Abstract
Despite increasing evidence indicating the essential involvement of selenium (Se) in the immune system, the effect of Se deficiency on the regulation of oxidative stress and heat shock proteins (Hsps) in broiler chickens is still unclear. In the present study, we established an exudative diathesis (ED) broiler chicken model caused by Se deficiency. We then analyzed histological observations and detected the expression levels of Hsps and antioxidant indexes in immune tissues. The antioxidant function declined remarkably, and most of the Hsp expression levels increased significantly in the spleen, thymus, and bursa of Fabricius of the broiler chicks with ED (except the messenger RNA (mRNA) levels of Hsp27, Hsp40, and Hsp70, which decreased in thymus tissues from the treatment groups); therefore, constitutive oxidation resistance and higher Hsps in broiler chicks with ED caused defects in immune organ morphology and function, as evidenced by abnormal histological structures: red pulp broadening and lymphocytes in the cortex and medulla of the thymic lobule decreased distinctly and distributed loosely. These results underscore the importance of Se in establishing an immune organ microenvironment conducive to normal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is a nutritionally essential trace element for a wide range of species [1], including broiler chickens. Se deficiency can cause oxidant stress and induce different tissue injuries, such as muscle necrosis, pancreas atrophy, and immune or nerve damage [2–5]. The immune system is particularly sensitive to Se deficiency. Previous studies suggested that Se deficiency could impair the functions of cellular immunity and humoral immunity in organisms [3, 6, 7]. The synthesis of immune globulin and T lymphocyte proliferation and differentiation [3, 8, 9] declined due to Se deficiency. Moreover, Zhang et al. proved that injury to the immune organs of chickens was caused by Se deficiency, and they suggested that oxidative stress inhibited immune organ growth and development and attenuated the immune function of chickens [10].
Heat shock proteins (Hsps) are known to play important roles in protecting cellular functions [11]. Hsps are divided into various types according to their different molecular weights, and Hsp90, Hsp70, and Hsp40 are important to immune tissues [12]. The overexpression of Hsps will exert its defensive function when organisms are under a variety of environmental stressors such as hyperpyrexia, cold stress, and intoxication [13–15]. Zhao et al. found that the messenger RNA (mRNA) expression levels of Hsp70, Hsp60, Hsp40, and Hsp27 increased to protect chicken heart tissue from cold stress [16]. Banerjee et al. demonstrated that heat stress upregulated Hsp70 in Chinese hamster lung fibroblast V79 cells [17]. Li et al. suggested that AVM exposure could cause a protective stress response by promoting both mRNA and protein expression of Hsp90, Hsp70, and Hsp60 under in vivo and in vitro conditions for neurons, thus easing the neurotoxic effects of AVM to some extent on brain tissues and brain neurons of pigeons [18]. Hsps also exert important functions in immune organs. J. Martimez et al. reported that Trichinella spiralis Hsp60, Hsp70, and Hsp90 were targets of the humoral immune response in a rat infection model [19]. Habich C. et al. indicated that Hsp60 plays a regulatory role in innate immune cells [20]. Wei et al. found that autologous Hsp60 could be considered a danger signal for the innate immune system; for example, local Hsp60 played an important role in expression/release in chronic Th1-dependent tissue inflammation [21]. In addition, Willem et al. proposed that Hsps could prevent or arrest inflammatory damage, and in initial clinical trials in patients with chronic inflammatory disease, Hsp-derived peptides have been shown to promote the production of anti-inflammatory cytokines, indicating that Hsps have immunoregulatory potential [22].
Studies have demonstrated that the expression of Hsps was correlated with the Se content in organisms [23, 24], and Se deficiency could induce oxidative stress. Based on these previous results, we sought to evaluate the effect of a low-Se diet on the immune organs of broiler chicks and to discuss the role of Hsps in immune organs of broiler chicks with Se deficiency. Although studies have addressed the effect of Se on antioxidant function in chicken immune organs and morphology changes, no studies have explored the effects on the immune organs of ED chickens. Therefore, the aim of this research was to investigate the parameters of oxidative stress, the expressions of Hsps, and tissue damage in broiler chick immune organs of ED due to Se deficiency.
