Abstract
For experiments of cadmium toxicity in animal models, cadmium (II) chloride is often used due to its solubility in water and its ability to produce high concentrations of cadmium at the target site. The present study was designed to investigate the potential inhibitory effect of the Fragaria ananassa fruit extract on cadmium (II) chloride-induced renal toxicity in rats. Tested animals were pretreated with the extract of F. ananassa and injected with cadmium (II) chloride (6.5-mg/kg body weight) for 5 days. Cadmium (II) chloride significantly increased kidney cadmium concentration, kidney weight, lipid peroxidation, and nitric oxide production. Plasma uric acid, urea, and creatinine levels also increased significantly, indicative of kidney dysfunction. These effects were accompanied by significantly decreased levels of nonenzymatic and enzymatic antioxidant molecules (i.e., glutathione content and the activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase). Moreover, messenger RNA (mRNA) expression of the antiapoptotic protein, Bcl-2, and the antioxidant proteins, superoxide dismutase 2 and glutathione reductase, were downregulated markedly, whereas mRNA expression of tumor necrosis factor-α was upregulated significantly in kidney tissues of cadmium-treated rats. Histology of kidney tissue demonstrated severe, adverse changes that reflected cadmium-induced tissue damage. Pretreatment of rats with the extract of F. ananassa ameliorated all aforementioned cadmium (II) chloride-induced changes. In conclusion, the present study showed acute renal toxicity in rats treated with cadmium (II) chloride. The study also revealed that pretreatment with the extract of F. ananassa could protect the kidney against cadmium (II) chloride-induced acute renal toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a dangerous metal, a potential poison originating from industrial and horticultural sources, and designated as an endocrine disruptor/endocrine-disrupting chemical (EDC) [1]. It is a contaminant in most human foodstuffs as a result of its high rates of soil-to-plant transmission, rendering diet as a major source of exposure [2]. The toxic levels of Cd in diverse tissues, such as kidney, lung, and testes, have already been determined [3]. Yang and Shu [4] reported many transport proteins that play vital roles in Cd accumulation in renal tissue, including metallothioneins, divalent metal-ion transporter-1, and Cd-binding proteins containing thiol (−SH) groups. The Cd that has accumulated in the kidney generates reactive oxygen species (ROS) that causes oxidative stress, inflammation, programmed cell death, and glomerular dysfunction [5], beside to the ability of Cd to interfere with essential elements, especially zinc and calcium [6]. The target sites of toxicity in kidney tissue are the S1 and S2 segments of the proximal convoluted tubules, whose damage accounts for kidney dysfunction [7]. Furthermore, Cd is thought to lead to lipid peroxidation and this has often been considered to be the main cause of its deleterious influence on membrane-dependent function [8]; this increases vulnerability of kidney to cadmium toxicity.
Because there is no adequate therapy for Cd nephrotoxicity, there has been a growing interest in the use of antioxidants that can prevent Cd toxicity. Fragaria ananassa is a natural antioxidant that has a high content of phenolic compounds [9]. There have been many investigations to understand the mechanisms responsible for augmentation of phenolic compounds in F. ananassa. Phenolic compositions vary among species and cultivars [10]; however, they could also be affected by growth conditions, such as environmental factors and agricultural techniques. F. ananassa has been consumed extensively as a fruit and as a constituent of processed foods. It is highly rich in active components, such as ascorbic acid, vitamin E, carotene, phenolic acids, flavonols, and anthocyanins [11]. Its main components are procyanidins, ellagitannins, catechins, and p-coumaroyl esters [12]. Several health advantages of F. ananassa consumption have been documented including increased antioxidant [13] and anticarcinogenic activities [14, 15]. Ibrahim and Abd El-Maksoud [16] demonstrated the nephroprotective potential of strawberry leaf extract in streptozotocin-induced diabetic nephropathy, and they found that strawberry leaf extract has antidiabetic, antioxidant, antiinflammatory, and antiapoptosis properties. Hence, we investigated the protective effect of the F. ananassa extract against cadmium (II) chloride-induced acute renal toxicity in rats.
