Abstract
The present study was performed to evaluate the protective effect of selenium (Se) against penconazole (PEN)-induced oxidative stress in the cardiac tissue of adult rats. Male Wistar rats were divided into four groups of six each. The first group represented the controls. For the second group (PEN), no treatment was performed during the first 6 days, and then, the rats received intraperitoneally 67 mg/kg body weight (bw) of PEN every 2 days from day 7 until day 15, the sacrifice day. For the third group (Se + PEN), Se was administered daily through the diet at a dose of 0.5 mg/kg of diet for 15 days. Rats of this group received also every 2 days PEN (67 mg/kg bw) from day 7 until day 15. The fourth group (Se) received daily, through the diet, Se (0.5 mg/Kg of diet) during 15 days. Our results showed that Se reduced significantly the elevated cardiac levels of malondialdehyde and protein carbonyl following PEN treatment, and attenuated DNA fragmentation induced by this fungicide. In addition, Se modulated the alterations of antioxidant status: enzymatic (superoxide dismutase, glutathione peroxidase, and catalase) and nonenzymatic (glutathione and vitamin C) antioxidants in the heart of PEN-treated rats. This trace element was also able to alleviate perturbations of lipid profile. The protective effect of selenium was further evident through the histopathological changes produced by PEN in the heart tissue. Taken together, our results indicated that Se might be beneficial against PEN-induced cardiac oxidative damage in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se), an essential biological trace element, has received considerable attention as an important micronutrient for human beings. It plays a key role in many biological processes such as thyroid hormone production and immune responses [1]. Se is also known to have beneficial antioxidant properties, protecting the organs and tissues from the harmful effects of free radicals, like reactive oxygen species (ROS) [2], thanks to its close localization in the active site of many antioxidant enzymes like glutathione peroxidase (GPx). Foods are the major natural source of this trace element. For example, seafood, cereals, and meat products contain relatively high levels of Se, while low levels are detected in milk, vegetables, and fruits [3]. Nutritional deficiency of this component has been linked with chronic degenerative diseases [4]. Due to its antioxidant properties, Se has long been the focus of observational studies and interventional trials in many pathophysiological conditions, including cardiovascular diseases [5]. Zhong et al. [6] have suggested that Se levels may be important in the heart function. In addition, previous studies have confirmed the protective effect of Se against free-radical-induced cardiac injury [7].
In this regard, the heart tissue is composed of postmitotic cells using fatty acids as the preferred substrate for energy production, which makes it more susceptible to oxidative stress than other tissues [8]. The excessive production of ROS in cardiac tissue may contribute to the development of many cardiovascular diseases including atherosclerosis, hypertension, heart failure, stroke, and diabetes [9]. An extensive survey of available literature indicates that oxidative stress can occur in the heart tissue following the exposure to many environmental pollutants and particularly pesticides [10].
Triazole fungicides represent one of the most important classes of pesticides in agriculture. They have the excellent protective and curative properties against a wide spectrum of crop diseases [11]. These fungicides are designed to inhibit the activity of lanosterol 14α-demethylase (CYP51), a key enzyme for ergosterol biosynthesis in fungi, causing membrane dysfunction and disability to ensure substrate intake [12]. Triazoles are increasingly used in many countries, including Tunisia. Penconazole (PEN) (1-(2,4-dichloro-β-propylphenethyl)-1 H-1,2,4-triazole) is considered as the active substance of a systemic triazole fungicide commonly used in horticultural, agricultural, and forestry industries for foliar pathogen control [13]. Its fungitoxic effectiveness against apple scab and powdery mildew has been confirmed under laboratory and field conditions [14]. PEN is normally sprayed directly onto plants and rapidly absorbed and distributed inside the leaves [15]. This fungicide has been shown as recalcitrant to degradation and susceptible to accumulate in soils [16]. Moreover, residual amounts above the maximum residue limits (MRL) of PEN are detected in some crops [17]. Its residues might affect the environmental safety and human health. In a recent toxicological study, PEN has been found to induce functional and structural testicular impairment in male albino rats [18]. Additionally, this fungicide is associated with endocrine disrupting mediated effects in T-47D cells, suggesting a possible mode of action in thyroid carcinogenesis [19].
To our knowledge, findings concerning the cardiotoxic effects of triazole fungicides remain scarce and appear to be lacking for PEN-induced cardiotoxicity. Besides, the potential ability of Se to attenuate toxicity of this fungicide in the heart has not been previously investigated. Therefore, the present study was carried out to determine the effects of PEN exposure on the oxidant status in the cardiac tissue of adult rats and to assess whether these effects could be ameliorated by Se supplementation.