Materials and Methods
Poultry and Tissue Collection
All procedures used in the present study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. One hundred and fifty broiler chicks (1 day old; Weiwei Co. Ltd., Harbin, China) were randomly divided into two groups (75 chickens per group). The chickens were maintained either on a diet supplemented with Se through the addition of 0.2 mg/kg Se (C group) via sodium selenite or on a Se-deficient granulated diet (L group, from the Se-deficient region of Heilongjiang Province in China, containing 0.008 mg/kg Se) until the broiler chicks exhibited ED onset. Feed and tap water were supplied ad libitum. In this study, when the symptoms of Se deficiency occurred in the L group at 20 days, we collected the samples. Following euthanasia, the immune tissues (including the spleen, thymus, and bursa of Fabricius) were quickly collected. The tissues were excised immediately on an ice-cold plate, washed in a physiological saline solution, and then divided into three portions: one stored at −80 °C until required to isolate the RNA and proteins, one fixed in 4 % buffered formaldehyde for histopathological examination, and the rest to determine the indexes of oxidative stress.
Histopathological Examination
To find the pathologic changes in immune organs caused by Se deficiency, the lesions were recorded and compared at the end of the experiment and were collected and fixed in 10 % buffered formalin for histopathological examination. Later, paraffin-embedded tissue sections were cut at 5-μm thicknesses, stained with H.E. and examined under a microscope.
Histological alterations were scored as (−), no histopathology; (+), histopathology in <20 % of the fields; and (++), histopathology in 20–60 % of the fields. This categorization was modified from methods described by Bernet and Figueiredo [25, 26].
Determination of Antioxidant Enzyme Activities
The tissues (spleen, thymus, and bursa of Fabricius) were homogenized (1:10 w/v) with a glass Teflon homogenizer (Heidolph SO1 10R2RO) in physiological saline. The homogenate was centrifuged at 700×g for 30 min at 4 °C to obtain the postmitochondrial supernatant to measure glutathione-peroxidase (GSH-Px) and inducible nitric oxide synthase (i-NOS) activities, nitric oxide (NO), GSH, and malondialdehyde (MDA) content levels.
Design of Primers and Quantitative PCR
Total RNA was isolated from the tissue samples using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, China). The dried RNA pellets were resuspended in 50 μl of diethyl-pyrocarbonate-treated water. The concentration and purity of the total RNA were spectrophotometrically determined at 260 nm/280 nm according to the spectrophotometer (Gene Quant 1300/100, General Electric Company, USA). First-strand complementary DNA (cDNA) was synthesized from 5 μg of total RNA using oligo dT primers and superscript II reverse transcriptase according to the manufacturer’s instructions (Roche, USA). The synthesized cDNA was diluted five times with sterile water and stored at −80 °C before use.
Primer Premier Software (PREMIER Biosoft International, USA) was used to design specific primers for Hsps based on known chicken sequences (Table 1). Quantitative real-time PCR was performed on an ABI PRISM 7500 Detection System (Applied Biosystems, USA). The reactions were performed in a 20-μl reaction mixture containing 10 μl of 2× SYBR Green I PCR Master Mix (TaKaRa, China), 2 μl of either diluted cDNA, 0.4 μl of each primer (10 μM), 0.4 μl of 50× ROX reference Dye II, and 6.8 μl of PCR-grade water. The PCR procedure for Hsps was 95 °C for 30 s followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 60 °C for 30 s. The melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed with the PCR products to verify primer specificity and product purity. The relative mRNA abundance was calculated according to the method of ΔΔCt, which accounts for gene-specific efficiencies and was normalized to the mean expressions of the above-mentioned index.
Western Blotting
Equal amounts of protein were resuspended in SDS-PAGE buffer (62.5 mM Tris-HCl, pH 6.8, 2 % SDS, 10 % glycerol, 5 % 2-mercaptoethanol, 1 mM PMSF) and boiled in a water bath for 5 min. Lysates were stored at −20 °C until further analysis. The proteins were separated under reducing conditions for 2 h at 120 V in 12 % SDS-polyacrylamide gels, and the gels were transblotted onto PVDF membranes at 100 V for 2 h. The membranes were blocked overnight in high salt buffer (50 mM Tris base, 500 mM NaCl, 0.05 % Tween-20) containing 5 % bovine serum albumin and then incubated for 1 h with anti-Hsp60 (1:1500), -Hsp70 (1:1500), and -Hsp90 (1:1000), followed by incubation with HRP-conjugated secondary antibodies (Pierce), goat anti-mouse, or anti-rabbit (1:2000). The protein bands were visualized by ECL, and the relative optical densities (arbitrary units) were obtained by normalizing each band for the β-actin band.
Statistical Analysis
All data were statistically analyzed using the SPSS statistical software for Windows (version 13; SPSS, Chicago, IL, USA). When a significant value (P < 0.05) was obtained by T test analysis of variance, further analysis was performed. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed using Tukey’s honest significant difference test for post hoc multiple comparisons. Data are expressed as the mean ± standard deviation. Differences were considered to be significant at P < 0.05 and highly significant at P < 0.01.