Material and Methods
Chemicals
Chemicals, including solvents, were all high-grade and were used without further purification. Anhydrous CdCl2 (cadmium (II) chloride) was supplied by Sigma-Aldrich (St. Louis, MO, USA). Kits to assess kidney function were all provided by Randox/Laboratory (Crumlin, UK). Sucrose, hydrochloric acid, and Tris were purchased from Fluka Chemie (Buchs, Switzerland). TRIzol reagent was supplied by Invitrogen (Carlsbad, CA, USA). RevertAid H Minus Reverse Transcriptase was obtained from Thermo Fisher Scientific Inc. (Waltham, MA, USA). PCR primers were prepared by Jena Bioscience GmbH (Jena, Germany).
Fruit Material
Fresh strawberries (F. ananassa) were collected from Cairo markets (Egypt) from April to May 2016. The fruit was authenticated by the Department of Botany at the Science Faculty in Helwan University on the basis its taxonomic features complemented by comparison with specimens from the herbarium at the Department of Botany. The fruit (500 g) was extracted with 70% methanol for 48 h, with blending every 2 h. The mixture was filtered, and the filtrate was evaporated to dryness by utilizing a vacuum evaporator (IKA, Germany). The residues were dissolved in distilled water and stored at −20 °C in an airtight bottle. The extract was designated as strawberry fruit extract (SFE). The amounts of phenolic and flavonoid compounds were determined in the SFE using standard methods as described by Abdel Moneim [17]; we found that each g of SFE contained from 98.3 to 112.7 mg phenolics and 44.2 to 57.1 mg flavonoids.
Animal Treatment
Thirty-two adult male Wistar rats (8–9 weeks old, weighing 160–180 g) were purchased from VACSERA (Cairo, Egypt). The rats were housed at the Department of Zoology at Helwan University (Cairo, Egypt) for 12-h light/dark cycles at 22–25 °C. The rats were given access to pelleted rodent feed and water ad libitum. To study the renal protective activity of strawberry extract on CdCl2-induced renal toxicity, rats were randomly divided into four groups (n = 8 per group). Control rats were intraperitoneally (i.p.) injected with 0.9% NaCl (physiological saline) daily for 5 days. The CdCl2 group was injected i.p. with 6.5 mg/kg CdCl2 daily for 5 days. The SFE group was orally administered SFE at 250 mg/kg, and the SFE + CdCl2 group was preadministered 250 mg/kg SFE 1 h before injecting 6.5 mg/kg CdCl2 i.p. daily for 5 days. CdCl2 was dissolved in physiological saline. SFE was orally administered at a dose of 250 mg/kg, according to a preliminary study that showed no toxicity at this dose, whereas CdCl2 was i.p. injected at 6.5 mg/kg according to the published results of Dkhil et al. [18]. Rats were euthanized (decapitation) 24 h after the final dosing. The kidneys were dissected, weighed, and immediately homogenized in ice-cold 10 mM phosphate buffer (pH 7.4) to produce a 10% (w/v) homogenate for biochemical analysis. All protocols and animal handling were approved by the Committee on Research Ethics for Laboratory Animal Care at the Department of Zoology, Faculty of Science, Helwan University, and were in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals, 8th edition (NIH Publication No. 85-23 revised 1985).
Cd Concentration in Kidney Tissue
The Cd concentrations in renal tissues were estimated utilizing a standard method. In summary, specimens of renal tissue were weighed and wet ashed with 1 M nitric acid at 150 °C for 2 h. The ashed specimens were diluted with deionized water to 50 ml. The levels of metal were measured by atomic absorption spectrophotometry (Perkin-Elmer 3100) in a graphite furnace at 228.8 nm. The Cd values are expressed as microgram per gram of wet renal tissue.
Biochemical Assays
Lipid peroxidation (LP) was determined based on the amount of malondialdehyde (MDA) formed, as assessed by the method of Ohkawa et al. [19]. Nitric oxide (NO) was measured using the Griess reagent [20]. The content of reduced glutathione (GSH) in the renal homogenates was estimated utilizing the method described by Ellman [21]. Catalase (CAT) activity was estimated by measuring the decomposition rate of hydrogen peroxide (H2O2) at 570 nm, as described by Aebi [22]. Superoxide dismutase (SOD) activity was determined to utilize the method of Nishikimi et al. [23], whereas the activities of glutathione peroxidase (GPx) and glutathione reductase (GR) were assayed using the methods of Paglia and Valentine [24] and De Vega et al. [25], respectively.
Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
Total renal RNA was extracted from frozen specimens with the TRIzol reagent. Approximately 5 μg of total RNA was reverse transcribed to cDNA. The sense/antisense primers for selected genes are shown in Table 1. The Power SYBR® Green Master Mix Kit was utilized for real-time PCR analysis. Gene expression was determined to utilize cycle time (Ct) values, and the relative differences among the groups were expressed as proportional changes relative to the untreated control (arbitrarily set as 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was utilized as a control and was shown to be unchanged by treatment.
Histological Examination
Renal specimens were selected and fixed in 10% formaldehyde in PBS for 24 h at room temperature (25 °C), and then treated for light microscopy. The specimens were embedded in paraffin, sectioned at a thickness of 4–5 μm, and then stained with hematoxylin/eosin.
Immunohistochemistry Analysis
Formaldehyde-fixed, paraffin-embedded sections (4 μm) were mounted on glass slides. Sections were deparaffinized and blocked with methanol containing 0.1% hydrogen peroxide for 10 min to destroy endogenous peroxidase activity. After blocking, the sections were incubated with a rabbit polyclonal antitumor necrosis factor-α (TNF-α) antibody at 4 °C overnight. Sections were then washed with buffer solution (phosphate-buffered saline) and incubated at 37 °C for 30 min using horseradish peroxidase-conjugated goat antirabbit antibody. The antibody binding sites were visualized by incubation at room temperature (25 °C) for 10 min with diaminobenzidine (DAB)-hydrogen peroxide. Images were taken at an original magnification of ×400 (Nikon Eclipse E200-LED, Tokyo, Japan).
Statistical Analysis
Values are expressed as the mean ± standard error of the mean (SEM) of seven rats. Data were analyzed with one-way analysis of variance (ANOVA) with post hoc Duncan multiple tests. The p values <0.05 were considered statistically significant.
Results
CdCl2 injection in rats significantly increased (p < 0.05) the concentration of Cd in renal tissue compared to that of control rats. The increased Cd concentration in the renal tissue was significantly (p < 0.05) reduced by preadministration of SFE to CdCl2-injected rats (Fig. 1). Furthermore, the kidney weights of rats exposed to CdCl2 alone significantly increased (p < 0.05) compared to those of the control rats. The preadministration of SFE, however, significantly alleviated the increase in kidney weights, decreasing these values close to those of control rats (Fig. 1).
Effect of strawberry fruit extract (SFE) on cadmium chloride (CdCl2) accumulation in kidney tissue and the kidney weights of rats treated with CdCl2. Data are expressed as the mean ± SEM (n = 8). ap < 0.05 with respect to control group; bp < 0.05 with respect to CdCl2 group, using Duncan’s post hoc test
Rats exposed to Cd-induced oxidative stress showed acute kidney injury, indicated by increases in the levels of serum uric acid, urea, and creatinine, which contrasted with the levels in control rats (Fig. 2). Treatment with SFE significantly ameliorated these metabolite increases (p < 0.05).
Strawberry fruit extract (SFE) pretreatment ameliorates plasma levels of uric acid, urea, and creatinine in rats exposed to cadmium chloride (CdCl2). Data are expressed as the mean ± SEM (n = 8). ap < 0.05 with respect to control group; bp < 0.05 with respect to CdCl2 group, using Duncan’s post hoc test
CdCl2 treatment significantly increased the production of MDA and NO in the kidney (p < 0.05). Rats subjected to Cd-induced oxidative stress showed acute kidney injury, as demonstrated by an apparent decline in the GSH content of the tissue (Fig. 3).
CdCl2 injection also significantly reduced the activities of the antioxidant enzymes, SOD, CAT, GR, and GPx (p < 0.05). The treatment with SFE significantly reduced the production of MDA and NO in the kidney and prevented the depletion of GSH, which were caused by CdCl2 injection (p < 0.05). Moreover, our results show that treatment with SFE enhanced SOD, CAT, and GPx activities (p < 0.05), although SFE failed to change GR activity significantly, compared to the enzyme activities in the CdCl2-treated group (Fig. 4). Consistent with the biochemical analyses, the quantitative PCR results showed that the messenger RNA (mRNA) expression of SOD2, CAT, GPx, and GR was downregulated after CdCl2 injection, and SFE treatment was able to upregulate these genes (Fig. 5).