Materials and Methods
Chemicals
A commercial formulation of PEN, with trade name Topas® (containing 100 g/L of the active ingredient PEN) and produced by Syngenta Company (Basel, Switzerland), was used in the present study. Sodium selenite (Na2SeO3), reduced glutathione (GSH), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), and thiobarbituric acid (TBA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals were of analytical grade and were purchased from standard commercial suppliers.
Animals and Treatments
Adult male rats of Wistar strain weighing 250–270 g and purchased from the Central Pharmacy (SIPHAT, Tunisia) were used in the present study. They were housed in plastic cages lined with husk and maintained under standard laboratory conditions (temperature 22 ± 2 °C, relative humidity 40 %, and 12-h light/dark cycle). The animals were provided ad libitum with a commercial standard pellet diet (SNA, Sfax, Tunisia) and drinking water. All the experimental procedures were performed according to the National Guidelines for Animal Care [20] and approved by the ethical Committee of Sfax Faculty of Sciences.
The animals were randomly divided into four groups of six rats each and treated as follows:
-
Group 1
(control)—considered as negative controls
-
Group 2
(PEN)—no treatment was performed during the first 6 days, then rats received intraperitoneally 67 mg/kg body weight (bw) of PEN every 2 days from day 7 until day 15, the sacrifice day
-
Group 3
(Se + PEN)—rats received daily via diet Se (0.5 mg Na2SeO3/kg of diet) for 15 days and received every 2 days intraperitoneally PEN (67 mg/kg bw) from day 7 until day 15
-
Group 4
(Se)—considered as positive controls where rats received daily via diet Se (0.5 mg Na2SeO3/kg of diet) for 15 days
The PEN dose used in the present study (67 mg/kg bw, equivalent to 1/30 LD50) was selected on the basis of the previous study of El-Sharkawy and El-Nisr [18]. These authors have demonstrated that PEN doses of 50 and 100 mg/kg bw induce structural and functional testicular impairment in adult rats. Therefore, we have chosen an intermediate dose of PEN which caused toxicity without lethality. A dose higher than 67 mg/kg bw provoked hemorrhage and diarrhea. Concerning Se, we have used in our experiment the dose 0.5 mg/kg of diet which was found to give high protection against stress conditions in several tissues [21]. As reported by Hotz et al. [22], lower doses of Se gave less protection while higher doses were not much effective.
At the end of the treatment period (15 days), animals of the different groups were sacrificed by cervical dislocation to avoid stress provoked by anesthesia. Trunk blood samples were collected into heparinized tubes. Plasma samples were separated after centrifugation at 2200×g for 10 min, and they were kept at −80 °C until biochemical analysis. The heart tissues were dissected out and cleaned. Some portions were rinsed and homogenized in an appropriate buffer (pH = 7.4). The homogenates were centrifuged, and the resulting supernatants were used for biochemical assays. Other heart tissue portions were immediately removed, cleaned, fixed in 10 % buffered formalin solution, and embedded in paraffin for histological studies.
Biochemical Analysis
Protein Estimation
Total protein content in heart homogenates was determined according to the Lowry et al. method [23], using bovine serum albumin as standard.
Determination of Lipid Peroxidation
Lipid peroxidation in the heart tissue was estimated spectrophotometrically by measuring malondialdehyde (MDA) according to the method described by Draper and Hadley (1990) [24]. The malonaldehyde values were expressed as nanomoles of malondialdehyde/milligram protein.
Determination of Protein Carbonyl Content
Protein carbonyl (PCO) content in the heart tissue was measured by the method of Reznick and Packer [25]. Results were expressed as nanomoles/milligram protein.
Estimation of Antioxidant Enzyme Activities
Catalase (CAT) activity was measured by using to the method of Aebi [26] and was calculated in terms of micromole H2O2 consumed/minute/milligram of protein.
Superoxide dismutase (SOD) activity was determined as described by Beauchamp and Fridovich [27]. SOD activity was the amount of enzyme required to inhibit the reduction of Nitroblue tetrazolium by 50 % and was expressed as enzyme units/milligram protein.
GPx activity was estimated according to Flohe and Gunzler [28]. The enzyme activity was expressed as nanomoles of GSH oxidized/minute/milligram protein.
Determination of Nonenzymatic Antioxidant Levels
GSH Level
The GSH content in the heart tissue was assayed according to the method of Ellman [29] modified by Jollow et al. (1974). Results were expressed as micromoles/gram tissue.
Vitamin C Level
The determination of vitamin C content in the heart tissue was performed as described by Jacques-Silva et al. [30], and results were expressed as micromoles/gram tissue.
