Abstract
Boric acid (BA) has positive effects on bone tissue. In this study, the effects of BA on fracture healing were evaluated in an animal model. Standard closed femoral shaft fractures were created in 40 male Sprague–Dawley rats under general anesthesia. The rats were allocated into five groups (n = 8 each): group 1, control with no BA; groups 2 and 3, oral BA at doses of 4 and 8 mg/kg/day, respectively; group 4, local BA (8 mg/kg); and group 5, both oral and local BA (8 mg/kg/day orally and 8 mg/kg locally). After closed fracture creation, the fracture line was opened with a mini-incision, and BA was locally administered to the fracture area in groups 4 and 5. In groups 2, 3, and 5, BA was administered by gastric gavage daily until sacrifice. The rats were evaluated by clinical, radiological, and histological examinations. The control group (group 1) significantly differed from the local BA-exposed groups (groups 4 and 5) in the clinical evaluation. Front-rear and lateral radiographs revealed significant differences between the local BA-exposed groups and the control and other groups (p < 0.05). Clinical and radiological evaluations demonstrated adequate agreement between observers. The average histological scores significantly differed across groups (p = 0.007) and were significantly higher in groups 4 and 5 which were the local BA (8 mg/kg) and both oral and local BA (8 mg/kg/day orally and 8 mg/kg locally), respectively, compared to the controls. This study suggests that BA may be useful in fracture healing. Further research is required to demonstrate the most effective local dosage and possible use of BA-coated implants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When a bone breaks, several complex cellular and biochemical processes occur to ensure integrity and fracture healing. Many factors can positively or negatively affect these processes [1, 2]. Boric acid (BA) is an important element in bone metabolism thought to affect bone fracture through many metabolic processes, and it interacts with calcium, vitamin D, and magnesium [3, 4]. Recent research into the effects of boron (B) on mice physiology has shown it to be helpful in bone growth and repair, including effects on the presence or activity of osteoblasts and osteoclasts, but not on bone calcium concentrations [5].
Boron is an essential trace element for vascular plants, and it is a ubiquitous element in the human environment [3]. It is present mostly in bodily tissues and fluids, such as in bone, hair, and nails, which tend to have higher levels of boron than other tissues in the human body [6]. The molecular effects of boron on bone metabolism have been observed. According to the study performed on cell culture of mouse osteoblasts, remarkable regulation in favor of osteoblastic function for collagen type I (COL I), osteopontin (OPN), bone sialoprotein (BSP), osteocalcin (OCN), and RunX2 mRNA expressions was observed in B treatment groups in comparison with untreated control groups [7]. Also, its effects on bone regeneration have also been demonstrated [8]. In another study, boron was doped on 45S5 bioactive glass and found that the controlled and localized release of borate ions could represent a promising alternative therapeutic strategy to achieve neovascularization in regenerative medicine and tissue engineering of vascularized tissues, such as the bone [9].
In this experimental study, we aimed to investigate whether local and oral administration of BA improves fracture healing, and we hypothesized that BA leads to positive effects on fracture healing.
Material and Methods
Animals
The protocol for this study was approved by the Institutional Animal Care and Use Committee at the Çanakkale Onsekiz Mart University Medical School. All animal experiments were conducted in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (revised, 1985). Forty male Sprague–Dawley rats weighing approximately 400–500 g were included. During the experimental procedure, all rats were housed under standard laboratory conditions with an artificial 12-h light/dark cycle. They were caged individually under a controlled temperature (22 ± 1 °C) and relative humidity and allowed free access to food and water in polycarbonate units. The rats were observed for 7 days in the animal care laboratory to exclude any possibility of underlying disease.