Strawberry fruit extract (SFE) pretreatment increases antioxidant enzyme activities of SOD, CAT, GPx, and GR in the kidneys of rats exposed to cadmium chloride (CdCl2). Data are expressed as the mean ± SEM (n = 8). ap < 0.05 with respect to control group; bp < 0.05 with respect to CdCl2 group, using Duncan’s post hoc test. SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase, GR glutathione reductase
Strawberry fruit extract (SFE) pretreatment ameliorates mRNA expression of SOD2, CAT, GPx, and GR genes in the kidneys of rats exposed to cadmium chloride (CdCl2). Data of the mRNA levels (mean ± SEM of three assays) were normalized to the GAPDH mRNA level and are shown as the fold induction relative to the control mRNA level. ap < 0.05 with respect to control group; bp < 0.05 with respect to CdCl2 group, using Duncan’s post hoc test. SOD2 superoxide dismutase 2, mitochondrial; CAT catalase; GPx1 glutathione peroxidase 1; GR glutathione reductase; GAPDH glyceraldehyde 3-phosphate dehydrogenase
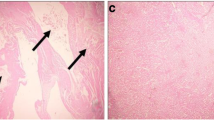
Rats that were poisoned with CdCl2 showed moderate-to-severe inflammation and widespread degeneration, as indicated by mononuclear cell infiltration, cytoplasmic vacuolation, and congested glomeruli (Fig. 6). Moreover, severe apoptosis was observed in the kidney sections, as indicated by the presence of karyomegaly and hyperchromatic nuclei in the tubular epithelial cells. Treatment of rats with SFE greatly improved this Cd-induced histopathology in the kidney tissue.
Light micrographs of the kidney. a The normal histological features of kidney tubules and glomeruli in the control rats. b Severe inflammation and extensive degeneration (black star), cytoplasmic vacuolation (black arrow), and congested glomeruli (white star) in the cadmium chloride (CdCl2)-treated rats. c The normal histological structure of kidney tubules and glomeruli in the strawberry fruit extract (SFE)-treated rats, similar to the histology of the control rats. d The normal histological structure of renal tubules and glomeruli in the rats where SFE was administered before CdCl2. However, some areas with vacuoles can be still seen (H&E staining, ×400)
To investigate whether the observed protective effects of SFE were due to its antiapoptotic and antiinflammatory characteristics, the mRNA levels of Bcl-2, Bax, and TNF-α in the kidney were quantified using real-time RT-PCR. The results demonstrated that the expression of Bcl-2 mRNA was downregulated considerably (Fig. 7), and the expression of Bax and TNF-α mRNA was upregulated in the CdCl2 treatment group (p < 0.05). However, these effects were ameliorated in the rats that received SFE prior to CdCl2 (p < 0.05).
Strawberry fruit extract (SFE) pretreatment ameliorates mRNA expression of Bcl-2, Bax, and TNF-α genes in the kidneys of rats exposed to cadmium chloride (CdCl2). Data of the mRNA levels (mean ± SEM of three assays) were normalized to the GAPDH mRNA level and are shown as the fold induction relative to the mRNA level in controls. ap < 0.05 with respect to control group; bp < 0.05 with respect to CdCl2 group, using Duncan’s post hoc test. TNF-α tumor necrosis factor-α, GAPDH glyceraldehyde 3-phosphate dehydrogenase
An immunohistochemical examination was performed to confirm that apoptosis was induced by CdCl2 in the kidney. In the CdCl2-injected group, the expression of the apoptotic protein, caspase-3, increased (Fig. 8). Preadministration of SFE to rats reduced the expression of caspase-3 in the cytoplasm compared with expression in the CdCl2-treated rats.