Plasma Lipid Profile
Levels of plasma lipid parameters such as total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were determined spectrophotometrically using commercially available diagnostic kits according to the standard procedures (Biomaghreb, Tunisia, ref. 20111, 20131, 20113, respectively). The low-density lipoprotein cholesterol (LDL-C) level and the atherogenic index (AI) were determined using the Friedewald equation [31]:
Determination of Heart Acetylcholinesterase Activity
Heart acetylcholinesterase (AChE) activity was determined according to the method of Ellman et al. [32] using acetylcholine iodide as a substrate. Results were expressed as micromoles/minute/milligram protein.
Qualitative DNA Fragmentation Assay by Agarose Gel Electrophoresis
Genomic DNA was isolated from the heart tissue using a commercial kit and electrophoresed on a 1 % agarose gel stained with ethidium bromide (Pure Link Genomic DNA Invitrogen ref. K 182001). The gel was then observed under ultraviolet lamp and photographed.
Histological Studies
Some heart tissues were fixed in 10 % of buffered formalin solution and then processed using graded ethanol series and embedded in paraffin. Sections of 3 μm thickness were stained with hematoxylin-eosin for light microscopic observation. Six slides were prepared from each heart tissue. All sections were evaluated for the degree of heart injury.
Statistical Analysis
The data were analyzed using the statistical package program Stat view 5 Software for Windows (SAS Institute, Berkley, CA). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test as a post hoc test for comparison between groups. Student unpaired t test was also used when comparison between two groups was required. All values were expressed as means ± S.D. Differences were considered significant if p < 0.05.
Results
Estimation of Lipid Peroxidation
Our results revealed an increase of lipid peroxidation in the heart of PEN-treated rats, as evidenced by the enhanced malondialdeyhde level in PEN group (+76 %) when compared to controls. Se supplementation alleviated lipid peroxidation without reaching normal values. In Se group (positive controls), heart MDA content was not significantly changed as compared to negative controls (Table 1).
PCO Content
PCO levels in the heart were significantly elevated by 42 % in PEN-treated rats, compared to those of controls. Treatment of PEN-exposed rats with Se restored the cardiac values of PCO to near normal values when compared to PEN-treated rats (Table 1).
Heart Antioxidant Status
Antioxidant Enzyme Activities
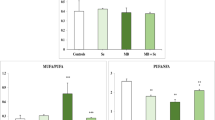
The activities of the cardiac antioxidant enzymes CAT, SOD, and GPx in control and treated rats were illustrated in Fig. 1. PEN treatment led to a significant increase in CAT, SOD, and GPx activities by 62, 16, and 40 %, respectively, as compared to controls. Supplementation of Se reduced significantly antioxidant enzyme activities in the heart of Se + PEN group as compared with PEN group.
Antioxidant enzyme activities CAT (a), SOD (b), and GPx (c) in the heart tissue of control and treated rats with PEN, Se, or their combination (Se + PEN). Values are means ± SD for six rats in each group. PEN and Se + PEN versus control: * p < 0.05; ** p < 0.01; *** p < 0.001. Se + PEN versus PEN: + p < 0.05; +++ p < 0.001
Nonenzymatic Antioxidant Levels
Heart GSH and vitamin C levels decreased significantly (−10 and −67 %, respectively) following PEN treatment when compared to controls. Supplementation of Se in the diet of PEN-exposed rats increased significantly the cardiac levels of GSH and vitamin C, as compared to those of PEN-treated group (Table 1).
Lipid Profile in Plasma
The changes in the levels of plasma lipids in control and experimental rats were shown in Table 2. No significant variations in TC and HDL-C levels were observed between control and PEN-treated rats. Nevertheless, compared to the control group, TG level decreased by 58 % while LDL-C level increased by 12 % in PEN group. Additionally, following PEN treatment, the AI as well as the atherosclerotic indexes (TC/HDL-C and LDL-C/HDL-C ratios) were enhanced significantly by 9, 7, and 11 %, respectively. These variations were markedly improved following the supplementation of Se via the diet of PEN-treated rats.
Heart AChE Activity
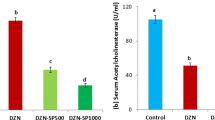
As shown in Fig. 2, rats treated with PEN provoked a significant inhibition of heart AChE activity (−29 %), when compared to controls. Supplementation of Se in the diet of PEN-treated rats did not alleviate AChE activity.
DNA Fragmentation
As shown in Fig. 3, a smear without ladder formation was observed in the heart of PEN-treated rats, indicating random DNA degradation. However, rats treated with Se and PEN showed a decreased DNA smearing as compared to rats treated with PEN alone. No DNA damage was observed in control or Se-treated groups.
