Abstract
Sources of supplemental minerals in the diet of animals are of important significance. Bio-availability of organic sources is believed to be more in the body as compared to regularly used inorganic sources and hence environment-friendly due to reduced mineral excretion, which in turn reduces their requirements in the diet as well. Twenty-four male Murrah buffalo (Bubalus bubalis) calves (about 18–20 months of age and 318.54 ± 8.85 kg body weight) were divided randomly into four groups of six animals each. In the control group (C, InOrg100) zinc (Zn), copper (Cu), and manganese (Mn) were supplemented through an inorganic source, while in treatment groups, organic source at the rate of 50, 75, or 100% (in groups T1 (Org50), T2 (Org75), and T3 (Org100), respectively) was fed at level as supplemented in the control group. Feeding was continued for a period of 180 days with blood sampling at day 0 followed by a regular interval of 45 days. Plasma samples were analyzed for trace elements Cu, Mn, Zn, and iron (Fe), total antioxidant status, ceruloplasmin, and superoxide dismutase (SOD) with cell-mediated and humoral immune response. Plasma levels of different trace minerals like Fe, Mn, and Cu remained unaffected with two sources and different levels of organic minerals, except the level of Zn, which showed higher (P < 0.05) levels in the group Org100 compared to others, and remained indicative of higher bio-availability through the organic source. The concentration of plasma total antioxidants indicated no adverse effect on the reduction of supplemental levels up to half of these minerals. Also, the level of plasma SOD was high (P < 0.05) at each level of the organic source as compared to the 100% level of the inorganic source. Immune response in respect of cell-mediated as well as humoral immunity did not show any reduction in different groups. The study indicated beneficial impacts of the organic source in the form of superior plasma Zn level as well as SOD concentrations. In addition, no negative effect on most of the studied parameters was observed after reducing supplemental trace minerals to half indicating higher bio-availability of organic trace minerals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu), zinc (Zn), and manganese (Mn) are incorporated with proteins and enzymes to help in the antioxidant defense system [1]. Cu and Zn together form the Cu–Zn superoxide dismutase (SOD), which converts superoxide to hydrogen peroxide, in the cytoplasm of the cell [2]. A similar enzyme, manganese superoxide dismutase, is present in the mitochondria. Copper and zinc can also be bound to metallothionein and ceruloplasmin, which are extracellular proteins that have antioxidant capabilities [2]. Ceruloplasmin, an essential Cu transport protein, exhibits oxidase activity and accounts for the majority of Cu present in circulating plasma [3, 4]. In addition, ceruloplasmin and metallothionein exhibit anti-inflammatory activity and may play critical roles in preventing oxidative tissue damage from inflammation as well as infection [5]. Role of copper is not limited to antioxidant defense, and it is also important for cellular respiration, cardiac function, bone formation, connective tissue development, and myelination of the spinal cord [6]. The micronutrient Zn is an essential component of the diet for maintaining health and performance [7]. Zinc is the second most abundant trace element in mammals and birds and makes up a structural component of over 300 enzymes [6], including those involved in DNA and RNA synthesis [3]. It plays a role in maintaining health and integrity of skin due to its role in cellular repair and replacement [8] and a component of thymosin, a hormone produced by thymic cells that regulate cell-mediated immunity [9]. Manganese is essential in the body, besides being a part of superoxide dismutase, due to its role in metabolism. It is an important part of a range of enzymes that are involved in antioxidant protection, bone growth, carbohydrate and lipid metabolism, reproduction, and immune and nerve function [6]. These enzyme functions may be affected by the quantity of mineral and protein availability.
Trace minerals contain antioxidant potential, and associated enzymes play an important role in neutralizing oxygen metabolites. In addition, they prevent damage to tissues and cells in the host, including protecting the neutrophil from self-destruction or damage prior to bacterial kill [10]. Excessive accumulation of reactive oxygen species (ROS) due to oxidative stress leads to mammalian tissue damage, and the main biological targets definitely include lipids, proteins, DNA, and other macromolecules [11].