Results
Histological Analysis
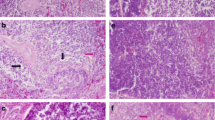
The general histological examination indicated that immune organs exhibited different changes due to Se deficiency (the histopathological changes were summarized in Table 2). We found that there were no obvious changes in the samples from the C group. Spleen tissues from the control group showed normal histological structures with red pulp and white pulp (Fig. 1a). In contrast, as shown in Fig. 1b–d, the number of lymphocytes in spleen tissues from the L group decreased, and we found a loosely distributed periarterial lymphatic sheath, full splenic sinus clearance, and broadening of the red pulp. We also observed lymphocyte necrosis (Fig. 1c) and cellular edema (Fig. 1d).
H&E staining revealed characteristic histopathological changes in the broiler chick spleen, thymus, and bursa of Fabricius with ED. a Spleen tissue of the control group (×200). b Spleen of broiler chickens in the low-dose group (×400). The lymphocytes in the spleen were significantly decreased. c Spleen of broiler chickens in the low-dose group (×400). Spleen tissue was identified with the emergence of lymphocyte necrosis (black arrow) and the loose arrangement of PALS cells. d Spleen of broiler chickens in the low-dose group (×400). Spleen tissue showed serious histopathological changes, such as red pulp broadening, the emergence of tawny hemosiderin (blue arrow), increases in the splenic sinus gap (black arrow), and lymphocyte necrosis. e Thymus tissue of the control group (×200). f Thymus of broiler chicks in the low-dose group (×400). Thymic corpuscles magnified. The lymphocytes in the thymic lobule cortex and medulla were significantly decreased and arranged loosely. g Thymus of broiler chickens in the low-dose group (×400). The thymic lobule medulla shrank, and angiotelectasis was present. h Thymus of broiler chicks in the low-dose group (×400). Thymus tissue was identified with the emergence of lymphocyte necrosis and nucleus fragmentation and pyknosis (black arrow). i Bursa of Fabricius tissue of the control group (×200). j Bursa of Fabricius of broiler chickens in the low-dose group (×400). The lymphoid follicle medulla and cortex became thin, and reticular cell hyperplasia was observed. k Bursa of Fabricius of broiler chickens in the low-dose group (400×). Bursa of Fabricius tissue was identified via the reduction of lymphoid follicle volume and a zigzag membrane epithelium. l Bursa of Fabricius of broiler chickens in the low-dose group (×400). Bursa of Fabricius tissue showed significant histopathological changes, such as a decrease in lymphoid follicle medulla cells and fibrous connective tissue hyperplasia
Thymus tissues from the control group showed normal histological structures with the cortex and medulla (Fig. 1e). However, we observed that the lymphocytes in the cortex and medulla of the thymic lobule decreased distinctly and were loosely distributed, and the volume of the thymus nodules became larger in thymus tissues from the L group (Fig. 1f). The medulla of the thymic lobule shrank, and capillary ectasia was observed (Fig. 1g). Figure 1h shows that the cell nuclei of lymphocyte pyknosis and fragmentation appeared in thymus tissues from the L group.
Bursa of Fabricius tissues from the control group showed normal histological structures (Fig. 1i). The cortex and medulla of the thymic lobule thinned, the lymphocytes were arranged sparsely, and reticular cells became hyperplastic (Fig. 1j). We observed that the volume of the lymphoid follicle diminished, the connective tissue among follicles increased, and the membrane epithelium appeared in a zigzag pattern in the bursa of Fabricius tissues from the L group (Fig. 1k). As shown in Fig. 1l, the lymphocytes in the lymphoid follicular medulla decreased obviously in the bursa of Fabricius tissues from the L group.
Analysis of the Effect of Low Dietary Se on the Oxidation Resistance Indexes in Immune Organs in Chickens
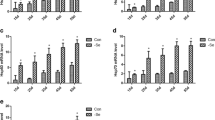
To determine the effect of low dietary Se on the oxidation resistance indexes in immune organs in broiler chicks, we measured the activities of i-NOS, GSH-PX, and contents of MDA, NO, and GSH (Fig. 2). The levels of MDA and NO significantly increased (P < 0.01) in the L groups in the immune organs. The level of MDA in the bursa of Fabricius changed the most, and the levels of NO exhibited the most changes in the thymus organs between the L groups and the C groups (P < 0.05). The activities of GSH-PX and the level of GSH significantly decreased (P < 0.01) compared with the C groups. However, the activity of i-NOS levels in the L group were significantly increased in the spleen and bursa of Fabricius (P < 0.01) and were significantly increased to a lesser degree in the thymus (P < 0.05).