Histopathological Findings
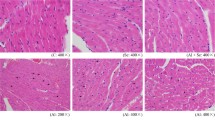
Histological assessment showed a normal structure of the heart in controls (Fig. 4a). Exposure to PEN induced structural changes in this tissue, characterized by cytoplasmic vacuolization of cardiac muscle cells (Fig. 4b). The latter was significantly decreased when Se was supplemented to the diet of PEN-treated rats when compared with those treated with PEN (Fig. 4c). In rats treated with Se alone, heart histoarchitecture was normal (Fig. 4d).
Discussion
Nowadays, the extensive use of triazole pesticide derivatives in agriculture emphasizes the need to investigate their potential hazardous effects on human health. Exposure to these fungicides was associated with a variety of toxicological outcomes in mammals, including thyroid tumors [33], mutagenicity [34], neurotoxicity [35], carcinogenicity, reproductive toxicity, and hepatotoxicity [36, 37]. Unfortunately, very little is known about the effects of these fungicides on the cardiovascular system. Research in this field has been limited to in vitro studies where, for example, the triazole fungicides prochloraz and miconazole have been reported to exert cardiotoxic effects [38, 39]. In this context, PEN is a typical triazole fungicide commonly used in Tunisia, whose cardiotoxicity has not been investigated yet. On the other hand, the trace element Se has been recognized for its cardioprotective effect. In the present study, we demonstrated for the first time that treatment with PEN induced in vivo oxidative stress in the heart of adult rats. Moreover, we showed that Se could play a positive role in mitigating PEN-induced cardiotoxicity and cell damage.
Recently, data indicate that toxic action of pesticides may include the induction of oxidative stress and the accumulation of oxygen free radicals, more generally known as ROS, in the cell. In this regard, it is worth mentioning that oxidative stress may be a major cause of myocardial cell injury. One of the main manifestations of cellular oxidative damage is lipid peroxidation. This process is initiated by the hydroxyl free radical through the extraction of hydrogen atom from polyunsaturated fatty acids of membrane phospholipids [40]. In the heart tissue, it plays an important role in myocardial membrane damage and accumulation of lipid hydroperoxides [41]. The results of the present study showed that the level of lipid peroxidation end product, MDA, was significantly increased in the heart of PEN-treated rats. This suggested the participation of free-radical-induced oxidative cell injury in mitigating PEN toxicity. Moreover, PEN is the most lipophilic triazole fungicide. So, it is supposed that this fungicide may bind extensively to myocardial membrane and cause damage by inducing lipid peroxidation. Similarly, other pesticides like diazinon are found to enhance lipid peroxidation in the heart of adult rats leading to an increase of free radical formation [42]. Meanwhile, Se supplementation to the diet of PEN-treated rats showed a protective role by reducing MDA levels, reflecting its anti-lipid peroxidative effect. Indeed, Se has been reported to be a protector against lipid peroxidation and useful in the management of myocardial injury [43]. Hence, this trace element prevents oxidative damage of heart membrane through a free-radical-scavenging mechanism, leading to the protection of tissues integrity and function.
Cellular proteins are also believed to be the target of ROS, giving rise to carbonyl group formation into side chains and/or to sulfhydryl groups’ reduction in susceptible amino acids [44]. Moreover, the formation of PCO derivatives was found to be associated with pathological conditions, including cardiovascular diseases [45]. In our study, ROS, probably generated as a result of PEN treatment, induced a rise in the cardiac level of PCO products, biomarkers of protein oxidative damage. The accumulation of oxidized proteins might impair myocardial cell function. The occurrence of protein oxidative damage in the heart tissue of experimental rats has been reported following exposure to the insecticide dimethoate, an organophosphorus compound [46]. Interestingly, when the diet of PEN-treated rats was supplemented by Se, the cardiac levels of PCO were restored to near-normal values. This finding could be explained by the ability of Se to counteract free radicals and protect the structure and function of proteins against oxidative injury as reported by Yuan and Tang [47] in chicken treated by lead.
In addition to cellular lipid and protein oxidation, it is well established that DNA may also be affected by free radical accumulation, giving rise to mutations and/or cell death [48]. In the literature reports, there is no evidence that PEN could exert genotoxic effects [49]. Yet, in the present study, PEN exposure induced DNA fragmentation in the heart of adult rats which was evidenced by the appearance on agarose gel of a DNA smear, a necrosis hallmark. It is well established that the biotransformation of many xenobiotics, including pesticides, often results in the production of reactive intermediates such as ROS, which are highly toxic causing DNA oxidative damage [50]. Moreover, several reactive mutagenic and genotoxic products of lipid peroxidation, such as MDA, have been identified to bind to DNA leading to its damage [51]. Thus, it is possible that the observed DNA fragmentation in the heart of PEN-treated rats could be due to ROS generated by fungicide metabolites, which could interact with DNA. Our findings were in accordance with those reported by other authors who have demonstrated that fungicide exposure induces genotoxicity [52]. Nevertheless, supplementation with Se was found to be effective in reducing DNA smearing effects induced by PEN. The beneficial effect of Se in terms of its DNA damage-reducing capacity is well known in mammalian studies [53]. According to Kara et al. [54], the mechanism of Se chemoprotection may be related to its antioxidant properties.