The quantity of mineral may not be as important as its form [7, 12]. Traditionally, micro minerals have been supplemented in the form of inorganic salts like sulfates. It has been highlighted in previous work [9] that most (80–99%) of the trace minerals ingested through inorganic source got excreted through feces of ruminants and remain a cause of excessive accumulation in soil and water. Excessive trace minerals lead to a detrimental effect on the health of different natural inhabitant flora and fauna of the soil and water. However, supplementation through organic source has potential due to the likelihood of increased bioavailability and absorption in the gut [6]. Organic trace minerals should undergo less dissociation in the reticulorumen, omasum, and abomasum than their inorganic counterparts [13]. Other reasons for investigating organic trace minerals include increased absorption in the gut, negative interactions between ingested metal ions and dietary factors when inorganic trace minerals are fed, and environmental impacts regarding undigested mineral compounds [6, 14]. Enhancing mineral absorption may have a positive effect on animal performance due to increased accessibility to minerals in the blood.
Long-term studies are scanty regarding the comparative availability of these three trace minerals through their organic and inorganic sources in Murrah buffalo. Therefore, the objective of the current study was to examine the effects of inorganic and organic supplementation of dietary Zn, Cu, and Mn on their plasma levels, antioxidant enzymes, and immune response in Murrah buffalo (Bubalus bubalis) calves.
Materials and Methods
Experimental Design
Twenty-four male Murrah buffalo (Bubalus bubalis) calves (18–20 months, 318.54 ± 8.85 kg body weight) were procured from the Institute’s Murrah herd and divided into four groups of six calves each on the basis of their body weights.
Housing and Management
Murrah buffalo calves were housed in a well-ventilated, clean, and concrete-floored shed and fed individually. Strict management and hygiene practices were adopted throughout the experimental period. Clean drinking water was provided ad libitum twice a day at about 9 a.m. and 3 p.m.
Feeds and Feeding
Calves were offered concentrate mixture (30% barley grain, 30% mustard cake, 19% wheat bran, 19% broken gram, 1% area-specific mineral mixture, and 1% common salt) and ad libitum wheat straw to meet their nutrient requirements for body weight gain of 500 g/day [15]. The amount of the concentrate mixture offered was revised fortnightly according to change in body weights. Calves were also provided about 2 kg of available green fodder (maize/oats/Egyptian clover) daily. Feeding schedule was the same for all four groups, except for replacement of zinc sulfate monohydrate, manganese sulfate monohydrate, and copper sulfate pentahydrate (in a mineral mixture of control group, InOrg100) with glycine amino acid-chelated Zn, Mn, and Cu (at 50, 75, and 100% amount of these elements, each as compared to that in control group) in treatment groups T1 (Org50), T2 (Org75), and T3 (Org100), respectively. Experimental feeding was done for a period of 180 days.
Feed Analysis
Concentrate mixture and wheat straw used in the experiment were analyzed for different chemical constituents after drying of feed at 60 °C and grinding to pass the 1-mm screen in a Wiley mill using standard procedures [16, 17], and nutrient composition of complete diet was calculated (on the basis of proportion of feed consumed during the entire experimental period). Calcium content in feed samples was analyzed by the classical method [18], and phosphorus was determined by the method of AOAC [16]. Elements like magnesium (Mg), Cu, Zn, Mn, and iron (Fe) were estimated using an atomic absorption spectrometer (AAS, model iCE 3300, Thermo Fisher Scientific) after dry ashing.
Blood Collection and Plasma Separation
Blood samples from buffalo calves were collected on day 0, i.e., before starting supplementation and subsequently at 45 days interval until the 180th day of study through jugular venipuncture, observing all aseptic precautions in the morning (before watering and feeding), into heparinised vacutainer. Plasma was collected following centrifugation at 700×g for 15 min. The plasma samples were stored at − 20 °C until further analysis.
Plasma Minerals
A suitable amount of plasma samples was taken in a 70-ml capacity glass digestion tube, soaked overnight in 10 ml double acid mixture (nitric and perchloric acid, 4:1) and digested. These digested plasma samples were analyzed for trace elements like Zn, Cu, Mn, and Fe by AAS.
