, we saw a dose-dependent significant increase (P < 0.05) in the cellular concentration of Cu in HT-29 cells which shows that as the concentration of Cu in the medium increased, the cellular concentration of Cu increased linearly. However, when Cu was supplemented in the presence of a fixed molar ratio of Zn, we found a significant decrease (P < 0.05) in Cu absorption at Cu:Zn ratio of 0.5:1 in comparison with when Cu was supplemented alone to HT-29 cells. Studies in the past have shown that the presence of high amount of zinc in the diet can lead to a reduction in the copper status in humans which can be explained on the basis of interference in Cu absorption by Zn at the intestinal level [19, 20]. Similar results carried out in an in vitro system by Reeves et al. (1998) [21] have shown significantly decreased cellular concentration of copper in Caco-2 cells in response to an increased media Zn concentration. However, a study carried out by Arredondo et al. [22] reported no inhibition in Cu uptake by the intestinal cells when supplemented with increased extracellular zinc. It is important to mention here that when Cu and Zn were present in equimolar ratios (1:1), no significant decrease in cellular Cu concentration was observed in the present study. Cu and Zn might be competing for the binding sites during their uptake, but since both of them were present in same concentrations in the extracellular medium, Cu uptake was not affected by the equal concentration of Zn in the medium.
When HT-29 cells were supplemented with a combination of Cu and Fe, with an increasing Cu concentration and a fixed Fe concentration, we observed a reduction in cellular Cu concentration at Cu:Fe ratios of 1:1, 2:1, and 4:1 with significant reduction at 2:1 and 4:1 molar ratio only. Cu-Fe interactions can be attributed mainly to their similar metabolic fates in addition to the similar atomic radii and positive charges [23]. The reciprocal relationship between Cu and Fe has been studied in many tissues [24, 25]. For example, during states of iron deficiency, Cu is known to accumulate in the liver and in the intestinal mucosa and its concentration increases during a state of Fe deprivation. Evidence from cell and molecular studies indicates that the antagonistic interaction between Cu and Fe can be explained on account of a competitive binding to a divalent metal transporter DMT-1 [26, 27]. The finding of the present study in addition to previous studies reporting decreased cellular Cu concentration in the presence of Fe raises a question mark on the use of Fe supplements during various diseased conditions of chronic kidney disease and malabsorptive disorders including Crohn’s disease and colitis.
Dose-Dependent Iron Uptake and Its Interactions with Zn and Cu
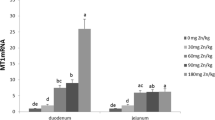
When Fe was supplemented alone at increasing concentrations for a period of 3 and 6 h to HT-29 cells, we found a significant dose-dependent increase in cellular Fe concentration. However, when Fe was supplemented in combination with a fixed molar ratio of Zn, we observed a significant reduction in cellular Fe concentration at all the ratios of Fe:Zn (0.5:1, 2: 1, and 4:1) except at 1:1 ratio.
Various studies were carried out in humans and cell culture systems to determine the effect of Zn on Fe absorption. A study by Donangelo et al. has reported a significant reduction in plasma ferritin and the percentage of transferrin saturation in young women with low iron reserves in the Zn-supplemented group [28]. Also, Rossander-Hulten et al. [29] reported a significant (56%) reduction in iron absorption using 5:1 ratio (Zn:Fe) when given in the form of water. Studies carried out in in vitro systems have also reported similar results. A study carried out by Arredondo et al. [22] and Iyengar et al. [30] using an in vitro cell culture system showed decreased intracellular Fe concentrations in Caco-2 cells in response to an increased extracellular Zn concentration in the medium. The possible reason for the negative interactions between Fe and Zn is the shared absorptive pathways employing DMT-1 as a common transporter for both Zn and Fe. Iyengar et al. [30] have also pointed to a concept of mixed inhibition of cellular Fe uptake by the cells in the presence of Zn as it caused a decrease in all the kinetic constants Km, Vmax, Umax, and Ku.
Expression of Metallothionein, Ferritin, and Ceruloplasmin as Biomarkers of Metals in HT-29 Cells
The synthesis of metallothionein is transcriptionally regulated by molecules like Zn, Cu, and Cd, and its levels are influenced by the nutritional status of humans. Studies carried out in the past have shown that the synthesis of metallothionein is induced within the intestinal cells by parenteral or oral Zn administration [31, 32].