The myocardium has a set of antioxidant defense to prevent free radical formation and to limit their damaging effects. Antioxidant enzymes are considered to be the first line of cell defense that prevents cellular ingredients from oxidative damage. Among them, SOD catalyzes the conversion of superoxide radicals to hydrogen peroxide, which is subsequently converted to water by CAT and GPx. Toxic oxygen species are, thereby, converted into less harmful products. In the present study, the myocardial SOD, CAT, and GPx activities increased significantly in PEN-treated rats, which might reflect an adaptive mechanism of the antioxidative defense system. Similar results have been reported in the cardiac tissue of rats exposed to other pesticides like lindane, an organochloride compound [55]. Yet, supplementation of Se to the diet of PEN-treated rats caused a significant decrease in SOD, CAT, and GPx activities, thus emphasizing its action as an antioxidant. Likewise, Ben Amara et al. [46] have demonstrated Se efficiency in reducing the cardiac activities increase of these antioxidant enzymes produced after dimethoate treatment.
In addition, nonenzymatic antioxidants such as GSH and vitamin C constitute a second line of cellular defense against free radicals. GSH is the most abundant non protein thiol in the cell which is considered to be the major cellular redox buffer. This tripeptide is involved in the removal of ROS and the maintenance of membrane protein thiols, and it serves as a substrate for GPx and glutathione S-transferase [56]. Additionally, its positive role in counteracting cardiotoxicity has been reported [57]. Regarding vitamin C, it is the most important antioxidant present in the hydrophilic compartment. It acts by direct scavenging of singlet oxygen, superoxide, and hydroxyl radicals. Moreover, it reduces the risk of cardiovascular diseases [58]. Under our experimental conditions, the depleted level of cardiac GSH following PEN treatment depicted probably the increased utilization of this biomolecule for the detoxification process. According to Hill and Singal [59] and Li et al. [60], cardiac GSH depletion occurs during oxidative stress and leads to cell function impairment due to the disturbed redox status in the heart. Another pesticide, the lindane, has been reported to decrease the level of GSH in cardiac tissues of rats [55]. Our results showed also a significant decrease in the cardiac level of vitamin C as a result of PEN exposure. Such finding could be due to the depletion of GSH since it is directly involved in recycling vitamin C [61]. With Se supply, the cardiac levels of GSH and vitamin C were significantly enhanced. This finding could be explained by the important role of Se in the metabolism of GSH, as reported earlier [62]. So, in the heart of PEN-treated rats, GSH concentration increased and antioxidant/prooxidant balance was improved.
To get a better evaluation of PEN effects on the cardiac function, it was necessary to examine plasma lipid profile. Results from the present study showed that PEN treatment did not affect plasma levels of TC and HDL-C. However, the plasma level of TG decreased significantly while that of LDL-C increased in PEN-treated rats, when compared to control rats. Moreover, our data demonstrated that PEN exposure increased the AI as well as the atherosclerotic indexes (LDL-C/HDL-C and TC/HDL-C ratios) which are the pertinent indices of the incidence of cardiovascular diseases. These changes in lipid concentrations indicated that PEN might alter lipid metabolism and contribute to the development of cardiac-related disorders. Our results showed that administration of Se through the diet of PEN-treated rats was able to act against the perturbation in plasma lipid profile induced by PEN. This indicated the important role of this trace element in maintaining tissue and cell integrity and function. The protective effect of Se could be attributed to its own antioxidant activity and cellular antioxidant enzyme improvement [63].
Another biochemical marker used in the present study to assess heart function is AChE. This enzyme is an important component of the heart’s cholinergic system known to regulate the cardiac parasympathetic responses via controlling acetylcholine levels [64]. Indeed, AChE rapidly catalyzes acetylcholine hydrolysis, thereby terminating its signaling action at the cholinergic neuroeffector junctions of the heart [65]. Previous studies suggest that free radical formation could be involved in AChE inhibition [66]. The results of the present study showed that PEN was able to reduce significantly AChE activity in rat heart tissue. The present inhibition of AChE activity may be related to the state of oxidative stress induced by PEN in the heart of adult rats. In line with this, the inhibition of blood AChE activity from cattle by tebuconazole-based fungicides has been described previously by Kolesárová et al. [67]. Even though Se has a well-established antioxidant role in living organisms [68], it failed to mitigate PEN-induced AChE inhibition, under our experimental conditions.