Assessment of Antioxidant Profile
Estimation of different oxidative stress-related parameters was carried out in plasma. Plasma total antioxidants were estimated with ferric-reducing antioxidant power (FRAP) method [19]. Superoxide dismutase (SOD) estimated [20] in plasma was expressed as SOD units (1 U of SOD is the amount (μg) of protein required to inhibit the MTT reduction by 50%). Ceruloplasmin has oxidase activity; hence, it was estimated in terms of its para-phenylenediamine (PPD) oxidase activity at an optimum pH and temperature [21] and expressed as mg/100 ml plasma.
Estimation of Immune Response
Before termination of experimental feeding, cell mediated immunity (CMI) response was assessed by in vivo delayed-type hypersensitivity (DTH) reaction against antigen phytohaemagglutinin-P (PHA-P, Sigma-Aldrich, St. Louis, MO, USA). The skin on both sides of the neck was cleaned and shaved 24 h prior to injection and on the right side 200 μg of PHA-P dissolved in sterile PBS (pH 7.4) solution was injected intra-dermally, while on the left side of neck 200 μl of PBS was injected as a control. The net increase in double fold skin thickness measured at the injected sites was evaluated in 24, 48, and 72 h post-injection using vernier caliper. The immune response to PHA-P was measured by subtracting the values obtained from the right side to that on the left side and values represented in centimeter (cm).
Humoral immunity was assessed in the form of total immunoglobulin (IgG + IgM) level determined in plasma samples by zinc sulfate turbidity test [22] using modified concentration [23] and represented in the form of mg/ml.
Statistical Analysis
Data generated were analyzed statistically using SPSS (version 16). Comparison among different groups and period within the same group was made using repeated measures (RM) GLM procedure.
Results
Chemical Composition of Feeds
The chemical composition of the concentrate mixture (CM), wheat straw (WS), and complete basal diet (calculated on the basis of the proportion of CM (53.9%) and WS (46.1%) consumed during the entire experimental period) is presented in Table 1. Average feed intake of buffalo calves during the complete experimental period was 8.79 ± 0.12 kg/day. The contents of crude protein, ether extract, neutral detergent fiber, and acid detergent fiber were comparable to the levels recommended for growing buffalo calves for 500 g daily gain [15]. The levels of Zn were 71.28, 49.23, 58.58, and 67.76 ppm, of Mn were 59.20, 47.39, 52.38, and 57.28, and of Cu were 7.73, 4.68, 6.07, and 7.42 ppm in the complete diet of InOrg100 (control), Org50, Org75, and Org100 groups, respectively.
Plasma Trace Minerals
The overall mean plasma Zn was significantly higher (P < 0.05) in Org100 group as compared to others. On day 135, values of Zn were high (P < 0.05) in the Org100 group as compared to other treatment groups. In group Org50, significant difference (P = 0.009) was also observed with time. Levels of Mn, as well as Cu, remained comparable (P > 0.05) among different groups at the different point of observations as well as their overall mean values, except on day 180 when both showed low (P < 0.05) plasma levels in Org100 group compared to InOrg100. Plasma Fe levels were higher at day 45 in organic groups; Org50 (P = 0.086), Org75 (P < 0.001), and Org100 (P = 0.001) as compared to InOrg100. The same pattern was observed at day 90, but non-significant among groups. At day 135, lower (P < 0.05) plasma Fe level was observed in group Org50 as compared to InOrg100. Plasma Fe levels were low (P < 0.05) in all three treatment groups at the end of the experiment. However, at another point of observation, values remained comparable (P > 0.05). Overall mean values among different groups were non-significant with the highest (P = 0.097) plasma Fe levels in group Org100 (7.59 ppm) as compared to InOrg100 (5.92 ppm). An interesting observation was made in the plasma Fe level of all three treatment groups. The levels initially increased until day 90, however, after that the values dropped to the same levels as that at the start of the experiment. This difference was significant (P = 0.008) for the Org75 group, but not significant for other treatment groups (Table 2).