Surgical Technique
The rats were operated on following an intramuscular injection of ketamine HCl (50 mg/kg, Ketalar; Parke-Davis, Morris Plains, NJ, USA) and xylazine anesthesia (10 mg/kg, Rompun; Bayer, Istanbul, Turkey), as described by Bonnarens [10]. A femoral channel was prepared by means of a 1-mm Kirschner wire (Hipokrat®, Izmir, Turkey) that was inserted through the femoral condyles, and a 0.8-mm Kirschner wire (Hipokrat®, Izmir, Turkey) was inserted into this channel (Fig. 1). To constitute a standard closed fracture after surgery, the guillotine method defined by Bonnarens and Einhorn [2, 10] was used. After closed fracture creation, the fracture line was opened with a lateral 1-cm mini-incision, and BA was locally applied to two groups (groups 4 and 5). For the other three groups, the fracture line was also opened by mini-incision, but no BA was applied. Then, the fascia and skin were closed with 3/0 absorbable suture (Vicryl Rapide). Fracture formation was confirmed clinically and radiologically.
Experimental Design
The rats were randomly divided into five independent groups, each containing eight rats: control group (closed femur fracture created), group 2 (oral 4 mg/kg BA per day), group 3 (oral 8 mg/kg BA per day), group 4 (local 8 mg/kg BA), and group 5 (oral 8 mg/kg BA per day + local 8 mg/kg). For oral BA-exposed groups, BA was dissolved in saline, and the BA exposure was initiated at the same hour every day. For local administration, the BA was used in a powder form and was measured with the aid of digital precision scales.
None of the animals received antibiotic prophylaxis during or after surgery, and none died after surgery. The rats were humanely euthanized 4 weeks postoperatively.
Clinical Evaluation of Union
After 4 weeks, the animals were sacrificed, and the right femur, knee, and hip joints were disarticulated. The muscles and other soft tissues were skimmed, and the K-wires were removed (Fig. 1). Bone union was clinically evaluated using the method of Dimar et al. [11]. A pathological movement examination was performed, and movement in the two planes was evaluated subjectively by macroscopic assessment. No movement in the sagittal and coronal planes of the fracture (front-rear and lateral) was defined as full bone union (two points), movement in only one plane was considered moderate bone union (one point), and movement in both planes was considered nonunion (zero point). Scoring was performed by two different independent orthopedists.
Radiographic Evaluation
After the clinical examination, the femur was evaluated radiographically. The fracture healing of the femur was examined by postero-anterior and lateral plain radiographs (Mammography Unit; Faxitron Corporation, Wheeling, IL, USA) at the end of the fourth week. These radiographs were scored using the Goldberg classification [12] by two different independent orthopedists who were blinded to group number of the rats. Femur nonunion was assigned one point; moderate union, two points; and union, three points. The anterior–posterior (Fig. 2) and lateral radiographs (Fig. 3) of all groups were evaluated separately.
Histological Evaluation
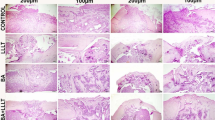
Histological evaluations were conducted after clinical and radiological assessments. The bones were fixed in 10 % formaldehyde solution. The specimens were decalcified in 10 % acetic acid, 85 % NaCl, and 10 % formaldehyde solution for 72 h. After decalcification, longitudinal sections were taken from the fracture site, including the proximal and distal sites of the fracture. All slides were stained with standard hematoxylin and eosin (H&E) methods and evaluated by a pathologist who was blinded to the groups (Fig. 4). Fracture healing was graded (1–10), as per Huo et al. [13] (Table 1), based on the ratios of the fibrous tissue, fibrocartilage, cartilage, and bone areas in the fracture site.
Histological grading of callus tissue according to the groups. Groups 4 and 5 have significantly higher grading scores compared to other groups. Group 1: sections showing fibrous tissue (thin arrow) in a grade 1 callus formation (H&E, ×10). Group 2: sections showing equal chondroid (thin arrow) and immature bone (thick arrow) in a grade 3 callus formation (H&E, ×10). Group 3: sections showing cartilage tissue (thin arrow) in a grade 5 callus formation (H&E, ×10). Group 4: sections showing more immature bone (thin arrow) and little cartilage tissue (thick arrow) in a grade 8 callus formation (H&E, ×10). Group 5: sections showing immature bone (thin arrow) in a grade 9 callus formation (H&E, ×10)
Statistical Analysis
SPSS software (SPSS 19.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Descriptive values were presented as mean, standard deviation, median, minimum, maximum, frequency, and percentage values. To compare the groups, a chi-square test was used for categorical variables, as well as the Kruskal–Wallis test for continuous variables, and a Mann–Whitney U test with a Bonferroni-corrected 95 % confidence interval. The threshold for statistical significance was p ˂ 0.05.