These biochemical perturbations were associated with histological alterations. In fact, examination of the heart histoarchitecture revealed that PEN treatment caused structural damage characterized by cytoplasmic vacuolization in cardiac muscle cells, a stage known to precede necrosis. This might result from an increased ROS generation in the heart tissue. Similar results have been observed in the heart of rats exposed to the organophosphorus insecticide, the chlorpyrifos [69]. Milder histopathological changes were shown when Se was supplemented to the diet of PEN-treated rats. So, it might be suggested that this trace element alleviated PEN-induced cardiac damage. In fact, Se is involved in function of GPx which protects membrane lipids against the oxidative damage generated by peroxides [70].
Conclusion
Taken together, our results revealed that PEN induced a state of oxidative stress in the heart of adult rats as evidenced by the increased lipid peroxidation, PCO formation, and the altered enzymatic and nonenzymatic antioxidant status. PEN exposure was found also to inhibit the cardiac AChE activity and to alter the metabolism of lipid. The toxic effects of PEN occurred probably through free radical generation, causing damage to various cardiomyocyte components. Diet supplementation with Se was quite effective in reducing PEN-induced cardiac disturbances due to its antioxidant properties. Our results reflected that Se could be used as an effective supplement in the appropriate management of PEN toxicity.
Abbreviations
- AChE:
-
Acetylcholinesterase
- AI:
-
Atherogenic index
- Bw:
-
Body weight
- CAT:
-
Catalase
- DTNB:
-
5,5′-dithiobis-2-nitrobenzoic acid
- GPx:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- MDA:
-
Malondialdehyde
- MRL:
-
Maximum residue limits
- Na2SeO3 :
-
Sodium selenite
- PCO:
-
Protein carbonyl
- PEN:
-
Penconazole
- ROS:
-
Reactive oxygen species
- Se:
-
Selenium
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
References
Tinggi U (2008) Selenium: its role as antioxidant in human health. Environ Health Prev Med 13:102–108
El-Demerdash FM, Nasr HM (2014) Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J Trace Elem Med Biol 28:89–93
Tinggi U, Reilly C, Patterson CM (1992) Determination of selenium in foodstuffs using spectrofluorometry and hydride generation atomic absorption spectrometry. J Food Comp Anal 5:269–280
Rayman M (2012) Selenium and human health. Lancet 379:1256–1268
Brigelius-Flohe R, Banning A, Schnurr K (2003) Selenium-dependent enzymes in endothelial cell function. Antioxid Redox Signal 5:205–215
Zhong GG, Jiang Y, Li ZB, Zhang BG, Zhang WJ, Yue G (1990) Protective action of selenium and manganese on xanthine and xanthine oxidase induced oxidative damage to cultured heart cells. Chinese Med J Peking 103:735–742
Danesi F, Malaguti M, Nunzio MD, Maranesi M, Biagi PL, Bordoni A (2006) Counteraction of adriamycin-induced oxidative damage in rat heart by selenium dietary supplementation. J Agric Food Chem 54:1203–1208
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction and aging. Science 273:59–63
Heistad DD (2006) Oxidative stress and vascular disease: 2005 Duff lecture. Arterioscler Thromb Vasc Biol 26:689–695
Jaiswal SK, Nj S, Sharma B (2013) Carbofuran induced oxidative stress in rat heart: ameliorative effect of vitamin C. ISRN Oxid Med ID 824102
Wang P, Jiang S, Liu D, Wang PI, Zhou Z (2005) Direct enantiomeric resolutions of chiral triazole pesticides by high-performance liquid chromatography. J Biochem Biophys Methods 62:219–230
Buchenauer H (1987) Mechanism of action of triazol fungicides and related compounds. In: Lyr H (ed) Modern selective fungicides: properties, applications, mechanisms of action. Longman Scientific and Technical, Co-published in the United States with John Wiley and Sons, Inc, New York, pp. 205–232
Kenyon DM, Dixon GR, Helfer S (1997) The repression and stimulation of growth of Erysiphe sp. on Rhododendron by fungicidal compounds. Plant Pathol 46:425–431
Percival GC, Boyle S (2005) Evaluation of microcapsule trunk injections for the control of apple scab and powdery mildew. Ann Appl Biol 147:119–127