Changes in caspase-3 expression in rat renal tissue after administration of strawberry fruit extract (SFE) and cadmium chloride (CdCl2). a Control, b CdCl2-treated, c SFE-treated, and d SFE + CdCl2-treated rats. In the control and SFE groups, apoptotic cells, i.e., those stained with caspase-3, were sparse and weakly stained. In the CdCl2 rats, many cells were apoptotic. In the SFE + CdCl2 group, the intensity of the caspase-3 immunostaining was decreased (×400)
Discussion
Consumption of berry fruits, including strawberries (F. ananassa), has been proposed to have beneficial effects against oxidative stress-related diseases, such as diabetes and cancer. Strawberries contain several phenolic molecules that are thought to contribute to their biological effects. In addition, novel studies suggest that strawberries have free radical scavenging, antilipidemic, and antidiabetic properties [16]. However, whether strawberries can ameliorate the damage caused by heavy metals is unclear. In the present study, we investigated the effects of SFE on Cd-induced renal injury in rats. Our investigations suggest that, even when Cd levels are high in the kidney tissue, SFE pretreatment reduces the levels significantly. This suggests that SFE may remove Cd from the kidney by chelation and increase the clearance of Cd from the kidney. SFE has many phenols that act as metal chelators because of multiple hydroxyl groups that can coordinate with Cd2+ [26]. The mechanism responsible for the metal chelation characteristics of SFE is not yet fully known; further studies are required. Increasing kidney weights, as noted in this investigation, reflect renal hypertrophy that is in agreement with previous investigations. Moreover, the loss of body weight of Cd2+-intoxicated rats is in line with the results of previous studies and is consistent with rats having renal hypertrophy [5, 18]. Pretreatment of rats with SFE prevented these weight alterations. This suggests that SFE has the ability to protect renal tissue from Cd2+-induced renal hypertrophy. The deleterious effects of Cd may be related to its capacity to generate reactive oxygen species (ROS) that oxidatively injure the kidney via two separate, but related, pathways [13]. The reduction of Cd-induced oxidative stress by strawberries affects both the generation and clearance of ROS. The improvement of renal functional parameters closer to control values demonstrates that SFE could stabilize the plasma membrane and induce cellular repair.
Furthermore, histological examinations of renal sections revealed acute damage in renal cells treated with Cd, while in sections from rats treated with SFE and Cd, renal cell damage had been alleviated. Thus, these results suggest that amelioration of renal functional markers and renal damage may participate in the protective effect of SFE on Cd-induced renal toxicity. Several investigations in animal species showed that Cd primarily accumulated in renal tissue [27, 28]. In agreement with these investigations, our studies showed that Cd can easily be detected in renal tissue. Moreover, the effect of Cd on the weight of the kidney has been previously studied. Many reports have indicated that Cd treatment causes kidney atrophy [29], while others have noted renal hypertrophy following Cd administration [30]. The data shown herein suggest that accumulated renal Cd has a deleterious effect on the weight of the kidneys. However, SFE pretreatment reverses the renal toxicity of Cd. SFE is rich in phenols, which act as chelators with metal ions, because they have hydroxyl groups forming coordinate bonds with Cd2+ [31]. However, the mechanisms involving the metal chelation characteristics of SFE are not clear and demand additional studies.
In the present work, we have also examined markers of kidney function, discovering that CdCl2 induced increases in serum uric acid, urea, and creatinine that demonstrated kidney dysfunction. Pretreatment of rats with SFE reduced the levels of these indicators of kidney dysfunction. In general, our results indicate that SFE can safeguard the ordinary capacity of the kidney and protect it from Cd-induced injury. Our histological findings bolster the previously mentioned effects, both the damaging effects of CdCl2 and the protective effects of SFE. The damaging effects of CdCl2 on renal tissue, because of its oxidative properties, are a consequence of the overproduction of ROS, which initiate cell damage and apoptosis. Once it has entered the renal cell, Cd can target the mitochondria, uncoupling the respiratory chain and causing cell death [31]. Pretreatment of rats with SFE serves to shield the kidney from this damage by, for example, chelating the Cd. Our outcomes, in this way, reinforce the hypothesis that SFE can shield the kidney from CdCl2-induced oxidative harm and, therefore, can avert kidney pathogenesis.
The first mechanism involved in Cd toxicity is its capability to increase lipid peroxidation (LPO). A second mechanism operates to deplete ROS scavenging capability, whereby Cd reacts with metal cofactors in various enzymatic and nonenzymatic antioxidants to reduce antioxidant enzyme activity and GSH levels. LPO is a prominent marker of oxidative stress, and it is known to increase cellular content of epoxides, hydroperoxides, and MDA, all of which can react with proteins, DNA, and RNA in the cell to cause renal tissue damage [32]. Herein, SFE appeared to decrease Cd-induced nephrotoxicity through its ability to decrease LPO. Increased NO generation, apparent in the present study, may add to the proinflammatory capacity of Cd by activating NF-κB, which upregulates inducible NO synthase in macrophages [33]. NF-κB usually aggravates inflammation through inducible NO synthase/NO generation by controlling transcription of the gene [34]. The poisonous quality of NO is enhanced considerably when it reacts with superoxide anion, generating the exceedingly reactive peroxynitrite anion (ONOO−). Phenols have been noted to specifically scavenge NO [35], which would clarify why SFE was so efficacious in decreasing NO.