We have tried to study the responsiveness of metallothionein to increasing and depleted Zn concentrations in HT-29 cells. We found that on increasing extracellular Zn concentration (0, 10, 20, 50, and 100 μM Zn) in the medium, the concentration of metallothionein in the HT-29 cells increased. Also, the presence of DTPA (1.2 mM), an extracellular Zn chelator of Zn, resulted in decreased metallothionein concentration in the cells. We found a highly significant correlation (P < 0.001) between the cellular Zn concentration and the metallothionein concentration in the cells. These findings suggest that metallothionein directly responds to extracellular Zn concentration and thus can be an indicator of Zn nutritional status in the body. Similar results have been reported in a study by Moltedo et al. [33] showing increased accumulation of metallothionein in Caco-2 cells as a response to increased Zn concentration in the medium.
To prove that ferritin can be used a direct marker of Fe status, we subjected HT-29 cells to increasing Fe (0, 25, 50, and 100 μM) concentrations for a period of 3 h. Ferritin expression increased in response to increased extracellular Fe concentration in the medium. Cellular Fe is mainly stored as ferritin, and its expression in intestinal epithelial cells increases in response to iron that has been taken up by the cell. On increasing Fe concentration in the medium, there is an increased uptake of Fe probably by the transporter located in the membrane of intestinal epithelial HT-29 cells which results in increased ferritin expression within the cells. We found a significant correlation (P < 0.05) between the cellular Fe concentration and ferritin concentration in the cultured HT-29 cells. A study carried out in Caco-2 cells by Glahn et al. [6] has shown that ferritin is a sensitive biomarker of Fe bioavailability which eliminates the need of using radiolabeled Fe to measure bioavailability of Fe in various foods thereby cutting cost.
Ceruloplasmin activity was tested in HT-29 cells subjected to increasing Cu concentrations (0, 25, and 50 μM) for a period of 3 h to establish its role as a potential biomarker which responds to dietary Cu levels. We found that on incubating HT-29 cells to increasing Cu concentration, we found a dose-dependent increase in the cellular concentration of ceruloplasmin although the changes were not significant. An in vitro study carried out in HepG2 cells have reported similar observations of increased ceruloplasmin expression when loaded with copper [34]. Also, a study carried out by Ranganathan et al. [35] showed that ceruloplasmin expression enhanced in response to higher dietary copper intake in serum.
Levels of Pro-inflammatory Cytokines in Response to Increasing Cu, Zn, and Fe Supplementation
A tightly regulated immune balance is required to maintain intestinal integrity and improve overall health. Therefore, it is important to maintain immune homeostasis within the intestine, and cytokines, one of the major molecules of the immune system, are involved in regulating such homeostasis. The cytokine milieu within the intestinal epithelial cells affects the immune regulatory processes and therefore promotes overall health. Micronutrients and vitamins modulate cytokine secretion, and their presence or absence in the diet may affect the overall cytokine response in the intestine. In an attempt to study the response of pro-inflammatory cytokines to increased levels of micronutrients, we subjected HT-29 cells to increased Cu, Zn, and Fe concentrations (0, 25, 50, 100, and 200 μM) for a period of 3 h. The addition of Cu in increasing concentration to the intestinal cells resulted in decreased levels of IL-1β and TNF-α with significant changes (P < 0.05) being observed in case of cells supplemented with 200 μM Cu in comparison with the control, 25 and 50 μM Cu. Also, supplementing cells with increasing Cu concentrations resulted in decreased levels of IFN-γ with increased Cu supplementation although the change was not significant. These findings provide a new insight into a role that Cu may play in improving gut health by decreasing the levels of pro-inflammatory cytokines. Cu supplementation may prove beneficial in patients with inflammatory bowel diseases like Crohn’s disease and ulcerative colitis. In a number of studies, it was reported that Cu was reduced in patients with Crohn’s disease [36, 37].
As regards the role of Zn in improving gut health, supplementing Zn in increasing concentrations to HT-29 cells in the present study did not result in any change in the levels of cellular pro-inflammatory cytokines. Zinc is known to have a dual effect on the secretion of pro-inflammatory cytokines because it is known to trigger as well as suppress the release of these cytokines by monocytic cell lineages [38, 39]. However, we did not find any such effect of supplementing Zn in our study. This is in contrast to the studies carried out in various other cell lines like THP-1 and HL-6 where the levels of pro-inflammatory cytokines like TNF-α and IL-1β were decreased in zinc-sufficient cells in comparison with zinc-deficient cells [40]. Zinc is an essential trace element for living organisms, and zinc deficiency is closely linked to the impaired mucosal integrity leading to induction of pro-inflammatory response. A growing body of evidence suggests that zinc deficiency increases the concentrations of inflammatory cytokines and oxidative stress; however, the effect of zinc on the gene expression and production of cytokines is cell lineage–specific, and studies of the past have shown conflicting data. We have used the HT-29 cell line to study cytokine changes in response to Zn levels, and to the best of our knowledge, this is the first study using this specific cell line for cytokine changes in response to extracellular metal levels.