Cohen’s kappa statistical analysis was used to evaluate inter-observed agreement, and the results were interpreted according to Landis and Koch’s guidelines [14], with 0.00–0.20 indicating very low agreement, 0.21–0.40 indicating minor agreement, 0.41–0.60 indicating moderate agreement, 0.61–0.80 indicating adequate agreement, and 0.80–1.00 indicating strong agreement.
The power analysis based on two-sided paired test with level of significance of 0.05 indicated that 38 rats should be included. We decided to include 40 rats to cover potential loss of animals during the course of the experiment.
Results
Clinical Evaluation and Analysis
The clinical evaluation assessed with Cohen’s kappa statistical method demonstrated adequate inter-observed agreement (kappa = 0.716). In total, 33 rats that demonstrated clinical examination agreement by the two observers were included. Groups were compared using the chi-square test. Clinical score was significantly higher in groups 4 and 5 which were the local BA (8 mg/kg) and both oral and local BA (8 mg/kg/day orally and 8 mg/kg locally), respectively, compared to the control group (p < 0.05).
Radiographic Analysis
The two orthopedists demonstrated adequate inter-observer agreement for front-rear and lateral radiographs (kappa = 0.693 and 0.667, respectively). To compare the radiological evaluation results of the front-rear radiographs among the groups, 33 rats that demonstrated agreement by the two observers were considered, and the chi-square test was used (Table 2). To compare the radiological evaluation results of the lateral radiographs, 33 rats that demonstrated agreement by the two observers were considered, and the chi-square test was again used to compare the groups (Table 3).
Histological Analysis
Kruskal–Wallis analysis of variance was used to compare the mean histological scores among the groups. A statistically significant difference was observed across the mean histological scores of the groups (Table 4). A Mann–Whitney U test was used to compare the groups, and a Bonferroni-corrected 95 % confidence interval was applied for statistical significance.
The histological grade scores in groups 4 and 5 were significantly higher than those of the control group. There were no significant differences between other groups (Fig. 5). The histological grade scores were also higher in other experimental groups compared to the control group, but these differences were not significant within the Bonferroni-corrected 95 % confidence intervals.
Discussion
Boron satisfies several of the criteria for essentiality in humans [15]. Recent studies have shown that boron may be an essential dietary component for animals and humans. Hunt claims that boron meets most of the criteria of a necessary nutrient [16]. In addition, Hunt et al. [17] also reported that a boron intake of 3.23 mg/day was associated with increased bone formation and bone mineral metabolism.
According to animal studies, physiological amounts of supplemental dietary boron influence a large number of metabolic parameters [18]. In the literature, the subtoxic dose of BA was reported as 8.7 mg/kg [6], and the preferred BA dose for experimental studies in rats was 3–4 mg/kg [5, 8]. Therefore, in the present study, 4 mg/kg/day boron was used as the therapeutic dose, and twice this amount (8 mg/kg/day) was established as a high but still subtoxic dose. In the literature, there is little data concerning the optimal dose for local BA application. We chose 8 mg/kg BA for local application, as it represented a high but still subtoxic dose.
Depending on the amount of the element supplied orally, boron is immediately and completely absorbed from the gastrointestinal tract into the bloodstream and accumulates in the bone. It also interacts with other significant bone metabolism agents including calcium, vitamin D, and magnesium [15]. The effects of dietary BA on bone strength have been researched in animals, and studies related to BA have shown beneficial effects on bone strength and bone formation [19]. Our results also support this evidence by showing clear positive effects of BA on fracture healing.