GSH is a noteworthy, nonenzymatic cell protective molecule, existing both intracellularly and extracellularly in living creatures and neutralizing both xenobiotics and ROS. Therefore, the level of GSH in the body is believed to be a marker of its antioxidative capacity, and reductions in GSH levels can have negative consequences for organic systems [36]. Oxidative stress is known to lower GSH content [37], and in the present study, GSH levels were clearly diminished in Cd2+-treated rats. This reduction in GSH levels may have enhanced LPO. Cd inactivates GSH biosynthesis from cysteine via the γ-glutamyl cycle, which additionally lowers GSH content. This study found, nonetheless, that the GSH content in the kidneys of rats pretreated with SFE was increased, indicating that SFE protects GSH from being diminished in the kidney; this maintains an endogenous antioxidant system to minimize oxidative stress.
Rats treated with CdCl2 were found to have significantly diminished activity of renal tissue antioxidant enzymes. Among these compounds, SOD catalyzes the transformation of superoxide anion to H2O2 through a dismutation reaction. Cd, in addition to interacting with critical sulfhydryl groups in the SOD protein, can interfere with other cofactors for SOD, consequently inactivating the enzyme. CAT is an essential antioxidant enzyme having heme as an active site prosthetic group. Cd is known to reduce iron assimilation and interfere with heme biosynthesis [38]. The increased renal LPO in Cd-intoxicated rats may be due to significant inhibition of the activities of free radical capturing enzymes such as SOD and CAT. In addition, Cd inactivates GPx and GR. The depletion in the content of GSH and increase in the level of LPO leads to decrease in the activities of GPx and GR during Cd intoxication. Adaramoye and Akanni [39] reported that the direct binding between Cd and –SH groups in these enzymes active sites as well as displacement of metal cofactors from the active sites of the enzymes may contribute to the inhibition. Pretreatment with SFE prevented each of these proteins from being altered, indicating a protective role of SFE on antioxidant enzymes, probably due to the free radical scavenging activity of SFE.
Cd injection for 5 days at 6.5 mg/kg induces a cascade of inflammatory reactions and modulates the immune cells with increased production of proinflammatory cytokines, particularly TNF-α, which accounts for further kidney damage. TNF-α is a cytokine produced by activated macrophages in response to pathogens and other injurious stimuli, like xenobiotics, and is a necessary and sufficient factor for local and systemic inflammation. TNF-α amplifies and prolongs the inflammatory response by triggering other cells to release both cytokines, such as interleukin-1β, and mediators, such as NO and ROS, all of which promote further inflammation and tissue injury [13]. Our results are supported by the findings of Alghasham et al. [40], who found that CdCl2-polluted drinking water (40 mg CdCl2/l) significantly increased TNF-α serum levels in rats. Fouad and Jresat [41] found that a single i.p. injection of CdCl2 (2 mg kg−1) markedly elevated TNF-α levels in the testes tissue of rats. Furthermore, Stosic et al. [42] found that an i.p. injection of Cd (1 mg/kg) in rats induced a significant elevation of TNF-α levels in lung tissue. Indeed, it has been assumed that TNF-α is an important mediator in the development of CdCl2-induced toxicity. However, pretreatment of SFE downregulated the expression of TNF-α compared to that of the CdCl2-induced group, demonstrating the antiinflammatory activity of SFE.
In the present study, both the caspase-3 protein level and Bax mRNA level were elevated in the renal tissue, while the Bcl-2 mRNA level was diminished. Hagar and Al Malki [43] determined that initiation of the caspase cascade and synchronous extracellular signal-regulated kinase dephosphorylation are the most noteworthy pathways connected with apoptotic signals activated in vitro. In addition, pretreatment with SFE reduced apoptosis in the kidney. The protective effect of SFE on kidney tissue is associated with inhibition of apoptosis, in light of the capacity of various phytochemicals to protect against stress-induced apoptosis. In the present study, CdCl2 increased caspase-3 expression, demonstrating that Cd activates cell death in the rodent kidney. Interestingly, SFE enhanced the kidney by diminishing levels of caspase-3. This concurs with the work of Al-Assaf et al. [44], who demonstrated that caspase-3 levels were increased in rats treated with metal. Caspase-3 assumes a noteworthy downstream role in mitochondrial pathway-induced cell death, after mitochondrial damage and the leakage of cytochrome c into the cytoplasm. It has been reported that caspase-3 activation occurs in Cd-initiated apoptosis in human hepatoma cells [45] and apoptosis in human leukemia (HL-60) cells [46]. Caspase-3 is known to be the final protease that activates apoptotic DNA fragmentation [47].