When HT-29 cells were supplemented with increasing Fe concentrations (0, 25, 50, 100, and 200 μM Fe), we found a decrease in the levels of TNF-α and IL-1β. Similar results were found in a study carried out by Foster et al. [15] who reported decreased cellular levels of TNF-α and IL-1β in Caco-2 cells supplemented with increasing Fe concentration (0.5 and 50 μmol/L). These findings point to an idea that iron supplementation may prove beneficial in inflammatory bowel disorders where it can reduce inflammation by decreasing the levels of pro-inflammatory cytokines. The destruction of tissue in the gut occurs when there is a constitutive activation of immune response, and Fe may help to overcome this excessive activation of immune response by bringing about a decrease in the level of IL-1β and TNF-α. Therefore, iron may play an important role in maintaining overall intestinal integrity and needs further exploration as dietary intervention for modulating immune response.
Summary
These findings on interactions between Cu, Zn, and Fe indicate that there are shared absorption pathways employing a common transporter for the uptake of metals, resulting in competition between the metals for binding to a common transporter. Metal-metal interactions at the intestinal level can help in defining bioavailability of a particular element from diet and multi mineral supplements. This part of the study also provided evidence to suggest the role of metallothionein as a biomarker for Zn which can be used to assess its toxicity and deficiency. The cellular concentrations of some of the cytokines like TNF-α, IL-1β, IFN-γ, and IL-6 responded to extracellular Fe and Cu concentrations, which implies that these metal elements may have some important role to play in gut health. Further research is needed to actually identify and ascertain the role of trace elements in promoting gut health.
Limitations of the Study
Besides metal-metal interaction, other factors like presence of macronutrients, trace elements, hormone profile, metabolic status of organism, and gut microflora will also affect the bioavailability. Being an in vitro study, interactions between two metals are direct, and to confirm the findings, in vivo studies will be required.
References
Windisch W (2002) Interaction of chemical species with biological regulation of the metabolism of essential trace elements. Anal Bioanal Chem 372:421–425
Sandstorm B (2001) Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr 85(2):S181–S185
Troost FJ, Brummer RJM, Dainty JR, Hoogewerff JA, Bull VJ, Saris WHM (2003) Iron supplements inhibit zinc but not copper absorption in vivo in ileostomy subjects. Am J Clin Nutr 78:1018–1023
Fogh J, Trempe G (1975) New human tumor cell lines. In: Fogh J (ed) Human tumor cell in vitro, 1st edn. Springer, New York, pp 115–159
Lenaerts K, Bouwman F, Lamers W, Renes J, Mariman E (2007) Comparative proteomic analysis of cell lines and scrapings of the human intestinal epithelium. BMC Genomics 8:91
Glahn RP, Lee OA, Yeung A, Goldman MI, Miller DD (1998) Caco-2 cell ferritin formation predicts nonradiolabelled food iron availability in an in vitro digestion/ Caco-2 cell culture model. J Nutr 128:1555–1561
Davis SR, Cousins RJ (2000) Metallothionein expression in animals: a physiological perspective on function. J Nutr 130:1085–1088
Maret W, Krezel A (2007) Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol Med 13(7 –8):371–375
Michalczyk AA, Ackland ML (2013) hZip 1 (HSLC39A1) regulates zinc homeostasis in gut epithelial cells. Genes Nutr 8(5):475–486
Heyman M (2001) Symposium on dietary influences on mucosal immunity. How dietary antigens access the mucosal immune system. Proc Nutr Soc 60(4):419–426
McGee DW (1999) Inflammation and mucosal cytokine production. In: Ogra PL, Mestecky J, Lamm M, Strober W, Bienenstock J (eds) . Academic Press, Mucosal Immunity, pp 559–573
Sun M, He C, Cong Y, Liu Z (2015) Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol 8(5):969–978
Shanahan F (2001) Inflammatory bowel disease: immunodiagnostics, immunotherapeutics and ecotherapeutics. Gastroenterol 20(3):622–635
Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347:417–429
Foster SL, Richardson SH, Failla ML (2001) Elevated iron status increases bacterial invasion and survival and alters cytokine/chemokine mRNA expression in Caco-2 human intestinal cells. J Nutr 131:1452–1458
