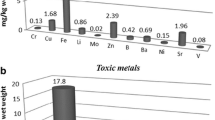
Abstract
The quantity of some essential and non-essential elements of wild edible mushroom samples collected from Tunceli Province of Turkey was determined by using flame and electrothermal atomic absorption spectrometer after microwave digestion. The method accuracy was corrected using standard reference material (NIST SRM 1547-Peach Leaves). The essential element concentrations of analyzed mushroom samples were determined in the range of 0.036–0.563 mg kg−1 for calcium, 1.28–2.55 mg kg−1 for magnesium, 0.054–0.188 mg kg−1 for sodium, 1.00–4.57 mg kg−1 for copper, 212–480 mg kg−1 for iron, and 75–151 mg kg−1 for zinc but cobalt and chromium were not detected. Based on results, there were statistically significant differences between the element contents of analyzed mushroom species. Consequently, according to this study results, the weekly intake and target hazard quotient values of the elements show that the consumption of these mushrooms does not threaten human health.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans have used edible mushrooms as food since ancient times due to their unique and elegant taste. Nowadays, mushrooms are one of the most widely used food sources, and in pharmacy also [1]. These products have been evidenced to be effective as antioxidant, antimicrobial, immunomodulatory, antiviral, anti-inflammatory, antitumor, antiaromatase, cytotoxic, and anticholesterole agents [2,3,4,5,6,7]. Therefore, mushrooms that can be eaten are important food sources which have an important place in balanced nutrition. They contain a wide variety of biomolecules with nutritional and bioactive properties [8, 9]. Edible mushrooms contain many biologically active materials, such as vitamins, nutritive compounds, proteins, polysaccharides, and mineral contents and in contrast, they are poor in calories and fat so they are evaluated as nutritious food all over the world [10,11,12,13,14,15].
Mushrooms are known to be a good mineral source containing Ca, Fe, K, Mg, Mn, Na, P, and Zn in particular. Living organisms require varying amounts of elements such as Ca, Co, Cr, Cu, Fe, K, Mg, Na, and Zn that are essential because they have important roles in biological systems. However, they can also be toxic if taken excessive quantities. Heavy metals including Cd, Ni, and Pb are non-essential; they are toxic and their accumulation in progress of time could cause serious illnesses in human bodies. To estimate the adequacy intake of essential metals and assessing exposure risks from intake of toxic non-essential metals, accurate data that include food composition have vital importance [16, 17].
Wild mushroom species that accumulate high amounts of heavy metals, as Cd, Co, Cu, Hg, Mn, Ni, Pb, and Zn, are important indicators for environmental pollution [18]. Researchers reported that the heavy metal content in edible mushrooms is higher than the other agricultural crops [19]. Among mushroom species, metal analysis is important in white rot fungi due to the ability of accumulate air and soil contaminants. Heavy metals accumulated with edible mushrooms are potentially hazard for human health. In wild edible mushrooms, therefore, determining essential and toxic element levels is very important.
The climate during the year is suitable for the growth of mushrooms in Tunceli region, exclusively in spring and autumn. For this reason, this area has a large mushroom diversity in these months and many people collect and consume edible mushrooms.
In this study, seven indigenous edible mushroom species which are Agaricus campestris, Morchella esculenta, Morchella vulgaris, Amanita vaginata, Langermannia gigantea, Pleurotus eryngii var. ferulae, and Pleurotus eryngii var. eryngii collected from three different regions of Tunceli were analyzed. Thus, the first aim of the present study was to determine the essential (Ca, Co, Cr, Cu, Fe, Mg, Na, and Zn) and non-essential elements (Cd, Ni, and Pb) concentrations using flame atomic absorption spectrometer (FAAS) and electrothermal atomic absorption spectrometer (ETAAS) in these mushroom species. For this reason, it was also aimed that there is a correlation between the metal components the mushroom species and the collected region. The second aim of the current study was to evaluate essential and non-essential element concentrations of analyzed mushrooms and their risk to human health. In the light of the above, these concentrations were then compared against the recommended maximum levels allowed in food. In addition, the quality of the mushroom for human consumption was assessed.