Analysis of the effect of low dietary Se on the oxidation resistance indexes in immune organs (a). The activity of GSH-Px in the immune tissues of broiler chickens with ED was significantly decreased in immune tissues. b The activity of i-NOS was significantly increased in the low-dose group compared with the control group. c–e The levels of NO, GSH, and MDA were significantly changed in immune tissues from low-dose groups. Asterisk significant differences (P < 0.05) between the control group and the low-dose group; each value represented the mean ± SD of six individuals
Effect of Dietary Se on the mRNA Levels of Hsps in the Immune Tissues of Chickens
To determine the effect of Se deficiency on the distribution of the Hsp mRNA in the immune tissues, quantitative PCR (qPCR) was performed, and the results are shown in Fig. 3. The mRNA levels of Hsp60 and Hsp90 significantly increased (P < 0.05) in the immune tissues from the L group (Fig. 3c, e), indicating that Hsps, as a type of stress protein, would be increased in immune organs when the broiler chicks faced the stress of Se deficiency. Both Hsp60 and Hsp90 changed the most in the spleen tissue. Interestingly, the mRNA levels of Hsp27, Hsp40, and Hsp70 significantly increased in the spleen and bursa of Fabricius tissues (P < 0.05) but decreased in thymus tissues (P < 0.05) in the L group (Fig. 3a, b, d). These three Hsps changed most in the spleen, similar to the other two Hsps.
Effects of Se deficiency on Hsps mRNA expression in broiler chick immune tissues The results are from at least five independent experiments. Data are represented as the mean ± SD (n = 6). Bars with different superscript letters represented statistically significant differences (P < 0.05) between the control group and the low-dose group
Effect of Dietary Se on Hsp Protein Expression in the Immune Tissues of Chickens
The protein levels of Hsps in immune tissues were tested using western blot. As shown in Fig. 4, Se deficiency treatment significantly increased the expression of Hsp60, Hsp70, and Hsp90 in thymus and spleen tissues in the L group. Meanwhile, compared with the control group, the expression of these Hsps in the bursa of Fabricius from the L group increased, but not significantly.
The levels of Hsp60, Hsp70, and Hsp90 in broiler chick immune tissues. a The results are from at least five independent experiments. Data are represented as the mean ± SD (n = 6). Bars with different superscript letters represented statistically significant differences (P < 0.05) between the control group and the low-dose group. b Immunoblotting of Hsp60, Hsp70, Hsp90, and β-Actin in broiler chick immune tissues
Discussion
Se deficiency may cause various health problems, such as ED, muscle necrosis, pancreas atrophy, and a series of disease in chickens. The effects of Se can be partially attributed to the properties of antioxidants that promote effective oxygen-free radical scavenging. Some reports clarified that a deficiency in Se might be the cause of certain diseases that develop from the harmful effects of free radical production [27]. Hsps are a group of highly conserved cellular stress proteins and play a pivotal role in the protection and maintenance of several vital cellular functions. Due to the lack of an effect of Se deficiency in broiler chick immune organs, we designed experiments to investigate the pathological changes, antioxidative parameters, and Hsps on immune organs of broiler chicks under Se deficiency. In the present study, the results showed that Se deficiency causes immune organ lesions and is responsible for the levels of NO, MDA content, and Hsps upregulation in the thymus, spleen, and bursa of Fabricius; meanwhile, the activities of i-NOS, GSH-Px, and the levels of GSH decreased. Our work sought to better understand the mechanisms of Se deficiency that induced abnormalities in broiler chicks by studying the immune tissues.