Antioxidant Enzymes
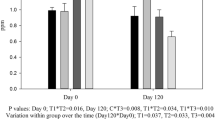
The activity of Cu containing enzyme ceruloplasmin (Fig. 1) remained comparable (P > 0.05) among different groups at the different point of collections with few exceptions. At day 45, values in group Org50 were lower (P < 0.05) as compared to InOrg100. At day 135, also values of ceruloplasmin were high in Org75 (P = 0.028) and Org100 (P = 0.030) groups as compared to group Org50. The increase in the activity of ceruloplasmin with time was observed in the groups fed higher amounts of the organic source (Org75; P = 0.025 and Org100; P = 0.049). SOD levels remained numerically higher in all the three organic groups (Fig. 2) almost throughout the experimental period with significant improvement at day 45, 90, or 135 in one or other organic mineral fed group over InOrg100. Thus, overall mean values of SOD in all the three organic mineral groups were high (P < 0.05) as compared to InOrg100. SOD levels were found to be higher within the groups (Org75; P = 0.003 and Org100; P = 0.025) at different points of recordings. The FRAP values (Fig. 3) remained comparable (P > 0.05) among different groups at the different point of observations as well as in their overall mean values, although the overall mean of FRAP values remained highest in the group fed the highest amount of organic minerals (Org100, 1161.67). In Org100 group, a periodical difference (P = 0.012) was observed in plasma FRAP values (Table 3).
Immune Responses of Buffalo Calves
Data showed comparable (P > 0.05) values at 24, 48, and 72 h as well as overall mean values after subcutaneous inoculation of antigen PHA-P, although values in all organic groups remained numerically higher as compared to InOrg100 group with highest values observed in group Org100 (3.01) and minimum in InOrg100 (2.74). The humoral immune response in terms of total immunoglobulin was statistically similar (P > 0.05) among different groups at the different point of observations except that at day 45, Org100 group had higher (P < 0.05) immunoglobulin as compared to group Org75. The same pattern was observed for overall mean values (Table 4; Figs. 4 and 5).
Discussion
Plasma Minerals
In experiments with organic and inorganic minerals, the concern is always about their bio-availability. Uninfluenced plasma levels of element Cu and Mn in the present study may be due to the initial (day 0) lower values of Mn and Cu in all three organic groups as compared to inorganic one. In earlier studies [24, 25], levels of Mn were found to be less conclusive in relation to its dietary level. In the present experiment, also variation in plasma level of these two minerals was not conclusive. As far as bio-availability is concerned, definitely, it was better at lower levels (Org50 and Org 75) of organic form as compared to the 100% level of inorganic one and that is the reason that plasma values of these minerals remained comparable among groups after even reduction of supplemental levels of minerals up to half as compared to inorganic group in a long-term (180 days) study. Higher (P < 0.05) plasma level of Zn in Org100 group as compared to InOrg100 was a result of higher bioavailability of zinc glycinate compared to zinc sulfate, inspit of the lesser Zn intake in Org100 group to that of InOrg100. In a short-term (30 days) study [26], also no effect was reported on serum levels of these minerals in Holstein cows. While in another study [27], supplementation of these three elements through two different sources in lactating Holstein cows had higher (P < 0.05) serum levels of Zn, Mn, and Cu in the organic minerals fed group. Higher Zn and Cu bio-availability through organic source was also reported [28] in sheep.
In another study [29], inorganic source of Cu and Zn was compared with an organic source at 75% level to that of the inorganic level, with Barbari male kids and reported no difference in plasma Zn and Cu levels, but they reported a low level of plasma Fe in groups fed organic Cu and Zn. However, in contrast, the present study showed a higher level of Fe in organic groups, though non-significant (P < 0.05) among groups. Higher Fe levels in organic groups also account for lower interaction among minerals when fed through the organic source. Over a period of time, Fe level showed a peculiar (sigmoid) pattern in the three groups fed organic source. Increased absorption (higher plasma values) until initial 90 days appears to be due to the lesser interaction among minerals, followed by saturation of intestinal mucosal cells [30] that leads to decreasing trend of absorption (lower plasma values) in organic groups until the end of the study (day 180). In contrast to the organic group, level of saturation was not achieved in the inorganic group until the end of the study to reduce the absorption of iron; thus, plasma levels were high until the end of the study.
Similar to present findings, a possibility was explored to reduce the level of inclusion of Cu and Zn up to 75% [29] in kids, while in another study [28], 33 and 52% higher bio-availability of Zn and Cu methionine were observed in ewes. Organically complexed minerals had a capacity to get easily absorbed from the intestine compared to the inorganic forms without interacting with other minerals.