Materials and Methods
Sample Collection and Preparation
The wild edible mushroom species [14, 20,21,22,23,24,25,26,27,28,29] used in this study (A. campestris, M. esculenta, M. vulgaris, A. vaginata, L. gigantea, P. eryngii var. ferulae, and P. eryngii var. eryngii) were collected from Tunceli region between the 2017 and 2018 years (Fig. 1). Each species of mushrooms were collected from three different regions for determining the correlation between the element components of species and the regions. Photos of mushroom species were taken in their natural habitats (Photo 1). The mushroom samples were first cleaned manually with soft brushes from forest and soil debris, then they were transported to the laboratory within 3 h of collection. Firstly, they were cleaned by tap water and then by ultrapure water. The mushroom fruit bodies were divided into several parts with plastic knife and then dried in an oven for 5 days at 70 °C to reach a constant weight before analysis. Then, the dried samples were powdered in mortar. Powdered mushroom samples were stored until they were analyzed in glass jars. Table 1 summarizes analyzed mushroom information including habitat, localization, harvest date, and the families.
Apparatus and Reagents
The measurements were performed using a PerkinElmer AAnalyst™ 800 model with deuterium background correction. Cd, Cr, Co, Cu, Ni, and Pb were determined by ETAAS using argon as inert gas. For Ca, Fe, Mg, Na, and Zn, measurements using air acetylene flame were carried out.
In all experiments, nitric acid, hydrogen peroxide, and metal standard solutions were of analytical grade, and they were obtained from Merck (Darmstadt, Germany) and all solutions were prepared using ultrapure water (Elga, PURELAB). For cleaning the glass and plastic wares, diluted nitric acid and then ultrapure water were used. The element standard solutions used for calibration were prepared by diluting a stock solution of 1000 mg L−1 for Ca, Fe, Mg, Na and Zn and 1000 μg L−1 for Cd, Cr, Co, Cu, Ni, and Pb.
Microwave Digestion Procedure
A microwave digestion system (Berghof, Germany) was used for mushroom samples digestion. About 0.2 g of mushroom samples was taken into the digestion vessel and 2 mL of concentrated nitric acid and 3 mL hydrogen peroxide were added. Mixture was shaken carefully and at least 20 min was waited before the vessel was closed and digestion program applied. After centrifugation, clear solutions were diluted to 30 mL with ultrapure water. Blanks were carried out in the same way. Table 2 represents the microwave digestion system conditions.
Analytical Procedure for Element Analysis and Accuracy
Cd, Co, Cr, Cu, Ni, and Pb concentrations were measured using ETAAS and others were measured using FAAS. The Cd, Cr, Co, Cu, Ni, and Pb in mushroom samples were determined by ETAAS using argon as inert gas. For Ca, Fe, Mg, Na, and Zn, measurements using air acetylene flame were carried out. Analyses were repeated triplicate. The analytical method accuracy was checked using the certified reference material (NIST SRM 1547-Peach Leaves).
Statistical Analysis
Results of analyses were evaluated statistically by using IBM SPSS Statistics 24 (USA). The one-way analysis of variance (ANOVA) was used for data analysis. Moreover, the correlation between the element components in the mushroom species and the collected regions were analyzed using a two-way ANOVA. The results of element contents were expressed as mean value ± standard error (SE) of triplicate measurements.
Risk Assessment
In this study, estimated weekly intake (EWI, mg kg−1 body weight week−1) of elements were calculated and the results were presented in Table 7. When calculating the EWI, concentrations of the studied heavy metals, for a standardized person weighing 70 kg with a consumption of 210 g of dried edible wild mushrooms per week, were considered. FAO/WHO (1993) and USEPA defined Cd, Cr, and Pb values as 7 μg kg−1, 9 μg kg−1, and 25 μg kg−1, respectively, for body weight of a consumer [30, 31]. On the other hand, for some elements, oral reference doses (RfDs) of Cu 40 mg kg−1 body weight/day, Ni 20 mg kg−1 body weight/day, and Zn 300 mg kg−1 body weight/day have been published by the Joint FAO/WHO Expert Committee on Food Additives [32].