The antioxidative/oxidative parameters are valuable to assess oxidative damage. GSH-Px is considered to be the first line of cellular defense against oxidative damage [28]. Our results showed that compared with the control groups, the activity of GSH-Px and the content of GSH in the immune organs of broiler chicks with ED significantly decreased. These results were similar to those of previous studies. Sunde and Hadley found that the activities of Gpx1 in the kidney and heart and Gpx4 in the liver were reduced in Se-deficient poultry [29]. Yao et al. found that Se deficiency could reduce the activity of GSH-Px in chicken muscles [30]. When the activity of GSH-Px was attenuated, excessive free radicals produced in the organism would attack polyunsaturated fatty acids and cause lipid peroxidation (LPO) to form lipid peroxide, such as MDA. In this study, compared to the control group, MDA content increased by greater than one or two times in the low-Se groups for immune organs, suggesting that a large amount of LPO occurred in the broiler chicks with ED. Liu et al. indicated that the MDA level increased in the chicken livers from the Se-deficient group (P < 0.05) [31]. Sun et al. also reported that Se deficiency caused the MDA content to increase in the chicken kidney [32] and intestinal tract [33]. Our results indicated that the effect of Se deficiency on immune tissues was similar to other organs in chickens. NO is a highly reactive free radical with an unpaired electron. NO is produced by a family of NO synthases (NOSs), especially i-NOS. Excessive NO was involved in lipid peroxidation-mediated injury and ultimately converted into peroxynitrite, which increased oxidative stress and cell injury. Tinkle et al. reported that Se deficiency is associated with an increase in reactive nitrogen species (RNS), such as NO and peroxynitrite production [34]. Zhao et al. noted that Se deficiency induced higher levels of NO and i-NOS in the pancreas of chickens [5]. Similar to these prior studies, our results showed that compared with the control groups, the levels of NO and i-NOS were significantly increased in the immune tissues of broiler chicks with ED. These results might explain the large extent of oxidative damage that occurs in Se-deficient broiler chickens.
Hsps are indispensable for maintaining normal cell function and directing the cell response [11]. Therefore, it is necessary to monitor Hsp expression levels in multiple tissues or organs. Hsps are upregulated in response to various forms of stress, such as oxidative stress and xenobiotic stresses. For example, Chen et al. indicated that Se deficiency induced the high expression of Hsps [35]. Liu et al. found that the levels of Hsp60 and Hsp70 significantly increased in the brains of common carp after exposure to atrazine, chlorpyrifos, and in combination [36]. Hsps are also considered to be biomarkers of immunological stress. Compared with the control group, most of the mRNA expression levels of Hsps were promoted in the thymus, spleen, and bursa of Fabricius of Se-deficient broiler chicks. Meanwhile, the protein levels of Hsp60, Hsp70, and Hsp90 were upregulated in the spleen and thymus. This indicated that an Se-deficient diet promoted the immune response as a type of stress. Mahmoud et al. noted that Pi deficiency might affect major cellular biochemical pathways via Hsp protein expression in broiler chicks [37]. It was interesting that in the present study, the protein levels of Hsp were not always consistent with Hsp mRNA expression. Several reasons may cause this difference: first, pre-mRNA splicing can influence subsequent RNA synthesis, resulting in a change in translational yields; second, many other mechanisms also activate the Hsp response when the organism undergoes a stressful situation [38]; and third, the high production of Hsps may be energetically expensive and could cause a negative effect if the high level of Hsps was not necessary to offset protein denaturation [39, 40]. Furthermore, in this study, the bursa of Fabricius was not consistent with the spleen and thymus. This difference depended on the different sensitivities to Se deficiency among immune organs. In the present study, the results showed that the mRNA levels of Hsp27 and Hsp40 were decreased by Se deficiency, yet their levels were increased in other organs. We observed that the degrees of injury in the thymus tissues from the L groups were more serious than those in the spleen and the bursa of Fabricius tissues with the naked eye and with a light microscope. Using the methods and standards described by D. Bernet, J. Mallatt, and A. Figueiredo-Silva to assess the degrees of injury, we obtained the results shown in Table 2. The structural damage of organs was closely related to their functions. The seriously injured organizational structure might lead to the reduction of Hsp mRNA expression levels in the thymus. Hsp27 and Hsp 40 are small Hsps, and their main functions are protecting the organism from oxidative stress [41]. When the broiler chicks underwent oxidative stress due to Se deficiency, other mechanisms might have caused the decrease in the mRNA levels of these two types of Hsps. Similar results were reported in previous studies. For example, Soldes OS et al. indicated that the level of Hsp27 decreased in esophageal adenocarcinomas [42] and Lo Muzi L et al. noted that decreased Hsp27 immunoexpression was found in aggressive and less differentiated oral squamous cell carcinoma [43]. Compared to the spleen and the bursa of Fabricius tissues, the mRNA expression of Hsp27 and Hsp40 in the thymus tissues showed a different trend. On the one hand, it showed that the thymus might be different from the spleen and the bursa of Fabricius in facing oxidative stress; on the other hand, it implied that the injury to the thymus was more serious than to the spleen and the bursa of Fabricius.