Kim IS, Beaudette LA, Shim JH, Trevors JT, Suh YT (2002) Environmental fate of the triazole fungicide propiconazole in a rice-paddy-soil lysimeter. Plant Soil 239:321–331
Singh N (2005) Mobility of four triazole fungicides in two Indian soils. Pest Manag Sci 61:191–196
Güdücü HE, İnam R, Aboul-Enein HY (2011) Determination of organophosphorus and triazole pesticides by gas chromatography and application to vegetable and commercial samples. J Liq Chrom Rel Technol 34:2473–2483
El-Sharkawy EE, El-Nisr NA (2013) Testicular dysfunction induced by penconazole fungicide on male albino rats. Comp Clin Path 22:475–480
Perdichizzi S, Mascolo MG, Silingardi P, Morandi E, Rotondo F, Guerrini A, Prete L, Vaccari M, Colacci A (2014) Cancer-related genes transcriptionally induced by the fungicide penconazole. Toxicol in Vitro 28:125–130
Council of European Communities (1986) Council instructions about the protection of living animals used in scientific investigations. Off J Eur Commun (JO 86/609/CEE) L358: 1–18
Ben Amara I, Fetoui H, Guermazi F, Zeghal N (2009) Dietary selenium addition improves cerebrum and cerebellum impairments induced by methimazole in suckling rats. Int J Dev Neurosci 27:719–726
Hotz CS, Fitzpatrick DW, Trick KD, L’Abbe MR (1997) Dietary iodine and selenium interact to affect thyroid hormone metabolism of rats. J Nutr 127:1214–1218
Lowry OH, Rosebrugh NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl. Method Enzymol. Academic Press, New York, p. 357
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Jacques-Silva MC, Nogueira CW, Broch LC, Flores EMM, Rocha JBT (2001) Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. J Pharmacol Toxicol 88:119–125
Friedewald WT (1972) Estimation of concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Ellman GL, Courtney KD, Andres V, Featherstone R (1961) A new and rapid colorimetric determination of acetyl cholinesterase activity. Biochem Pharmacol 7:88–95
Hester SD, Nesnow S (2008) Transcriptional responses in thyroid tissues from rats treated with a tumorigenic and a non-tumorigenic triazole conazole fungicide. Toxicol Appl Pharm 227:357–369
Ross JA, Moore T, Leavitt SA (2009) In vivo mutagenicity of conazole fungicides correlates with tumorigenicity. Mutagenesis 24:149–152
Moser VC, Barone S, Smialowicz RJ, Harris MW, Davis BJ, Overstreet D, Mauney M, Chapin RE (2001) The effects of perinatal tebuconazole exposure on adult neurological, immunological, and reproductive function in rats. J Toxicol Sci 62:339–352
Georgopapadakou NH, Walsh TJ (1996) Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents CH 40:279–291
Peffer RC, Moggs JG, Pastoor T, Currie RA, Wright J, Milburn G, Waechter F, Rusyn I (2007) Mouse liver effects of cyproconazole, a triazole fungicide: role of the constitutive androstane receptor. J Toxicol Sci 99:315–325
Papaefthimiou C, Theophilidis G (2001) The cardiotoxic action of the pyrethroid insecticide deltamethrin, the azole fungicide prochloraz, and their synergy on the semi-isolated heart of the bee Apis mellifera macedonica. Pestic Biochem Phys 69:77–91
Won KJ, Lin HY, Jung S, Cho SM, Shin HC, Bae YM, Lee SH, Kim HJ, Jeon BH, Kim B (2012) Antifungal miconazole induces cardiotoxicity via inhibition of APE/Ref-1-related pathway in rat neonatal cardiomyocytes. J Toxicol Sci 126:298–305
Farber JL, Kyle ME, Coleman JB (1990) Biology of disease: mechanisms of cell injury by activated oxygen species. La Inves 62:670–679
Radhiga T, Rajamanickam C, Senthil S, Pugalendi KV (2012) Effect of ursolic acid on cardiac marker enzymes, lipid profile and macrosco. Food Chem Toxicol 50:3971–3977
Ogutcu A, Uzunhisarcikli M, Kalender S, Durak D, Bayrakdar F, Kalender Y (2006) The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of vitamin E. Pestic Biochem Phys 86:93–98
Gan L, Liu Q, Xu HB, Zhu YS, Yang XL (2002) Effects of selenium overexposure on glutathione peroxidase and thioredoxin reductase gene expressions and activities. Biol Trace Elem Res 89:165–175
Halliwell B, Gutteridge JMC (2001) Oxidative stress: adaptation, damage, repair and death. In: Halliwell B, Gutteridge JMC (eds), Free Radic Biol Med. 3rd Ed. Oxford, UK: Oxford University Press, Oxford, pp 246–350.