The EWI of elements were calculated using the following equation:
Target Hazard Quotient
The target hazard quotient (THQ) has been used as marker because it connects the element concentrations in food with their toxicity, quantity and quality of food consumption, and consumers’ body mass. The combination of many complex parameters provides more comprehensive information to assess the potential health risk of elements in various foods.
An equation used by Ihugba et al. (2018) was preferred to evaluate THQ and it was presented at below [33]:
Exposure frequency (365 days year−1), exposure duration (equivalent to the average lifetime, 70 years), and ingestion rate of mushrooms (g/person/day), assuming 0.5 g and 1.0 g for an average-level consumer and a high-level consumer, are represented by EF, ED, and FIR, respectively. Also, C represents mushroom element concentration as mg kg−1 dry weight. While RfDo is the oral reference dose (mg/kg/day), WAB and TA represented average body weight and averaged exposure time for non-carcinogens (365 days year−1 × ED), respectively. The US EPA stated that an index more than 1 is considered as not safe for human health. Also, for some elements including Cu, Ni, Pb, and Zn, RfDo values were 0.04, 0.02, 0.0035, and 0.3, as mg kg−1 body weight day−1 [33, 34].
Results and Discussion
In the current study, the contents of the essential elements (the macroelements Ca, Mg, and Na and the microelements Cu, Co, Cr, Fe, and Zn) and non-essential elements (Cd, Ni, and Pb) were determined and they were compared in terms of mushroom species in Table 3. In all tested species, the macroelement Mg (1.28 ± 0.03–2.55 ± 0.02 mg kg−1 dw) had the highest concentration; the microelements Fe and Zn were in the range between 212 ± 8 mg kg−1 dw–480 ± 25 mg kg−1 dw and 75 ± 2 mg kg−1 dw–151 ± 7 mg kg−1 dw, respectively. From the data given in Table 3 describing the concentrations of essential elements, M. esculenta has the highest concentration of essential elements as Ca (0.563 ± 0.018 mg kg−1 dw), Fe (480 ± 25 mg kg−1 dw), and Na (0.172 ± 0.004 mg kg−1 dw). Also, A. vaginata is rich in terms of Cu (5.17 ± 0.11 mg kg−1 dw) and Fe (462 ± 10 mg kg−1 dw) contents.
It was detected that there is a close relationship between the amount of mineral content and the mushroom species statistically (Table 3). The macroelement Ca content of across all the tested mushroom species were in the following order: M. esculenta ˃ M. vulgaris ˃ A. vaginata ˃ P. eryngii var. eryngii ≥ P. eryngii var. ferulae ≥ A. campestris ≥ L. gigantea; the amounts of Na were in the following order: P. eryngii var. eryngii ≥ M. esculenta ˃ M. vulgaris ˃ P. eryngii var. ferulae ≥ A. vaginata ≥ A. campestris ≥ L. gigantea; and the amounts of Mg were, respectively, P. eryngii var. eryngii ˃ P. eryngii var. ferulae ˃ A. campestris ˃ A. vaginata ˃ L. gigantean ≥ M. esculenta ≥ M. vulgaris (p < 0.05).
On the other hand, the microelement contents of all mushroom species were examined. According to Table 3, there were statistically significant differences between the analyzed mushroom species as regards content of Cu, Fe, and Zn in their fruit bodies (p < 0.05). The microelement contents of all the mushroom studied were in following the order: for the amount of Cu content, A. vaginata ˃ A. campestris ˃ L. gigantea ˃ M. vulgaris ≥ M. esculenta ˃ P. eryngii var. eryngii ≥ P. eryngii var. ferulae; for the amount of Fe content, M. esculenta ≥ A. vaginata ≥ M. vulgaris > A. campestris ≥ P. eryngii var. ferulae ˃ P. eryngii var. eryngii ˃ L. gigantean; and for the amount of Zn content, L. gigantea ˃ M. vulgaris ≥ A. vaginata ≥ M. esculenta ≥ A. campestris ˃ P. eryngii var. eryngii ≥ P. eryngii var. ferulae. Besides, it was also found that these three mushroom species have the highest amount of Fe contents among all studied species. Also, essential elements Co and Cr and non-essential elements Pb, Cd, and Ni concentrations were found under the detection limit (Table 3).