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Kambe T, Weaver B, Andrews G (2008) The genetics of essential metal homeostasis during development. Genesis 46:214–228
Nishito Y, Kambe T (2018) Absorption mechanisms of Iron, copper, and zinc: an overview. J Nutr Sci Vitaminol 64:1–7
King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ (2016) Biomarkers of nutrition for development (BOND)-zinc review. J Nutr 146:858S–885S
Ozden TA, Gokçay G, Cantez MS, Durmaz O, Işsever H, Omer B, Saner G (2015) Copper, zinc and iron levels in infants and their mothers during the first year of life: a prospective study. BMC Pediatr 15:157
Reeves PG, Briske-Anderson M, Johnson L (1998) Physiological concentrations of zinc affect the kinetics of copper uptake and transport in the human intestinal cell model, Caco-2. J Nutr 128:1794–1801
Arredondo M, Martinez R, Nunez MT, Ruz M, Olivares M (2006) Inhibition of iron and copper uptake by iron, copper and zinc. Biol Res 39(1):95–102
Ha JH, Doguer C, Wang X, Flores SR, Collins JF (2016) High iron consumption impairs growth and causes copper deficiency anaemia in weanling Sprague Dawley rats. PLoS One 11(8):1–19
Fox PL (2003) The copper-iron chronicles: the story of an intimate relationship. Biometals 16(1):9–40
Ravia JJ, Stephen RM, Ghishan FK, Collins JF (2005) Menkes copper ATPase (Atp7a) is a novel metalresponsive gene in rat duodenum, and immunoreactive protein is present on brush border and basolateral membrane domains. J Biol Chem 280(43):36221–36227
Jiang L, Garrick MD, Garrick LM, Zhao L, Collins JF (2013) Divalent metal transporter 1 (Dmt1) mediates copper transport in the duodenum of iron-deficient rats and when overexpressed in iron-deprived HEK-293 cells. J Nutr 143(12):1927–1933
Tennant JP, Jai T, Sharp PA (2004) Mechanisms involved in copper uptake by human intestinal epithelial cells. Proc Nutr Soc 63:35A
Donangelo CM, Woodhouse LR, King SM, Viteri FE, King JC (2002) Supplemental zinc lowers measures of iron status in young women with low iron reserves. J Nutr 32(7):1860–1864
Rossander-Hultén L, Brune M, Sandström B, Lonnerdal B, Hallberg L (1991) Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr 54:152–156
Iyengar V, Pullakhandam R, Nair KM (2009) Iron-zinc interaction during uptake in human intestinal Caco-2 cell line: kinetic analysis and possible mechanism. Indian J Biochem Biophys 46:299–306
Marreiro DD, Cruz KJ, Morais JB, Beserra JB, Severo JS, de Oliveira AR (2017) Zinc and oxidative stress: current mechanisms. Antioxidants (Basel) 6(2):24
Grungreiff K, Reinhold D, Wedemeyer H (2016) The role of zinc in liver cirrhosis. Ann Hepatol 15(1):7–16
Moltedo O, Verde C, Capasso A, Parisi E, Remondelli P, Bonatti S, Alvarez-Hernandez X, Glass J, Alvino CG, Leone A (2000) Zinc transport and metallothionein secretion in the intestinal human cell line Caco-2. J Biol Chem 275(41):31819–31825
Fosset C, Danzeisen R, Gambling L, McGaw BA, McArdle HJ (2009) Cu loading alters expression of non-IRE regulated, but not IRE regulated Fe dependent proteins in HepG2 cells. J Inorg Biochem 103:709–716
Ranganathan PN, Lu Y, Jiang L, Kim C, Collins JF (2011) Serum ceruloplasmin protein expression and activity increases in iron-deficient rats and is further enhanced by higher dietary copper intake. Blood 118:3146–3153
Hwang C, Ross V, Mahadevan U (2012) Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm Bowel Dis 18:1961–1981
Filippi J, Al-Jaouni R, Wiroth JB, Hebuterne X, Schneider SM (2006) Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis 12:185–191
Bao B, Prasad AS, Beck FW, Godmere M (2003) Zinc modulates mRNA levels of cytokines. Am J Phys 285:E1095–E1102
Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ (2004) Abrogation of nuclear factor-ƙB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-α. Am J Pathol 164:1547–1556
Prasad AS (2014) Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr 1:1–10
Acknowledgments
The authors are highly grateful to Indian Council of Medical Research, Department of Science & Technology and Defence Research and Developmental Organisation (DRDO), for award of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rakhra, G., Masih, D., Vats, A. et al. Study of Metal-Metal Interactions and Their Biomarkers Using an Intestinal Human Cell Line. Biol Trace Elem Res 195, 95–104 (2020). https://doi.org/10.1007/s12011-019-01831-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01831-2