Organ structure is the basis of its functions. Histological changes in animal tissues provide a rapid method for detecting the effects of irritants. In this study, histological observations showed that the volume of thymus corpuscles became larger and the lymphocytes in the cortex and medulla of thymic lobules decreased distinctly and were loosely distributed in the thymus tissues of broiler chicks from the L group. The main cause of the decline in the thymus visceral index might be the decrease in the cortex and medulla lymphocytes of the thymic lobules. The thymus is the main immune organ, so the normal structure of thymus is the basis of the organism’s maintenance of normal immune function. The impaired development of thymus lymphocytes induced by Se deficiency was associated with damage to thymic corpuscles. Aita et al. reported that thymic corpuscles might take part in the cellular immunologic response and could secrete thymic hormone [44]. Molinero et al. suggested that there were type II thyroxine 5′-deiodinases in both lymphocytes and the matrix cells of the thymus, and when Se was lacking, the damaged activity of type II thyroxine 5′-deiodinase might influence the development and function of thymus cells [45]. Therefore, histological changes in the thymus caused by Se deficiency might result in changes in the biological function of the thymus. The number of lymphocytes in spleen tissues also decreased in the L group, and there was sparse distribution of the periarterial lymphatic sheath, full splenic sinus clearance, and broadening of the red pulp. Similar to our results, Liu et al. discovered that AVM altered the structure of pigeon spleen tissues, as evidenced by red pulp broadening, mangy tawny hemosiderin emergence, lymphocyte necrosis, and nucleus fragmentation in spleen tissues [15]. In the present study, histological observation showed that the spleen structure was damaged, indicating that the function of the spleen is influenced by Se deficiency by changes in its structure. In the present study, the lymphocytes in the thymus and the bursa of Fabricius decreased, manifesting in the reduction of spleen follicles and the constriction of PALS in the L group was connected with lesions in the thymus and bursa of Fabricius. The bursa of Fabricius, the characteristic immune organ in poultry, is where B lymphocytes undergo differentiation and maturation. In this study, histopathological observation showed that Se deficiency decreased lymphocytes at different levels, and the lymphocytes were loosely arranged in the cortex and medulla of the lymphoid follicle in the bursa of Fabricius. Similar to our results, Marsh et al. indicated that the lymphocytes decreased in the bursa of Fabricius of chickens with Se deficiency [7]. The ripe B lymphocytes in the bursa of Fabricius decreased, and the immune globulin synthesized in the organism decreased as well. The morphological findings in this study indicated that a series of lesions in Se-deficient immune organs influence normal physiological function.
Conclusion
In summary, the present study showed that Se deficiency could lead to oxidative injury and alterations in the histology and morphology of the immune organs of broiler chickens. We speculated that increased Hsps in the immune organs of broiler chickens might play roles in protecting immune organs due to Se deficiency. Meanwhile, we observed that the mRNA expression levels of Hsp27, Hsp40, and Hsp70 decreased idiosyncratically in the thymus. However, our results provided complementary data concerning the effect of Se deficiency on immune organs in broiler chickens.
References
Lenz M, Lens PN (2009) The essential toxin: the changing perception of selenium in environmental sciences. Sci Total Environ 407(12):3620–3633. doi:10.1016/j.scitotenv.2008.07.056
Ruan H, Zhang Z, Wu Q, Yao H, Li J, Li S, Xu S (2012) Selenium regulates gene expression of selenoprotein W in chicken skeletal muscle system. Biol Trace Elem Res 145(1):59–65. doi:10.1007/s12011-011-9166-y
Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G (1992) Regulation of cellular immune responses by selenium. Biol Trace Elem Res 33:23–35