As stated in several studies in the literature, the element contents are related to mushroom species and their ecosystems [35,36,37]. This study results revealed that there is a close relationship between the amount of essential element contents and their growing region of the examined edible mushroom species.
All analyzed mushroom species’ element composition were compared in terms of both species and collected area. Their results were presented in Tables 4 and 5. It was found out that macroelement contents of four mushroom species and different regions where these are collected were statistically different. Each P.erygii var. eryngii sample has the highest Ca and Na content collected from Nazımiye. Moreover, this mushroom collected from Pülümür has the highest Mg content then other samples (Table 4).
Microelement amount of mushroom species were determined statistically different. In addition, it was also detected that the microelement concentration of these mushrooms changes correspondingly with their geographical habitat (Table 4). Concentration of Cu and Zn were statistically different in A. campestris, L. gigantea, P. eryngii var. eryngi, and P. eryngii var. ferulae species. However, there is no difference in terms of Cu and Zn concentrations for the each these mushroom species in three regions. Finally, A. campestris and P. eryngii var. ferulae Fe concentrations were the highest collected from Nazımiye and Ovacik.
M. esculenta’s, M. vulgaris’, and A. vaginata’s macro- and microelement contents were compared and shown in Table 5. M. esculenta which collected from Kocakoç and Güleç village has the highest content in terms of Ca. Moreover, this mushrooms collected from Kocakoç and Karşılar village have the highest Na content. Lastly, the Mg amount of A. vaginata was the highest collected from Kocakoç and Güleç village. There is no difference when it was compared in terms of microelement concentration of mushrooms in the collected regions. Fe concentrations in M. esculenta, M. vulgaris, and A. vaginata were statistically similar (p ˃ 0.05). When compared the regions, there were no difference in terms of Fe concentrations, too. It was observed that Zn concentrations in M. vulgaris and A. vaginata were statistically similar when assessed in terms of species. On the other hand, these species have the highest Zn content and there was no difference in Zn content among the samples collected from different areas. And, also, A. vaginata has the highest Cu content among these three species. In addition, it was detected that the Cu concentrations of the mushroom did not change correspondingly with their collected region.
Element concentrations of mushrooms have been reported from several studies in Table 6. Also, mushrooms were reported to be an accumulator for toxic metals [38, 51]; white rot fungus are especially good bioaccumulators because of the cell walls of these mushrooms. In white rot fungi, the existence of toxic metals is important because they affect the growth of the fungus and the biodegradation process [52]. Also, they constitute a risk for human health in the case of consumption. In the present study, concentration of non-essential elements as Cd, Ni, and Pb were under the detection limit.
Mushroom Consumption Safety
As consumption of wild edible mushroom is a possible source of metal accumulation in humans, there is great interest in estimation of the weekly intakes of elements through mushroom consumption. The EWI (mg/week/person) and THQs of elements through consumption of mushroom species by Turkish people in the Tunceli Province are illustrated in Table 7. The EWI of elements was calculated on the basis of the concentrations measured in mushroom and daily mushroom consumption rate. As can be seen in Table 7, the values of EWI of Ca, Cu, Fe, Mg, Na, and Zn in mushroom in this study are well below their corresponding permissible tolerable weekly intake for 70 kg person values.