Plasma Antioxidant Enzymes
The balance between the activity and intracellular antioxidant enzyme levels is important for the health and survival of living organisms. When ROS formation is higher than the capacity of their detoxification by cellular antioxidant mechanisms, oxidative stress occurs and inevitably leads to cellular damage [31]. Enhancement of ROS formation alters the antioxidant enzyme activity of blood and tissue, causing lipid peroxidation and destruction of membranes of the cells and subcellular organelle. Increase in levels of plasma ceruloplasmin with time in organic trace minerals fed groups (Org75 and Org100) compared to inorganic is indicative of the superior bio-availability of organic form of minerals. Ceruloplasmin is a copper-glycoprotein and is indicative of peroxidase activity. Most changes observed in plasma copper levels are associated with changes in ceruloplasmin [32]. A similar observation was reported in the present study.
Zn, Cu, and Mn play a key role in the antioxidant process [33]. Zn plays an important role in the induction and activation of GSH-Px in liver cells, thereby reducing free radicals. Analysis of data regarding dominant antioxidant enzyme SOD, an early (by day 45) improvement after supplementation commenced, as well as overall mean, despite the reduction of the supplemental level of these minerals up to 75 and 50% as compared to inorganic one indicates higher bio-availability of these three minerals through their organic source, as all the three elements are associated with SOD and had a potential role to scavenge superoxide radicals [34, 35]. In the past, it is shown that organic sources of these elements were helpful in reducing oxidative stress [36, 37]. Similar to present findings, supplementation of Zn, Mn, and Cu through inorganic sulfate salts or chelated form in lactating Holstein cows [27] and broiler birds [38] (even at reduced levels of organic source) reported higher levels of blood SOD and reduced level of lipid peroxidation in organic minerals fed group as compared to the group fed inorganic mineral source. Higher activity of SOD in liver was also reported in organic minerals fed crossbred pigs [39] as compared to inorganic minerals fed group.
Despite the reduction of organic mineral supplemental levels, no reduction was found in FRAP values. These observations are important for using lower levels of organic minerals and support the idea that low level of organic Zn, Cu, and Mn is required for the activity of the antioxidant system. It is a well-known fact that element copper is associated with catalytic action, while zinc plays role in the stability of the enzyme [40] and thus high bio-availability will be capable to cater higher capacity of scavenging free radicals with reduced dose rate.
Immune Response
Improvement (though non-significant) in CMI indicates the superior bio-availability of organic source over inorganic, even at lower levels of supplementation. Similar to the present findings, comparable CMI was also observed in grazing, growing heifers [41] and mid-lactation Holstein cows [26] by use of Cu, Mn, and Zn through inorganic or organic sources. Similar to CMI, no negative effect on levels of total immunoglobulins was observed despite lower levels of supplemental minerals, even up to half in a ration of buffalo calves. It is known that trace minerals (particularly Zn, Cu, Mn) could activate T cells and affect antibody responses in the body [42]. Organic trace minerals have been shown to increase antibody titer in cows [27, 43, 44], while no difference on antibody titer against Mannheimia haemolytica, bovine viral diarrhea types 1 and 2 viruses, was reported between two sources in Angus × Hereford calves in a recent study [45]. On the basis of findings of above workers and observations recorded in the present experiment, it can be inferred that requirement of calves is definitely lower than cows and levels of minerals used in the present study were sufficient to take care of the requirement of CMI as well as a humoral immune response in buffalo calves. Alternatively, it can be stated that the 50% level of the inorganic source is sufficient and higher levels are not required for any better performance.
It can thus be concluded that reducing the supplementation levels of Cu, Zn, and Mn through an organic source in the ration of buffalo calves as compared to that of inorganic source did not adversely affect the plasma mineral levels, antioxidant defense system, and an immune response. These results, therefore, indicate that Cu, Zn, and Mn from organic sources can be incorporated at much lower levels in place of inorganic forms of those minerals.