Estevez AO, Mueller CL, Morgan KL, Szewczyk NJ, Teece L, Miranda-Vizuete A, Estevez M (2012) Selenium induces cholinergic motor neuron degeneration in Caenorhabditis elegans. Neurotoxicology 33(5):1021–1032. doi:10.1016/j.neuro.2012.04.019
Zhao X, Yao H, Fan R, Zhang Z, Xu S (2014) Selenium deficiency influences nitric oxide and selenoproteins in pancreas of chickens. Biol Trace Elem Res 161(3):341–349. doi:10.1007/s12011-014-0139-9
Hawkes WC, Kelley DS, Taylor PC (2001) The effects of dietary selenium on the immune system in healthy men. Biol Trace Elem Res 81(3):189–213. doi:10.1385/BTER:81:3:189
Marsh JA, Combs GF Jr, Whitacre ME, Dietert RR (1986) Effect of selenium and vitamin E dietary deficiencies on chick lymphoid organ development. Proc Soc Exp Biol Med Soc Exp Biol Med 182(4):425–436
Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G (1990) Selenium and immune cell functions. I. Effect on lymphocyte proliferation and production of interleukin 1 and interleukin 2. Proc Soc Exp Biol Med Soc Exp Biol Med 193(2):136–142
Ongele EA, Ashraf M, Nesbitt RA, Humphrey PA, Lee CM (2002) Effects of selenium deficiency in the development of trypanosomes and humoral immune responses in mice infected with Trypanosoma musculi. Parasitol Res 88(6):540–545. doi:10.1007/s00436-002-0617-4
Zhang ZW, Wang QH, Zhang JL, Li S, Wang XL, Xu SW (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149(3):352–361. doi:10.1007/s12011-012-9439-0
Yu J, Bao E, Yan J, Lei L (2008) Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperones 13(3):327–335. doi:10.1007/s12192-008-0031-7
Lei L, Yu J, Bao E (2009) Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br Poult Sci 50(4):504–511. doi:10.1080/00071660903110851
Wang S, Edens FW (1998) Heat conditioning induces heat shock proteins in broiler chickens and turkey poults. Poult Sci 77(11):1636–1645
Zhao FQ, Zhang ZW, Qu JP, Yao HD, Li M, Li S, Xu SW (2014) Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones 19(5):635–648. doi:10.1007/s12192-013-0489-9
Liu C, Wang XS, Xu Z, Li M, Zhang ZW, Min YH, Khoso PA, Li S (2014) Effects of avermectin on heat shock proteins expression and histopathology in spleen tissues of pigeon. Chem Biol Interact 224C:176–182. doi:10.1016/j.cbi.2014.10.035
Zhao FQ, Zhang ZW, Wang C, Zhang B, Yao HD, Li S, Xu SW (2013) The role of heat shock proteins in inflammatory injury induced by cold stress in chicken hearts. Cell Stress Chaperones 18(6):773–783. doi:10.1007/s12192-013-0429-8
Banerjee Mustafi S, Chakraborty PK, Dey RS, Raha S (2009) Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones 14(6):579–589. doi:10.1007/s12192-009-0109-x
Li M, Wang XS, Xu FP, Liu S, Xu SW, Li S (2014) The change in heat shock protein expression in avermectin induced neurotoxicity of the pigeon (Columba livia) both in vivo and in vitro. Ecotoxicol Environ Saf 110:95–102. doi:10.1016/j.ecoenv.2014.08.015
Martinez J, Perez-Serrano J, Bernadina WE, Rodriguez-Caabeiro F (2001) HSP60, HSP70 and HSP90 from Trichinella spiralis as targets of humoral immune response in rats. Parasitol Res 87(6):453–458
Habich C, Burkart V (2007) Heat shock protein 60: regulatory role on innate immune cells. Cell Mol Life Sci: CMLS 64(6):742–751. doi:10.1007/s00018-007-6413-7
Chen W, Syldath U, Bellmann K, Burkart V, Kolb H (1999) Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol 162(6):3212–3219
van Eden W, van der Zee R, Prakken B (2005) Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol 5(4):318–330. doi:10.1038/nri1593
Kojouri GA, Faramarzi P, Ahadi AM, Parchami A (2013) Effect of selenium nanoparticles on expression of HSP90 gene in myocytes after an intense exercise. J Equine Vet Sci 33(12)):1054–1056. doi:10.1016/j.jevs.2013.04.001
Kumar GS, Kulkarni A, Khurana A, Kaur J, Tikoo K (2014) Selenium nanoparticles involve HSP-70 and SIRT1 in preventing the progression of type 1 diabetic nephropathy. Chem Biol Interact 223:125–133. doi:10.1016/j.cbi.2014.09.017
Bernet D, Schmidt H, Meier W, Burkhardt‐Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22(1):25–34
Figueiredo‐Silva A, Rocha E, Dias J, Silva P, Rema P, Gomes E, Valente L (2005) Partial replacement of fish oil by soybean oil on lipid distribution and liver histology in European sea bass (Dicentrarchus labrax) and rainbow trout (Oncorhynchus mykiss) juveniles. Aquac Nutr 11(2):147–155