Another evaluation factors for risk assessment of metal intake associated with food and foodstuff are values of THQ. In fact, the THQ values express estimated dose of a contaminant and the reference dose ratio. When it is below the reference dose, there will be no appreciable risk. Because the US EPA stated that an index lower than 1 is considered as safe for human health [34, 53]. Table 7 presents the THQ values on the consumption of meals prepared with analyzed mushroom. Here, ALC (average-level consumer) and HLC (high-level consumer) values represent consuming 0.5 g and 1 g of mushroom from a meal, respectively. In the present study, the range of ALC mean values of Cu, Fe, and Zn of analyzed mushroom is from 1.3E−2 to 6.5E−2, from 1.5E−1 to 3.4E−1, and from 1.3E−1 to 2.5E−1, respectively. Similarly, the range of HLC mean values of Cu, Fe, and Zn of analyzed mushroom is from 2.5E−2 to 1.3E−1, from 3.0E−1 to 6.9E−1, and from 2.5E−1 to 5.0E−1, respectively. When consuming habits are considered, it can be mentioned that the weekly intake of mushroom has no risk for average- and high-level mushroom consumption originating from the local area on Tunceli Province people’s health. Therefore, consumption of mushroom collected from the areas does not constitute a toxicological risk to human health in terms of Cd, Ni, and Pb level.
Conclusion
Regarding the daily intake and safety aspects, the investigated mushroom species were safe for human consumption, because the EWI for an adult consuming 210 g of mushroom, having 70 kg of body weight, was found to be lower than provisional tolerable weekly intake (PTWI) for Ca, Cu, Fe, Mg, Na, and Zn. Also, the value of THQ was found to be below the RfDo and also less 1 (THQ < 1). When mushrooms are consumed at an average level, the concentrations of the other elements have no health risks. But a continuous monitoring of heavy metals in mushroom consumer in Tunceli Province is necessary to insure the prescribed worldwide limit.
References
Tong H, Xia F, Feng K, Sun G, Gao X, Sun L, Jiang R, Tian D, Sun X (2009) Structural characterization and in vitro antitumor activity of a novel polysaccharide isolated from the fruiting bodies of Pleurotus ostreatus. Bioresour Technol 100(4):1682–1686. https://doi.org/10.1016/j.biortech.2008.09.004
Turkoğlu A, Duru ME, Mercan N, Kivrak I, Gezer K (2007) Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem 101(1):267–273. https://doi.org/10.1016/j.foodchem.2006.01.025
Moradali MF, Mostafavi H, Ghods S, Hedjaroude GA (2007) Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int Immunopharmacol 7(6):701–724. https://doi.org/10.1016/j.intimp.2007.01.008
Gern RMM, Wisbeck E, Rampinelli JR, Ninow JL, Furlan SA (2008) Alternative medium for production of Pleurotus ostreatus biomass and potential antitumor polysaccharides. Bioresour Technol 99:76–82. https://doi.org/10.1016/j.biortech.2006.11.059
Chen S, Oh SR, Phung S, Hur G, Ye JJ, Kwok SL, Shrode GE, Belury M, Adams LS, Williams D (2006) Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res 66(24):12026–12034
Zhang M, Cui SW, Cheung PCK, Wang Q (2007) Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Technol 18(1):4–19
Jeong SC, Jeong YT, Yang BK, Islam R, Koyyalamudi SR, Pang G, Cho KY, Song CH (2010) White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr Res 30:49–56
Kalac P (2009) Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem 113(1):9–16
Ferreira ICFR, Barros L, Abreu RMV (2009) Antioxidants in wild mushrooms. Curr Med Chem 16(12):1543–1560
Purkayastha RP, Chandra A (1985) Manual of Indian Edible mushrooms. Today & Tomorrow’s Printers and Publishers, New Delhi, pp 226–244
Sanmee R, Dell B, Lumyong P, Izumori K, Lumyong S (2003) Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chem 84(4):527–532
Manzi P, Marconi S, Aguzzi A, Pizzoferrato L (2004) Commercial mushrooms: nutritional quality and effect of cooking. Food Chem 84:201–206