Jawalekar SL, Kulkarni UJ, Surve VT, Deshmukh YA (2010) Status of lipid profile, MDA and protein carbonyl in patients with cardiovascular diseases. Arch Appl Sci Res 2:8–14
Ben Amara I, Soudani N, Hakim A, Troudi A, Zeghal KH, Boudawara T, Zeghal N (2011) Protective effects of vitamin E and selenium against dimethoate-induced cardiotoxicity in vivo: biochemical and histological studies. Environ Toxicol. doi:10.1002/tox.20759
Yuan X, Tang C (1999) Lead effect on DNA and albumin in chicken blood and the protection of selenium nutrition. J Environ Sci Health 34:1875–1887
Scott D, Galloway SM, Marshall RR, et al (1991) International commission for protection against environment mutagens and carcinogens. Genotoxicity under extreme culture conditions. A report from ICPEMC task Group 9. Mutat Res 257:147–204
Jawich D, Lteif R, Pfohl-Leszkowicz A, Strehaiano P (2006) Effects of penconazole on two yeast strains: growth kinetics and molecular studies. Mol Nutr Food Res 50:552–556
Cavalcantea DGSM, Martinez CBR, Sofiaa SH (2008) Genotoxic effects of Roundup® on the fish Prochilodus lineatus. Mutat Res 655:41–46
Eder E, Wacker M, Lutz U, Nair J, Fang X, Bartsch H, Beland F, Schlatter J, Lutz W (2006) Oxidative stress related DNA adducts in the liver of female rats fed with sunflower-, rapeseed-, olive-or coconut oil supplemented diets. Chem Biol Interact 159:81–89
Soloneski S, González M, Piaggio E, Reigosa MA, Larramendy ML (2002) Effect of dithiocarbamate pesticide zineb and its commercial formulation azzurro. III. Genotoxic evaluation on Chinese hamster ovary (CHO) cells. Mutat Res 514:201–212
El-Bayoumy K (2001) The protective role of selenium on genetic damage and on cancer. Mutat Res 475:123–139
Kara H, Cevik A, Konar V, Dayangac A, Yilmaz M (2007) Protective effects of antioxidants against cadmium-induced oxidative damage in rat testes. Biol Trace Elem Res 120:205–211
Ananya R, Subeena S, Kumar DA, Kumar DT, Kumar MS (2005) Oxidative stress and histopathological changes in the heart following oral lindane (gamma hexachlorohexane) administration in rats. Med Sci Monit 11:325–329
Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57:144–155
Priscilla DH, Prince PSM (2009) Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact 179:118–124
May JM (2000) How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med 28:1421–1429
Hill MF, Singal PK (1997) Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation 96:2414–2420
Li S, Li X, Rozanski GJ (2003) Regulation of glutathione in cardiac myocytes. J Mol Cell Cardiol 35:1145–1152
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci U S A 100:3525–3530
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Said L, Banni M, Kerkeni A, Said K, Messaoudi I (2010) Influence of combined treatment with zinc and selenium on cadmium induced testicular pathophysiology in rat. Food Chem Toxicol 48:2759–2765
Hoover DB, Ganote CE, Ferguson SM, Blakely RD, Parsons RL (2004) Localization of cholinergic innervation in guinea pig heart by immunohistochemistry for high-affinity choline transporters. Cardiovasc Res 62:112–121
Lefkowitz RJ, Hoffman BB, Taylor P (1996) Neurotransmission: the autonomic and somatic motor nervous systems. In: Hardman JG, Limbird LL, Molinoff PB, Ruddon RW, Gilman AG (eds) Goodman and Gilman’s the pharmacological basis of therapeutics, 9th edn. McGraw-Hill, New York, pp. 105–139
Tsakiris S, Angelogianni P, Schulpis KH, Stavridis C (2000) Protective effect of L-phenylalanine on rat brain acetylcholinesterase inhibition induced by free radicals. Clin Biochem 33:103–106
Kolesárová V, Šinko G, Šiviková K, Dianovský J (2013) In vitro inhibition of blood cholinesterase activities from cattle by triazole fungicides. Int J Cytol Cytosystematics Cytogenet 66:346–350
Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215:213–219
Bas H, Kalender Y (2011) Chlorpyrifos induced cardiotoxicity in rats and the protective role of quercetin and catechin. Gazi Univ J Sci 24:387–395
Hsu YT, Wolter KG, Youle RJ (1997) Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A 94:3668–3672
Acknowledgments
This work was supported by the Ministry of Higher Education and Scientific Research [UR/11ES-70], Tunisia. The authors are thankful to Mr. Chedli Tmar for the maintenance of the laboratory animals and to Mrs. Raoudha Ben Amar Abdennadher for her skillful technical assistance.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaâbane, M., Tir, M., Hamdi, S. et al. Improvement of Heart Redox States Contributes to the Beneficial Effects of Selenium Against Penconazole-Induced Cardiotoxicity in Adult Rats. Biol Trace Elem Res 169, 261–270 (2016). https://doi.org/10.1007/s12011-015-0426-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0426-0