References
Hilal EY, Elkhairey MAE, Osman AOA (2016) The role of zinc, manganese, and copper in rumen metabolism and immune function: a review article. Open J Anim Sci 6:304–324. https://doi.org/10.4236/ojas.2016.64035
Nockels CF (1996) Antioxidants improve cattle immunity following stress. Anim Feed Sci Technol 62:59–68
Spears JW, Weiss WP (2008) Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J 176:70–76
Harris ED (1993) The transport of copper. Prog Clin Biol Res 380:163–179
Stabel JR, Spears JW, Brown TT (1993) Effect of copper deficiency on tissue, blood characteristics, and immune function of calves challenged with infectious bovine rhinotracheitis virus and Pasteurella hemolytica. J Anim Sci 71:1247–1255
Andrieu S (2008) Is there a role for organic trace element supplements in transition cow health? Vet J 176:77–83
Cope CM, MacKenzie AM, Wilde D, Sinclair LA (2009) Effects of level and form of dietary zinc on dairy cow performance and health. J Dairy Sci 92:2128–2135
Sordillo LMK, Shafer-Weaver DRD (1997) Immunobiology of the mammary gland. J Dairy Sci 80:1851–1865
National Research Council (2001) Nutrient requirements of dairy cattle, 7th edn. National Academy of Science, Washington, DC
Arthur JR, Boyne R (1985) Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci 36:1569–1575
Sordillo LM, Aitken SL (2009) Impact of oxidative stress on the health and immune function of dairy cattle. Vet Immunol Immunopathol 128:104–109
Spears JW (1996) Organic trace minerals in ruminant nutrition. Anim Feed Sci Technol 58:151–163
Gressley TF (2009) Zinc, copper, manganese, and selenium in dairy cattle rations. Mid-Atlantic Nutrition Conference, pp 65–71
Nemec LM, Richards JD, Atwell CA, Diaz DE, Zanton GI, Gressley TF (2012) Immune responses in lactating Holstein cows supplemented with Cu, Mn, and Zn as sulfates or methionine hydroxyl analog chelates. J Dairy Sci 95:4568–4577
Kearl LC (1982) Nutrient requirements of ruminants in developing countries. International Feedstuffs Institute, Utah State University, Logan
AOAC (2005) Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Arlington
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Talapatra SK, Ray SN, Sen KC (1940) Estimation of phosphorus, chlorine, calcium, magnesium, sodium, and potassium in foodstuffs. Indian J Vet Sci Anim Husb 10:243–246
Benzie IFF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Packer L (ed) Methods in enzymology. Academic Press, Orlando, pp 15–27
Madesh M, Balasubramaniam KA (1998) Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys 35:184–188
Sunderman FW, Nomoto S (1970) Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin Chem 16:903–910
McEwan AD, Fisher EW, Selman IE, Penhale WJ (1970) A turbidity test for the estimation of immune globulin levels in neonatal calf serum. Clin Chim Acta 27:155–163
Hudgens KA, Tyler JW, Besser TE, Krytenberg DS (1996) Optimizing performance of a qualitative zinc sulfate turbidity test for passive transfer of immunoglobulin G in calves. Am J Vet Res 57:1711–1713
Underwood EJ, Suttle NF (1999) The mineral nutrition of livestock, 3rd edn. CABI Publishing, Wallingford
Marques RS, Cooke RF, Rodrigues MC, Cappellozza BI, Larson CK, Moriel P, Bohnert DW (2016) Effects of organic or inorganic Co, Cu, Mn, and Zn supplementation to late-gestating beef cows on productive and physiological responses of the offspring. J Anim Sci 94:1215–1226
Dietz AM (2015) Effects of dietary Cu, Zn, and Mn on bovine neutrophil function. Thesis, Graduate School of the Ohio State University, USA
Zhao XJ, Li ZP, Wang JH, Xing XM, Wang ZY, Wang L, Wang ZH (2015) Effects of chelated Zn/Cu/Mn on redox status, immune responses and hoof health in lactating Holstein cows. J Vet Sci 16:439–446. https://doi.org/10.4142/jvs.2015.16.4.439
Pal DT, Gowda NKS, Prasad CS, Amarnath R, Bharadwaj U, Suresh Babu G, Sampath KT (2010) Effect of copper- and zinc-methionine supplementation on bioavailability, mineral status and tissue concentrations of copper and zinc in ewes. J Trace Elem Med Biol 24:89–94