El-Demerdash FM, Nasr HM (2014) Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J Trace Elements Med Biol: Organ Soc Miner Trace Elements 28(1):89–93. doi:10.1016/j.jtemb.2013.10.001
Ferreccio C, Gonzalez Psych C, Milosavjlevic Stat V, Marshall Gredis G, Sancha AM (1998) Lung cancer and arsenic exposure in drinking water: a case-control study in northern Chile. Cad Saude Publica 14(Suppl 3):193–198
Sunde RA, Hadley KB (2010) Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med 235(1):23–31. doi:10.1258/ebm.2009.009262
Yao HD, Zhao WC, Zhao X, Fan RF, Khoso PA, Zhang ZW, Liu W, Xu SW (2014) Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol Trace Eleme Res 161(3):318–327. doi:10.1007/s12011-014-0125-2
Liu CP, Fu J, Xu FP, Wang XS, Li S (2015) The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers. Biometals : Int J Role Met Ions Biol Biochem Med 28(1):163–173. doi:10.1007/s10534-014-9812-x
Sun D, Li C, Gao J, Li S, Wang H (2015) Effects of selenium deficiency on principal indexes of chicken kidney function. Biol Trace Elem Res 164(1):58–63. doi:10.1007/s12011-014-0196-0
Yu J, Yao HD, Gao XJ, Zhang ZW, Wang JF, Xu SW (2015) The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol Trace Elem Res 163(1-2):144–153. doi:10.1007/s12011-014-0164-8
Tinkel J, Hassanain H, Khouri SJ (2012) Cardiovascular antioxidant therapy: a review of supplements, pharmacotherapies, and mechanisms. Cardiol Rev 20(2):77–83. doi:10.1097/CRD.0b013e31823dbbad
Chen X, Yao H, Yao L, Zhao J, Luan Y, Zhang Z, Xu S (2014) Selenium deficiency influences the gene expressions of heat shock proteins and nitric oxide levels in neutrophils of broilers. Biol Trace Elem Res 161(3):334–340. doi:10.1007/s12011-014-0150-1
Liu T, Zhang ZW, Chen DC, Wang LL, Yao HD, Zhao FQ, Xing HJ, Xu SW (2013) Effect of atrazine and chlorpyrifos exposure on heat shock protein response in the brain of common carp (Cyprinus carpio L.). Pestic Biochem Phys 107(2):277–283. doi:10.1016/j.pestbp.2013.09.002
Mahmoud KZ, Edens FW, Eisen EJ, Havenstein GB (2004) The effect of dietary phosphorus on heat shock protein mRNAs during acute heat stress in male broiler chickens (Gallus gallus). Comp Biochem Physiol Toxicol Pharmacol: CBP 137(1):11–18. doi:10.1016/j.cca.2003.10.013
Sorensen JG, Loeschcke V (2007) Studying stress responses in the post-genomic era: its ecological and evolutionary role. J Biosci 32(3):447–456
Lewis S, Donkin ME, Depledge MH (2001) Hsp70 expression in Enteromorpha intestinalis (Chlorophyta) exposed to environmental stressors. Aquat Toxicol 51(3):277–291
Jing J, Liu H, Chen H, Hu S, Xiao K, Ma X (2013) Acute effect of copper and cadmium exposure on the expression of heat shock protein 70 in the Cyprinidae fish Tanichthys albonubes. Chemosphere 91(8):1113–1122. doi:10.1016/j.chemosphere.2013.01.014
Schlenk D (1996) The role of biomarkers in risk assessment.
Soldes OS, Kuick RD, Thompson IA 2nd, Hughes SJ, Orringer MB, Iannettoni MD, Hanash SM, Beer DG (1999) Differential expression of Hsp27 in normal oesophagus, Barrett's metaplasia and oesophageal adenocarcinomas. Br J Cancer 79(3-4):595–603. doi:10.1038/sj.bjc.6690094
Lo Muzio L, Leonardi R, Mariggio MA, Mignogna MD, Rubini C, Vinella A, Pannone G, Giannetti L, Serpico R, Testa NF, De Rosa G, Staibano S (2004) HSP 27 as possible prognostic factor in patients with oral squamous cell carcinoma. Histol Histopathol 19(1):119–128
Aita M, Amantea A (1991) Distribution of anti-keratins and anti-thymostimulin antibodies in normal and in Down's syndrome human thymuses. Thymus 17(3):155–165
Molinero P, Osuna C, Guerrero JM (1995) Type II thyroxine 5′-deiodinase in the rat thymus. J Endocrinol 146(1):105–111
Acknowledgments
The authors thank the members of the Veterinary Internal Medicine Laboratory, College of Veterinary Medicine, and Northeast Agriculture University for their help with sample collection.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31472161).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, Z., Liu, C., Zheng, W. et al. The Functions of Antioxidants and Heat Shock Proteins Are Altered in the Immune Organs of Selenium-Deficient Broiler Chickens. Biol Trace Elem Res 169, 341–351 (2016). https://doi.org/10.1007/s12011-015-0407-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0407-3