Agrahar-Murugkar D, Subbulakshmi G (2005) Nutritional value of edible wild mushrooms collected from the Khasi hills of Meghalaya. Food Chem 89(4):599–603
Pereira E, Barros L, Martins ARL, Ferreira ICFR (2012) Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem 130(2):394–403
Reis FS, Barros L, Martins A, Ferreira ICFR (2012) Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food Chem Toxicol 50(2):191–197
Obodai M, Ferreira ICFR, Fernandes A, Barros L, Mensah DLN, Dzomeku M, Urben AF, Prempeh J, Takli RK (2014) Evaluation of the chemical and antioxidant properties of wild and cultivated mushrooms of Ghana. Molecules 19(12):19532–19548
Gebrelibanos M, Megersa N, Taddesse AM (2016) Levels of essential and non-essential metals in edible mushrooms cultivated in Haramaya, Ethiopia. Int J Food Contam 3(2):1–12
Baba H, Ergün N, Özcubukcu S (2012) Antakya (Hatay)’dan toplanan bazı makrofungus türlerinde ağır metal birikimi ve mineral tayini. BİBAD 5(1):5–6
Manzi P, Aguzzi A, Pizzoferrato L (2001) Nutritional value of mushrooms widely consumed in Italy. Food Chem 73:321–325
Meng F, Zhou B, Lin R, Jia L, Liu X, Deng P, Fan K, Wang G, Wang L, Zhang J (2010) Extraction optimization and in vivo antioxidant activities of exopolysaccharide by Morchella esculenta SO-0. Bioresour Technol 101:4564–4569
Oke F, Aslim B (2011) Protective effect of two edible mushrooms against oxidative cell damage and their phenolic composition. Food Chem 128:613–619
Alam N, Yoon KN, Lee JS, Cho HJ, Lee TS (2012) Consequence of the antioxidant activities and tyrosinase inhibitory effects of various extracts from the fruiting bodies of Pleurotus ferulae. Saudi J Biol Sci 19:111–118
Giri S, Biswas G, Pradhan P, Mandal SC, Acharya K (2012) Antimicrobial activities of basidiocarps of wild edible mushrooms of west Bengal, India. Int J Pharm Tech Res 4(4):1554–1560
Badshah H, Qureshi RA, Khan J, Ullah F, Fahad S, Ullah F, Khan AM, Hussain I, Khan N (2012) Pharmacological screening of Morchella esculenta (L) Pers., Calvatia gigantea (Batsch ex Pers.) Lloyd and Astraeus hygrometricus Pers., mushroom collected from South Waziristan (FATA.). J Med Plants Res 6(10):1853–1859
Heleno SA, Stajkovic D, Barros L, Galamoclija J, Sokovic M, Martins A, Queiroz MJ, Ferreira ICFRA (2013) Comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. From Portugal and Serbia. Food Res Int 51:236–243
Li S, Shah NP (2014) Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem 165:262–270
Ren L, Hemar Y, Perera CO, Lewis G, Krissansen GW, Buchanan PK (2014) Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact Carbohydr Diet Fibre 3:41–51
Woldegiorgis AZ, Abate D, Haki GD, Ziegler GR (2014) Antioxidant property of edible mushrooms collected from Ethiopia. Food Chem 157:30–36
Elmastas M, Turkekul I, Ozturk L, Gulcin I, Isildak O, Aboul-Enein HY (2006) Antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta) from North Turkey. Comb Chem High Throughput Screen 9:443–448
WHO (1993) Evaluation of certain food additives and contaminants. Technical Report No. 837. World Health Organization, Genewa
USEPA. USEPA Regional Screening Level (RSL) Summary Table: November 2011
JECFA—Joint Expert Committee for Food Additives (2003) Summary and Conclusions of the 61st Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). JECFA/61/SC. Rome, Italy
Ihugba UA, Nwoko CO, Tony-Njoku FR, Ojiaku AA, Izunobi L (2018) Heavy metal determination and health risk assessment of oyster mushroom Pleurotus tuberregium (Fr.) Singer, collected from selected markets in Imo State. Nigeria. Am J Environ Protect 6(1):22–27
US EPA (Environmental Protection Agency) (2002) A review of the reference dose and reference concentration processes. Risk Assessment Forum, Washington, DC; EPA/630/P-02/002F. Available online at http://www.epa.gov/ncea/raf