In conclusion, the fruit extracts of F. ananassa preadministration exhibited renoprotective effects against CdCl2-induced nephrotoxicity. These renoprotective effects could be achieved by the antioxidant and antiapoptotic activities of the extracts. Thus, for Cd-induced nephrotoxicity, the use of SFE could be beneficial.
References
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208
Satarug S, Nishijo M, Ujjin P, Vanavanitkun Y, Moore MR (2005) Cadmium-induced nephropathy in the development of high blood pressure. Toxicol Lett 157(1):57–68
Meyer KJ, Reif JS, Veeramachaneni DN, Luben TJ, Mosley BS, Nuckols JR (2006) Agricultural pesticide use and hypospadias in eastern Arkansas. Environ Health Perspect 114(10):1589–1595
Yang H, Shu Y (2015) Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int J Mol Sci 16(1):1484–1494
Ansari MA, Raish M, Ahmad A, Alkharfy KM, Ahmad SF, Attia SM, Alsaad AM, Bakheet SA (2017) Sinapic acid ameliorate cadmium-induced nephrotoxicity: in vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-kappaB downregulation. Environ Toxicol Pharmacol
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health res 24(4):378–399. doi:10.1080/09603123.2013.835032
Dorian C, Gattone Ii VH, Klaasen CD (1992) Renal cadmium deposition and injury as a result of accumulation of cadmium-metallothionein (CdMT) by the proximal convoluted tubules—a light microscopic autoradiography study with 109CdMT. Toxicol Appl Pharmacol 114(2):173–181. doi:10.1016/0041-008X(92)90066-2
El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA (2007) Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 235(3):185–193
Aaby K, Skrede G, Wrolstad RE (2005) Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J Agric Food Chem 53(10):4032–4040. doi:10.1021/jf048001o
MilivojeviĆ J, MaksimoviĆ V, NikoliĆ M, BogdanoviĆ J, MaletiĆ R, MilatoviĆ D (2011) Chemical and antioxidant properties of cultivated and wild fragaria and rubus berries. J Food Qual 34(1):1–9. doi:10.1111/j.1745-4557.2010.00360.x
Hamed SS, Al-Yhya NA, El-Khadragy MF, Al-Olayan EM, Alajmi RA, Hassan ZK, Hassan SB, Abdel Moneim AE (2016) The protective properties of the strawberry (Fragaria ananassa) against carbon tetrachloride-induced hepatotoxicity in rats mediated by anti-apoptotic and upregulation of antioxidant genes expression effects. Front Physiol 7:325. doi:10.3389/fphys.2016.00325
Oszmiański J, Wojdyło A (2009) Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Eur Food res Technol 228(4):623–631. doi:10.1007/s00217-008-0971-2
Elkhadragy MF, Abdel Moneim AE (2017) Protective effect of Fragaria ananassa methanolic extract on cadmium chloride (CdCl2)-induced hepatotoxicity in rats. Toxicol Mech Methods:1–27. doi:10.1080/15376516.2017.1285973
Carlton PS, Kresty LA, Siglin JC, Morse MA, Lu J, Morgan C, Stoner GD (2001) Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis 22(3):441–446
Naemura A, Mitani T, Ijiri Y, Tamura Y, Yamashita T, Okimura M, Yamamoto J (2005) Anti-thrombotic effect of strawberries. Blood Coagul Fibrinolysis 16(7):501–509
Ibrahim DS, Abd El-Maksoud MA (2015) Effect of strawberry (Fragaria × ananassa) leaf extract on diabetic nephropathy in rats. Int J Exp Pathol 96(2):87–93. doi:10.1111/iep.12116
Abdel Moneim AE (2013) The neuroprotective effects of Purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. CNS Neurol Disord Drug Targets
Dkhil MA, Al-Quraishy S, Diab MM, Othman MS, Aref AM, Abdel Moneim AE (2014) The potential protective role of Physalis peruviana L. fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Food Chem Toxicol 74:98–106
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J lab Clin med 70(1):158–169
De Vega L, Fernandez RP, Mateo MC, Bustamante JB, Herrero AM, Munguira EB (2002) Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail 24(4):421–432