Several possible mechanisms that explain the effects of BA on bone healing have been suggested. As a possible mechanism underlying the effects of BA on bone healing is the increased presence and activity of osteoblasts and osteoclasts. [5].
Bone formation and fracture repair are not yet fully understood. The administration of various drugs to patients with fractures can alter the duration of treatment until a solid bony union is attained [20]. Factors affecting fracture healing and the acceleration of healing have been frequent subjects of research. Studies have particularly focused on the acceleration of fracture healing. The effects of frequently used drugs on fracture healing comprise an important part of the literature, and the effects of some drugs, such as montelukast, recombinant human parathyroid hormone, and vitamin C, on fracture healing have been clearly established [21–23]. In this study, we aimed to demonstrate the possible contribution of BA to fracture healing because of its known effects on bone metabolism. Although several studies have reported the effects of BA on bone metabolism, its effects on fracture healing have remained unclear. However, BA has been shown to decrease periodontal inflammation, alveolar bone loss, and the severity of alveolar disease [24].
BA was shown to increase osteogenic effects by stimulating osteogenic-differentiation-related marker gene synthesis during the proliferation and differentiation cycle in human bone marrow stromal cells [25]. On histological examination, we also found that fracture healing was better in locally applied BA groups. However, as a limitation of our study, we did not investigate any bone metabolic markers, tissue-associated genes (BSP, OCN, RunX2), and RANKL/OPG levels or ratio for bone formation/remodeling. Another limitation of the study is that the study did not evaluate the toxicity of the BA for internal organs like the kidney or liver.
The micronutrients of particular interest with regard to bone health are magnesium, vitamin D, phosphorus, and calcium. Boron has been observed to positively influence the metabolism of these dietary substances, which all play a role in maintaining bone health. As a result, boron may be nutritionally significant in preventing osteoporosis in humans [26]. Besides, boron supplementation has shown advantageous effects on bone characteristics in hens, and BA is commonly acknowledged to increase the strength of the tibia and femur [17]. In our study, two independent orthopedists evaluated the radiological and clinical effects of boron on fracture healing, with the agreement between them assessed statistically, and the results were similar to the histological evaluation results. Although humans and animals absorb nearly 90–100 % of supplemental inorganic boron [27], the positive effects of BA were most prominent in the locally administered BA groups in our study. The same positive effect was not seen in oral BA groups. We think that this difference may be the result of the fracture damaging nutritional arteries, with subsequent insufficient transfer of BA to the fracture line.
Conclusions
BA may be useful as a therapeutic agent in fracture healing. However, further research is required to demonstrate the most effective local dosage of BA for fracture healing, as well as whether BA affects fracture healing in the human body. Due to the local effect of BA, BA-coated implants should also be considered in the future.
References
Buckwalter JA, Einhorn TA, Marsh JL (2006) Bone and joint healing. In: Rockwood CA, Green DP, Bucholz RW, Heckman JD (eds) Fracture in adults, vol I, 6th edn. Lippincott Williams-Wilkins, Philadelphia, pp 297–311
Einhorn TA (1995) Enhancement of fracture-healing. J Bone Joint Surg Am 77:940–956
Benderdour M, Hess K, Gadet MD, Dousset B, Nabet P, Belleville F (1997) Effect of boric acid solution on cartilage metabolism. Biochem Biophys Res Commun 234:263–268
Devirian TA, Volpe SL (2003) The physiological effects of dietary boron. Crit Rev Food Sci Nutr 43:219–231
Gorustovich AA, Steimetz T, Nielsen FH, Guglielmotti MB (2008) A histomorphometric study of alveolar bone modelling and remodelling in mice fed a boron-deficient diet. Arch Oral Biol 53:677–682