Woldegiorgis AZ, Abate D, Haki GD, Ziegler GR (2015) Major, minor and toxic minerals and anti-nutrients composition in edible mushrooms collected from Ethiopia. J Food Process Technol 6(3):2–8
Gast CH, Jansen E, Bierling J, Haanstra L (1988) Heavy metals in mushrooms and their relationship with soil characteristics. Chemosphere 17(4):789–799
Kalac P, Burda J, Staskova I (1991) Concentrations of lead, cadmium, mercury, and copper in mushrooms in the vicinity of a lead smelter. Sci Total Environ 105:109–119
Cocchi L, Vescovi L, Petrini LE, Petrini O (2006) Heavy metals in edible mushrooms in Italy. Food Chem 98:277–284
Tuzen M, Sesli E, Soylak M (2007) Trace element levels of mushroom species from East Black Sea region of Turkey. Food Control 18:806–810
Gursoy N, Sarikurkcu C, Cengiz M, Solak MH (2009) Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem Toxicol 47:2381–2388
Isildak Ö, Turkekul I, Elmastas M, Tuzen M (2004) Analysis of heavy metals in some wild-grown edible mushrooms from the middle black sea region, Turkey. Food Chem 86:547–552
Liu H, Zhang J, Li T, Shi Y, Wang Y (2012) Mineral element levels in wild edible mushrooms from Yunnan, China. Biol Trace Elem Res 147:341–345
Li T, Wang Y, Zhang J, Zhao Y, Liu H (2011) Trace element content of Boletus tomentipes mushroom collected fromYunnan, China. Food Chem 127:1828–1830
Gençcelep H, Uzun Y, Tunçtürk Y, Demirel K (2009) Determination of mineral contents of wild-grown edible mushrooms. Food Chem 113:1033–1036
Sarikurkcu C, Copur M, Yildiz D, Akata I (2011) Metal concentration of wild edible mushrooms in Soguksu National Park in Turkey. Food Chem 128:731–734
Kosanic M, Rankovic B, Rancic A, Stanojkovic T (2016) Evaluation of metal concentration and antioxidant, antimicrobial, and anticancer potentials of two edible mushrooms Lactarius deliciosus and Macrolepiota procera. J Food Drug Anal 24:477–484
Vimala R, Das N (2009) Biosorption of cadmium (II) and lead (II) from aqueous solutions using mushrooms: a comparative study. J Hazard Mater 168:376–382
Ivanić M, Fiket Z, Medunić G, Turk MF, Marović G, Senčar J, Kniewald G (2019) Multi-element composition of soil, mosses and mushrooms and assessment of natural and artificial radioactivity of a pristine temperate rainforest system (Slavonia, Croatia). Ecotoxicol Environ Saf 137:265–271
Falandysz J, Drewnowska M, Chudzińska M, Barałkiewicz D (2017) Accumulation and distribution of metallic elements and metalloids in edible Amanita fulva mushrooms. Ecotoxicol Environ Saf 137:265–271
Šíma J, Vondruška J, Svoboda L, Šeda M, Rokos L (2019) The accumulation of risk and essential elements in edible mushrooms Chlorophyllum rhacodes, Suillus grevillei, Imleria badia, and Xerocomellus chrysenteron growing in the Czech Republic. Chem Biodivers 16:e1800478
Chen XH, Zhou HB, Qiu GZ (2009) Analysis of several heavy metals in wild edible mushrooms from regions of China. Bull Environ Contam Toxicol 83(2):280–285
Baldrian P, Gabriel J (2003) Lignocellulose degradation by Pleurotus ostreatus in the presence of cadmium. FEMS Microbiol Lett 220(2):235–240
Saba M, Falandysz J, Nnorom IC (2016) Mercury bioaccumulation by Suillus bovinus mushroom and probable dietary intake with the mushroom meal. Environ Sci Pollut Res 23:14549–14559
Acknowledgments
We thank Abdunnasir Yildiz, Professor (Department of Biology, Faculty of Science, Dicle University, Diyarbakir, Turkey) for the diagnosis of the mushroom species.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study does not involve human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alp, H., Ince, M., Ince, O.K. et al. Evaluation the Weekly Intake of Some Wild Edible Indigenous Mushrooms Collected in Different Regions in Tunceli, Turkey. Biol Trace Elem Res 195, 239–249 (2020). https://doi.org/10.1007/s12011-019-01814-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01814-3