Chaudhary UB, Tripathi MK, Gupta B, Dutta TK, Sirohi HV (2013) Effect of inorganic and organic zinc and copper supplementation on performance, nutrient utilization, rumen fermentation and blood biochemistry of kids Ind. J Anim Sci 83:1313–1322
Hahn PF, Bale WF, Rose JF, Balfour WM, Whipple GH (1943) Radioactive iron absorption by gastrointestinal tract. J Exp Med 78:169–188
Reiter RJ, Carneiro RC, Oh CS (1997) Melatonin in relation to cellular antioxidative defense mechanisms. Horm Metab Res 29:363–372
Milne DB (1994) Assessment of copper nutritional status. Clin Chem 40:1479–1484
Naziroğlu M, Yürekli VA (2013) Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol 33:589–599
Lean IJ, Westwood CT, Golder HM, Vermunt JJ (2013) Impact of nutrition on lameness and claw health in cattle. Livest Sci 156:71–87
Tapiero H, Tew KD (2003) Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother 57:399–411
Campbell MH, Miller JK (1998) Effect of supplemental dietary vitamin E and zinc on reproductive performance of dairy cows and heifers fed excess iron. J Dairy Sci 81:2693–2699
Miller JK, Brzezinska-Slebodzinska E, Madsen FC (1993) Oxidative stress, antioxidants, and animal function. J Dairy Sci 76:2812–2823
Aksu D, Aksu T, Ozsoy B, Baytok E (2010) The effect of replacing inorganic with a lower level of organically complexed minerals (Cu, Zn, and Mn) in broiler diets on lipid peroxidation and antioxidant. Asian Australas J Anim Sci 23:1066–1072
Liu B, Xiong P, Chen N, He J, Lin G, Xue Y, Li W, Yu D (2016) Effects of replacing of inorganic trace minerals by organically bound trace minerals on growth performance, tissue mineral status, and fecal mineral excretion in commercial grower-finisher pigs. Biol Trace Elem Res 173:316–324. https://doi.org/10.1007/s12011-016-0658-7
Forman HJ, Ridovich I (1973) On the stability of bovine superoxide dismutase: the effects of metals. J Biol Chem 248:2645–2649
Ahola JK (2004) Copper, zinc, and manganese in beef cattle production: effects of supplementation and source on reproduction, mineral status, feedlot performance, immunity, and carcass characteristics. Ph.D. thesis, Colorado State University, Fort Collins, Colorado
Fraker PJ, King LE, Laakko T, Vollmer TL (2000) The dynamic link between the integrity of the immune system and zinc status. J Nutr 130:1399S–1406S
Kinal S, Korniewicz A, Jamroz D, Zieminski R, Slupczynska M (2005) Dietary effects of zinc, copper and manganese chelates and sulfates on dairy cows. J Food Agric Environ 3:168–172
Nemec LM, Richards JD, Atwell CA, Diaz DE, Zanton GI, Gressley TF (2012) Immune responses in lactating Holstein cows supplemented with Cu, Mn, and Zn as sulfates or methionine hydroxy analog chelates. J Dairy Sci 95:4568–4577
Lippolis KD, Cooke RF, Silva LGT, Schubach KM, Brandao AP, Marques RS, Larson CK, Russell JR, Arispe SA, DelCurto T, Bohnert DW (2017) Effects of organic complexed or inorganic Co, Cu, Mn and Zn supplementation during a 45-day preconditioning period on productive and health responses of feeder cattle. Animal 11:1949–1956. https://doi.org/10.1017/S1751731117001033
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Before starting the experiment, prior approval was obtained for conducting the experiment from the Institute Animal Ethical Committee (IAEC). The guidelines followed by the Institutional Animal Ethics Committee (IAEC) are governed by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) under Ministry of Environment, Forest and Climate Change, Government of India.
Rights and permissions
About this article
Cite this article
Mudgal, V., Saxena, N., Kumar, K. et al. Sources and Levels of Trace Elements Influence Some Blood Parameters in Murrah Buffalo (Bubalus bubalis) Calves. Biol Trace Elem Res 188, 393–403 (2019). https://doi.org/10.1007/s12011-018-1439-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1439-2