Gallardo-Williams MT, Maronpot RR, Turner CH et al (2003) Effects of boric acid supplementation on bone histomorphometry, metabolism, and biomechanical properties in aged female F-344 rats. Biol Trace Elem Res 93:155–170
Hakki SS, Bozkurt BS, Hakki EE (2010) Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J Trace Elem Med Biol 24:243–250
Uysal T, Ustdal A, Sonmez MF, Ozturk F (2009) Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. Angle Orthod 79:984–990
Haro Durand LA, Vargas GE, Romero NM, Vera-Mesones R, Porto-López JM, Boccaccini AR et al (2015) Angiogenic effects of ionic dissolution products released from a boron-doped 45S5 bioactive glass. J Mater Chem B 3:1142–1148
Bonnarens F, Einhorn TA (1984) Production of a standard closed fracture in laboratory animal bone. J Orthop Res 2:97–101
Dimar JR 2nd, Ante WA, Zhang YP, Glassman SD (1996) The effects of nonsteroidal anti-inflammatory drugs on posterior spinal fusions in the rat. Spine (Phila Pa 1976) 21:1870–1876
Goldberg VM, Powell A, Shaffer JW, Zika J, Bos GD, Heiple KG (1985) Bone grafting: role of histocompatibility in transplantation. J Orthop Res 3:389–404
Huo MH, Troiano NW, Pelker RR, Gundberg CM, Friedlaender GE (1991) The influence of ibuprofen on fracture repair: biomechanical, biochemical, histologic, and histomorphometric parameters in rats. J Orthop Res 9:383–390
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Gorustovich AA, Steimetz T, Nielsen FH, Guglielmotti MB (2008) Histomorphometric study of alveolar bone healing in rats fed a boron-deficient diet. Anat Rec (Hoboken) 291:441–447
Hunt CD (1998) Regulation of enzymatic activity: one possible role of dietary boron in higher animals and humans. Biol Trace Elem Res 66:205–225
Wilson JH, Ruszler PL (1998) Long term effects of boron on layer bone strength and production parameters. Br Poult Sci 39:11–15
Gallardo-Williams MT, Chapin RE, King PE et al (2004) Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma (LNCaP) tumors in nude mice. Toxicol Pathol 32:73–78
Armstrong TA, Spears JW (2001) Effect of dietary boron on growth performance, calcium and phosphorus metabolism, and bone mechanical properties in growing barrows. J Anim Sci 79:3120–3127
Pountos I, Georgouli T, Blokhuis TJ, Pape HC, Giannoudis PV (2008) Pharmacological agents and impairment of fracture healing: what is the evidence? Injury 39:384–394
Alkhiary YM, Gerstenfeld LC, Krall E et al (2005) Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34). J Bone Joint Surg Am 87:731–741
Cakici H, Hapa O, Gideroglu K et al (2011) The effects of leukotriene receptor antagonist montelukast on histological, radiological and densitometric parameters of fracture healing. Eklem Hastalik Cerrahisi 22:43–47
Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B (2001) The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg 121:426–428
Demirer S, Kara MI, Erciyas K, Ozdemir H, Ozer H, Ay S (2012) Effects of boric acid on experimental periodontitis and alveolar bone loss in rats. Arch Oral Biol 57:60–65
Ying X, Cheng S, Wang W et al (2011) Effect of boron on osteogenic differentiation of human bone marrow stromal cells. Biol Trace Elem Res 144:306–315
Nielsen FH (1990) Studies on the relationship between boron and magnesium which possibly affects the formation and maintenance of bones. Magnes Trace Elem 9:61–69
Hunt CD, Herbel JL, Nielsen FH (1997) Metabolic responses of postmenopausal women to supplemental dietary boron and aluminum during usual and low magnesium intake: boron, calcium, and magnesium absorption and retention and blood mineral concentrations. Am J Clin Nutr 65:803–813
Acknowledgments
The authors thank the Experimental Research Center of Çanakkale Onsekiz Mart University.
Conflict of Interest
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gölge, U.H., Kaymaz, B., Arpaci, R. et al. Effects of Boric Acid on Fracture Healing: An Experimental Study. Biol Trace Elem Res 167, 264–271 (2015). https://doi.org/10.1007/s12011-015-0326-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0326-3