Emamverdian A, Ding Y, Mokhberdoran F, Xie Y (2015) Heavy metal stress and some mechanisms of plant defense response. Sci World J 2015:18. doi:10.1155/2015/756120
Haouem S, El Hani A (2013) Effect of cadmium on lipid peroxidation and on some antioxidants in the liver, kidneys and testes of rats given diet containing cadmium-polluted radish bulbs. J Toxicol Pathol 26(4):359–364. doi:10.1293/tox.2013-0025
Chen J, Du L, Li J, Song H (2016) Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem Toxicol 96:70–78
Hwang DF, Wang LC (2001) Effect of taurine on toxicity of cadmium in rats. Toxicology 167(3):173–180
Pari L, Murugavel P (2005) Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ Toxicol Pharmacol 20(3):493–500
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal 2013:162750. doi:10.1155/2013/162750
Kehrer JP, Klotz LO (2015) Free radicals and related reactive species as mediators of tissue injury and disease: implications for health. Crit rev Toxicol 45(9):765–798. doi:10.3109/10408444.2015.1074159
Reyes JL, Molina-Jijon E, Rodriguez-Munoz R, Bautista-Garcia P, Debray-Garcia Y, Namorado Mdel C (2013) Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed res Int 2013:730789. doi:10.1155/2013/730789
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651
Boora F, Chirisa E, Mukanganyama S (2014) Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J Food Process 2014:7. doi:10.1155/2014/918018
Pastore A, Federici G, Bertini E, Piemonte F (2003) Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 333(1):19–39
Bast A, Haenen GR (1988) Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim Biophys Acta 963(3):558–561
Schauder A, Avital A, Malik Z (2010) Regulation and gene expression of heme synthesis under heavy metal exposure—review. J Environ Pathol Toxicol Oncol 29(2):137–158
Adaramoye OA, Akanni OO (2016) Modulatory effects of methanol extract of Artocarpus altilis (Moraceae) on cadmium-induced hepatic and renal toxicity in male Wistar rats. Pathophysiology 23(1):1–9
Alghasham A, Salem TA, Meki AR (2013) Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-alpha, interleukin-6 and oxidative status biomarkers in rats: protective effect of curcumin. Food Chem Toxicol 59:160–164. doi:10.1016/j.fct.2013.05.059
Fouad AA, Jresat I (2015) Thymoquinone therapy abrogates toxic effect of cadmium on rat testes. Andrologia 47(4):417–426. doi:10.1111/and.12281
Stosic J, Mirkov I, Belij S, Nikolic M, Popov A, Kataranovski D, Kataranovski M (2010) Gender differences in pulmonary inflammation following systemic cadmium administration in rats. Biomed Environ Sci 23(4):293–299
Hagar H, Al Malki W (2014) Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: role of oxidative stress and caspase-3. Environ Toxicol Pharmacol 37(2):803–811
Al-Assaf AH, Alqahtani AM, Alshatwi AA, Syed NA, Shafi G, Hasan TN (2013) Mechanism of cadmium induced apoptosis in human peripheral blood lymphocytes: the role of p53, Fas and caspase-3. Environ Toxicol Pharmacol 36(3):1033–1039
Elmallah MIY, Elkhadragy MF, Al-Olayan EM, Abdel Moneim AE (2017) Protective effect of Fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int J Mol Sci 18 (5)
Kondoh M, Araragi S, Sato K, Higashimoto M, Takiguchi M, Sato M (2002) Cadmium induces apoptosis partly via caspase-9 activation in HL-60 cells. Toxicology 170(1–2):111–117
Abdel Moneim AE (2016) Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One 11(7):e0158965. doi:10.1371/journal.pone.0158965
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research through Research Group Project No. RG-1435-016
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Elkhadragy, M.F., Al-Olayan, E.M., Al-Amiery, A.A. et al. Protective Effects of Fragaria ananassa Extract Against Cadmium Chloride-Induced Acute Renal Toxicity in Rats. Biol Trace Elem Res 181, 378–387 (2018). https://doi.org/10.1007/s12011-017-1062-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1062-7